Abstracts
Over the last few decades, evidence has been emerging that the pathogenesis of psychiatric disorders such as schizophrenia can involve perturbations of the hypothalamic-pituitary-adrenal (HPA) axis. Variations in the manifestation of these effects could be related to the differences in clinical symptoms between affected individuals as well as to differences in treatment response. Such effects can also arise from the complex interaction between genes and environmental factors. Here, we review the effects of maternal stress on abnormalities in HPA axis regulation and the development of psychiatric disorders including schizophrenia. Studies in this area may prove critical for increasing our understanding of the multi-dimensional nature of schizophrenia. Further research in this area could ultimately lead to the development of improved diagnostics and novel therapeutic approaches for treating this debilitating psychiatric condition.
Psychiatric disorders; schizophrenia; HPA axis dysfunction; diagnosis; biomarkers
Nas últimas décadas, têm surgido evidências sugerindo que a patogênese de desordens psiquiátricas, tais como a esquizofrenia, pode envolver perturbações no eixo hipotalâmico-pituitário-adrenal (HPA). Variações na manifestação desses efeitos poderiam estar relacionadas a diferenças em sintomas clínicos entre os indivíduos afetados, assim como a diferenças na resposta ao tratamento. Tais efeitos podem também ser originados de complexas interações entre genes e fatores ambientais. Aqui, revisamos os efeitos do estresse maternal em anormalidades na regulação do eixo HPA e desenvolvimento de desordens psiquiátricas, incluindo a esquizofrenia. Estudos nessa área podem gerar o aumento do nosso entendimento da natureza multidimensional da esquizofrenia. Posterior pesquisa nesse campo poderia, em última instância, levar ao desenvolvimento de melhores diagnósticos e novas abordagens terapêuticas para essa debilitante condição psiquiátrica.
Desordens psiquiátricas; esquizofrenia; disfunção do eixo HPA; diagnóstico; biomarcadores
The effects of stress on hypothalamic-pituitary-adrenal (HPA) axis function in subjects with schizophrenia
Francesca L. GuestI; Daniel Martins-de-SouzaII, III; Hassan RahmouneII; Sabine BahnII, IV; Paul C. GuestII
IFaculty of Medicine and Dentistry, University of Bristol, Bristol, UK
IIDepartment of Chemical Engineering and Biotechnology, University of Cambridge, Cambridge, UK
IIIMax Planck Institute of Psychiatry & Ludwig Maximilians University (LMU), Munich, Germany; Laboratory of Neurosciences (LIM-27), Institute of Psychiatry, School of Medicine, University of Sao Paulo (IPq-FMUSP), Sao Paulo, SP, Brazil
IVDepartment of Neuroscience, Erasmus Medical Centre, The Netherlands
Correspondence
ABSTRACT
Over the last few decades, evidence has been emerging that the pathogenesis of psychiatric disorders such as schizophrenia can involve perturbations of the hypothalamic-pituitary-adrenal (HPA) axis. Variations in the manifestation of these effects could be related to the differences in clinical symptoms between affected individuals as well as to differences in treatment response. Such effects can also arise from the complex interaction between genes and environmental factors. Here, we review the effects of maternal stress on abnormalities in HPA axis regulation and the development of psychiatric disorders including schizophrenia. Studies in this area may prove critical for increasing our understanding of the multi-dimensional nature of schizophrenia. Further research in this area could ultimately lead to the development of improved diagnostics and novel therapeutic approaches for treating this debilitating psychiatric condition.
Keywords: Psychiatric disorders, schizophrenia, HPA axis dysfunction, diagnosis, biomarkers.
Introduction
During a stressful event, chemicals such as adrenaline and glucocorticoids are released into the bloodstream leading to a chain reaction of events inside the body. The effect of these chemicals from stress situations experienced during pregnancy has also been investigated. David Barker proposed the "fetal programming hypothesis" - also known simply as the Barker hypothesis - in his paper of 1989, which details the importance of the intrauterine environment in the development of the fetal organs and tissues1. This hypothesis suggests that deviation from the ideal intrauterine environment may have lasting effects on organ structure and function1,2. Extrapolation of this concept to the brain suggests that the intrauterine environment during the first and second trimesters of pregnancy will have direct effects on the structure of the brain in the fetus, which may continue on into adulthood.
Correlations between prenatal maternal stress and behavioral and psychological abnormalities in animal offspring have been widely documented. It has been proposed that hormones released into the bloodstream in response to a stressful stimuli perceived by the mo-ther, have a direct effect on development of the brain and other organs in the fetus. The correct regulation of hormone release in pregnancy is critical for correct fetal development and any deviation from the normal concentration of these hormones can produce microscopic and macroscopic changes in the brain, particularly in synaptic connectivity within and across distinct brain regions3. However, it is not one acute stressor, but chronic stress which predisposes to psychiatric disorders in the offspring. The development of behavioral and psychological problems including autism, attention-deficit hyperactivity disorder (ADHD), major depressive disorder, bipolar disorder and schizophrenia are thought to be linked to such perturbations in the hypothalamic-pituitary-adrenal (HPA) axis and in other organs of the diffuse neuroendocrine system.
From the research carried out by others it is clear that there is some correlation between increased maternal stress and behavioural and psychological problems in the offspring, but this is not thought to be purely hormonal. It also speculated that genetic links may exist for many of these diseases and that these may be, in some way, hereditary. It should also be considered that mothers with psychological problems or who have problems with stress management may be more likely to become stressed during pregnancy and thus induce a similar condition in their offspring. What is more likely is that these problems have both hormonal and genetic causes. The two hit hypothesis4 suggests that a genetic susceptibility exists in the fetus and then an anomalous hormonal environment in the uterus allows an abnormality to come to fruition.
This review aims to summarize and correlate the existing literature on the effect of prenatal stress on development of the fetal brain and how this may affect the behavior, psychology and overall health of the offspring later on in life. As much of the current literature reports the use of animal models to test these hypotheses, we will speculate on the extent to which these findings can be extrapolated to humans. We will also propose the likely effects of prenatal stress on the development of psychiatric disorders in humans and discuss the reported effects of this on the function of the HPA axis and other neuroendocrine systems. Finally, we will address the importance of the timing of the stress stimuli with respect to the gestational period in the mother as well as the impact of this on the existence and severity of behavioral problems in the offspring.
Stress: definitions
The book Neuroendocrinology, an integrated approach describes stress as "any event, whether real or perceived, that acts to disturb the homeostatic balance"5. When we think of stress in everyday life, we tend to think of emotional stress. This could mean the effects of big deadlines, crucial examinations, overbearing bosses and important decisions which can all be included as stressful stimuli6. Not everyone responds to stress in the same way nor do they have the same stress threshold7,8. Some people will become stressed only in extreme situations, whereas others could become overly-stressed over seemingly small matters. Stress can be evoked by fear of the unforeseen, the unknown or even possible bad outcomes. For example, the fear that a loved one will have a serious injury, even though there is no evidence to suggest that this may happen. The most intense emotional stressors occur in close relationships, such as those between family members or partners9. Other types of stress include those of a mechanical or physical nature, such as impact injuries. Although this can lead to emotional stress in many circumstances, mechanical stress is not necessarily relevant to the subject of this review since this is focused more on biochemical and emotional stressors. Simple definitions of biochemical stress could be the addition of a harmful substance, deprivation of a substance that the body needs or any deviation from the norm. Examples include life at high altitude, a lack of protein in the diet, or enduring extremes of temperature.
The extent that a particular stress will affect an individual can depend simply on that individual's perception of whether they can cope with it. A person who feels trapped and unable to cope with a stressful event may experience depression or distress, whereas a small amount of stress may in fact boost another person to work harder, not to lose hope or to promote a feeling of well-being10. An example of the latter would be that completion of a particular project before a deadline will be helped by the pressure to do so. This has been described as "eustress"11. Some people describe themselves as working well under pressure and, therefore, may be among those who have a higher stress threshold.
The stress response in humans
In reaction to a stress-producing stimulus, the body produces a response via activation of the HPA axis. This is a necessary mechanism as it prepares the body for the well-known "fight or flight" reaction to a potentially dangerous situation12. However, the stress response that is produced does not differ greatly depending on the stressor. In humans the molecules greatly responsible for the stress response are adrenaline and glucocorticoids13. There are three main phases of the generalized stress response that are set into motion when a stressor is detected. The first phase involves the production of the catecholamine molecules noradrenaline and adrenaline. These are examples of monoamine hormones, which are derived from the amino acid tyrosine. The conversion from tyrosine to dihydroxyphenylalanine (DOPA) takes place in chromaffin cells found in the adrenal medulla14. Noradrenaline and adrenaline are released from the adrenal medulla almost instantaneously into the blood stream via the autonomic nervous system and affect a number of responses in the body. For example, the release of noradrenaline and adrenaline causes an increase in heart rate, constriction of blood vessels, widening of the pupils (midriasis), and an opening of respiratory passages. In addition insulin secretion is inhibited and glycogenolysis is stimulated in the muscles and liver, leading to a higher concentration of glucose in the blood stream15.
Secondly, around 20 minutes afterwards, corticotrophin-releasing factor (CRF) is released from the paraventricular nucleus (PVN) of the hypothalamus into the portal vessel system and median eminence of the hypothalamus. This, in turn, stimulates the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary directly into the blood stream. ACTH travels through the circulation to the adrenal glands, culminating in the release of glucocorticoids such as cortisol from the adrenal cortex. With respect to prenatal stress in humans, cortisol is the most important glucocorticoid. The elevation of cortisol levels in the blood results in the inhibition of the release of ACTH and CRF in a negative feedback loop mechanism5.
In the third stage, glucocorticoids produce similar responses to those of the catecholamines, including increased gluconeogenesis and increased lipolysis, leading to increased volumes of glycerol and fatty acids in the blood stream. Glucocorticoids also stimulate the mobilization of proteins from skeletal muscle and their deamination5.
Fetal programming in response to stress
Chronic prenatal stress is linked with morphological changes in the fetal brain but the mechanisms underlying these effects are not well known. The maternal and fetal HPA axes and placenta have been studied to determine whether these can serve as conduits for such morphological effects16. During pregnancy the mother's body undergoes many physical and chemical changes, including altered production of certain hormones. The levels of cortisol are typically found to be elevated in a pregnant mother17. Such an elevation of cortisol is essential for the growth of the fetus and it stimulates the production of surfactant. However excessively elevated levels of cortisol can permanently modify the growth of the fetus18. Stress alters the levels of many hormones and other molecules found in the blood and this can lead to changes in the fetus. Prenatal stress affects the placenta in particular, decreasing the function of this organ via decreased levels of nutrients and oxygen that reaches the fetus. The mechanism of this effect results from increased levels of adrenaline in the blood which induces increased vascular resistance, and restriction of the flow of blood to the placenta16.
The fetus has a natural barrier enzyme to maternal cortisol called 11β-hydroxysteroid dehydrogenase-2 (11β-HSD2)16. This molecule is down-regulated in instances of maternal stress, leaving the fetus more susceptible to cortisol exposure. The reduction in 11β-HSD2 also leads to an increase in the production of other hormones such as prostaglandins, oestrogens, and placental lactogen16. Also, placental CRH levels are subject to regulation by the maternal HPA axis19. Studies have shown that stress-induced increases in CRH concentrations can lead to macroscopic and microscopic changes in the hippocampus of the offspring20-22.
Behavioral and psychological problems
A number of different psychological problems have been shown to be linked to antenatal stress. An increased incidence in attention deficit hyperactivity disorder (ADHD) has been found in rhesus monkey offspring when mothers were exposed to loud noises during pregnancy23,24. Studies have shown that the offspring of mothers exposed to long term stress showed greater responses to stressors, abnormalities in coping with stress and disturbed social behaviour25,26. The incidence of ADHD was also found to be increased in offspring whose mothers experienced bereavement during pregnancy, as shown in a Danish study27. In this case, unexpected bereavement resulted in a 72% increase in the likelihood of ADHD occurring in the offspring. Many other researchers have reported a greater prevalence of ADHD in the offspring of stressed mothers28,29.
A number of reports have suggested that there is an increased incidence of schizophrenia in the offspring of prenatally-stressed mothers. The German army invasion of the Netherlands in May 1940, led to nationwide stress. A cohort consisting of the offspring of mo-thers exposed to this stress during the first, second and third trimesters of pregnancy were followed up and the prevalence of schizophrenia observed30. This revealed a greater incidence of schizophrenia in these subjects, with the largest increase seen in those whose mo-thers were exposed in the first trimester31-34. Similar findings resulted from studies of the Chinese Famine of 1958-196135. Again, in those individuals conceived at the peak of the famine showed an increased risk of schizophrenia and other conditions. Increased incidence of schizophrenia in the offspring of stressed mothers has also been reported by Huttenen et al.36, Kinney et al.37, and others.
Further evidence supporting the importance of intrauterine environment in the precipitation of psychiatric illnesses is seen in animal models. For example, studies involving the deprivation of protein in prenatal rats have shown that this can have long term effects on the brain in the offspring. These effects include changes in hippocampal morphology and glutamate and dopamine receptor binding38. Other studies have shown that restriction of protein intake during pregnancy may have a negative effect on fetal brain development. This is thought to be due to changes in maternal metabolism of lipids, which are an essential component of cellular membranes39. Interestingly, the behavioural abnormalities seen in these protein restricted models do not manifest until early adulthood, including effects on brain-regulated activities such as decreased pre-pulse inhibition of the startle response and hyperlocomotion, which are traditionally used as a measure of schizophrenia-like behavior40,41. A pre-pulse is a reduced version of a stimulus delivered to an individual shortly before a full version of a stimulus (pulse) is applied. The brain will normally down regulate its startle response to a stimulus if this is preceded by a pre-pulse. However, individuals with schizophrenia or Alzheimer's disease will show a startle response of the same magnitude regardless of the presence of a pre-pulse40-42.
Watson et al.43 reported a 13.3% incidence of depression in the offspring of mothers who were exposed to a severe earthquake compared to an incidence of 5.5% seen in the offspring on non-exposed mothers. Depression has also been linked to abnormalities seen in the HPA axis of rat dams suffering from prenatal stress44. After Louisiana was hit by a bout of hurricanes and tropical storms, a study examining the difference between the incidences of autism in the offspring of mothers was carried out45. This showed an incidence of 26.59/10,000 in the high exposure group, compared with that of 3.72/10,000 in the low exposure group. Beversdorf et al.46 also established a link between prenatal stress and autism. A number of other noteworthy fetal abnormalities have been linked with prenatal stress seen in the mother including cognitive, behavioural and emotional issues47-51. Interestingly, rat studies have shown that the offspring of mothers who were restrained in the last week of pregnancy are more likely to self-administer drugs, such as cocaine and amphetamines, than those from non-restrained mothers52.
Microscopic and macroscopic changes in the brain
Specific regions of the brain have been shown to be affected by prenatal stress both macroscopically and microscopically. These regions include the hippocampus53-57, amygdala58-60, corpus callosum61, cerebral cortex62,63, cerebellum64,65 and hypothalamus66,67. Since morphological changes in the above brain regions have been linked with certain psychological and behavioural problems, it is possible that prenatal stress affects the neurodevelopmental formation of these areas. This would, in turn, be expected to have behavioral effects, such as those seen in psychiatric disorders.
Changes in the size of the corpus callosum and the number of specific cell types within this brain structure have been linked with autism68, ADHD69 and schizophrenia70. Links of autism, ADHD and schizophrenia to prenatal stress have been detailed above. It may therefore be reasonable to extrapolate these findings to suggest that prenatal stress directly affects the structure of the corpus callosum. However, no studies in this area have been carried out in humans.
The hippocampus is known to be involved in the formation of memories and plays a major role in learning. Lemaire et al.71 reported that prenatal stress had a negative effect on the memory of rats. The hippocampal granule neurons continue to be created throughout life and are responsible for the formation of memories. However, the number of such granule neurons was reduced in adult rats with mothers that had been exposed to stress while pregnant. In addition, the authors showed that prenatal stress reduced hippocampal cell proliferation and survival rate of newborn cells, along with a reduced number of differentiated neurons. Interestingly, all of these deleterious effects could be counteracted by neonatal handling.
Although animal models have given us some insight into the connection between morphological changes in the brain, prenatal stress and behavioural disorders, it cannot be assumed that the same effects occur in humans. In fact, this is not likely to occur in some cases due to the fact that there are large differences with respect to time taken and the extent to which the brain undergoes development before, during and after birth in different animal species.
Timing and severity
In rats and non-human primates, the timing of the stressor in the pregnancy has been shown to be important58-60. A study using rats has shown a 64% increase in the production of corticosterone - a similar hormone to aldosterone in humans - after handling pregnant rats in the last week of gestation and placing them in new, unfamiliar cages72. Although some evidence has been put forward in the case of animal models, the timing and severity of a stressor which affects the human fetus is harder to determine. This difficulty is mainly due to the fact that it would be unethical to expose pregnant mothers to stress at different points during their pregnancy. However, natural disasters can be used for this purpose; such events can provide a large cohort of people affected by an identical stress. This allows retrospective assessment of those who were pregnant during the natural disaster and follow up studies of the offspring. In this way, we can assess whether any of the patterns seen in animal models can be extrapolated to humans.
A study termed "Project Ice Storm" attempted to determine the timing of the effects of maternal stress on the offspring using human subjects. In this case over 3 million people were unfortunately exposed to extreme cold due to power outages47. This study revealed an abnormality in fingerprint formation in offspring of mothers exposed to the storm between weeks 14 and 22 of pregnancy. Interestingly, the period of fingerprint formation is known to overlap with the formation of the hippocampus73,74. Therefore, it is possible that changes in the hippocampus may be seen in the same individuals. The project is still ongoing and is currently utilizing a magnetic resonance imaging (MRI) approach to determine whether behavioural and psychological problems seen in the offspring are linked to any changes in brain morphology. As the hippocampus is responsible for memory formation and cognitive reasoning, this region is of primary interest.
It must be acknowledged, that the effects of a particular stressor on a mother depends not only on the nature of the stressor itself but also on the severity of the perceived threat of the stressor by the mother and on the mother's stress tolerance and behavior in response to that stressor.
Potential advantages of stress
An interesting and potentially useful aspect of the stress response is that during acute stress the hippocampal dentate gyrus region is more active. This is the area of the brain responsible for learning, memory and neurogenesis. In response to a stressful stimulus the neural progenitor cells in the dentate gyrus are transformed into neurons and glia, which eventually lead to new synaptic connections. It is supposed that this is at least part of the mechanism of how memories are made and this process enables subjects to remember the object or event that caused the stress. An emotional input from the amygdala and the recognition of stress by the hippocampus cooperate to form a memory of fear and this will hopefully lead the individual to avoid similar dangerous situations in the future75.
In contrast, chronic stress appears to decrease the ability to form new memories. Conrad et al.76 showed that chronically-stressed rats performed less well on a spatial maze test than their non-stressed counterparts. In addition, studies in humans have shown that years of stressful living can lead to increased incidence of depression and other psychiatric conditions77. This leads to the possibility that acute stress can be beneficial and chronic stress detrimental.
Effects of stress on insulin resistance and the HPA axis
In humans, prenatal maternal stress during pregnancy has been shown to predict low birth weight and pre-term delivery21. Studies have shown that this is, in turn, linked with metabolic dysfunctions such as decreased glucose tolerance and increased insulin resistance78. Using an oral glucose tolerance test, a recent study analyzed the glucose levels of young adults whose mothers had experienced stressful life events during pregnancy such as relationship conflicts, death or severe illness of a close relative or friend, severe financial difficulties and car accidents, in comparison to control subjects whose mothers had relatively stress-free pregnancies79. The results showed no significant difference between the glucose levels of the two groups, although the subjects with mothers that suffered from prenatal stress had significantly higher insulin levels at the 120 minute time point after administration of the glucose tolerance test.
Interestingly, another study by the same authors showed that adults whose mothers were stressed during pregnancy had increased cortisol levels in response the Trier Social Stress Test (TSST) with a decreased cortisol response after administration of an ACTH stimulation test, indicating a possible dysregulation of the HPA axis80. This provided evidence in humans of an association between prenatal stress exposure and alterations of HPA axis function in the offspring (Figure 1).
A study using rat models showed that prenatal stress induced long-term changes in feeding behavior, glucose metabolism and insulin signalling81. These deviations from normal glucose and insulin handling are similar to problems seen in type II diabetes. The authors speculated that this was linked to the increased levels of glucocorticoids in the intrauterine environment that accompanies prenatal stress. Another study tested the effects of administering stress hormones directly into sheep in the early stages of pregnancy, with particular interest in the effect that this had on the regulation of glucose and insulin signalling in adult male offspring82. The study showed that the adult offspring of mothers which were administered stress hormones during pregnancy had an impaired glucose tolerance and hyperinsulinaemia. This suggested that glucocorticoid exposure in early pregnancy might lead to long-term pancreatic problems and diabetes, consistent with the fact that stress during early pregnancy contributes to such outcomes in humans.
Effects on the insulin signalling and the HPA axis in schizophrenia
Despite decades of research, the pathophysiology and aetiology of schizophrenia and other psychiatric disorders are not completely understood. The main hypotheses have focused on alterations in neurotransmitter systems such as the glutamatergic and dopaminergic pathways and current antipsychotic medications mainly target these systems83. However, schizophrenia is often associated with peripheral manifestations including hyperinsulinaemia and type II diabetes mellitus84. Although these effects can result from antipsychotic medications, they were also observed decades before the development and clinical use of antipsychotics. In addition, recent evidence has emerged that patients can show these effects at the first clinical presentation and prior to receiving medication85,86 and blood-based analyses have demonstrated hyperinsulinaemia and abnormalities in secretion of other endocrine factors at first presentation of symptoms87,88.
In a recent study of 66 first onset schizophrenia patients and 68 matched control subjects, we used a series of immunoassays to measure the levels of insulin, proinsulin and des 31,32 proinsulin using two-site time-resolved fluorescence assays employing different combinations of monoclonal antibodies that discriminate between the specific forms of the molecule87,89. Also, C-peptide and the insulin secretory granule protein chromogranin A were measured using commercially-available immunoassays. All of these molecules were found at significantly elevated levels in the circulation of the schizophrenia patients. In contrast, the glucose levels were relatively normal which was consistent with the possibility that at least some of these patients were insulin resistant at the onset of the disease. This could have important implications since elevated insulin can have deleterious effects on brain function90.
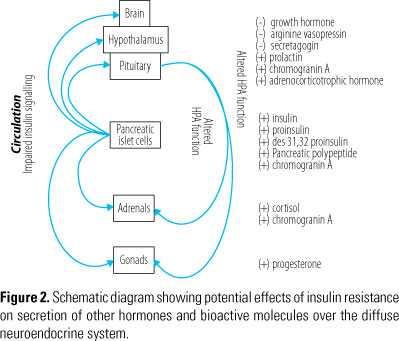
Assuming that the finding of increased insulin levels in schizophrenia patients is associated with impaired insulin signalling, we also tested the possibility that secretion of other hormones of the diffuse neuroendocrine system and the HPA axis are affected in schizophrenia. We carried out multiplex immunoassay analysis of 21 hormones and hormone-related molecules using blood serum from 236 first onset schizophrenia patients and 230 matched controls using the Multi-Analyte Profiling (MAPTM) platform from Rules Based Medicine (Austin, TX, USA). Analysis using the multiplex immunoassay technology revealed that the serum concentrations of insulin and chromogranin A were increased in schizophrenia subjects, consistent with the above findings. In addition, we found elevated concentrations of pancreatic polypeptide, prolactin, progesterone and cortisol, and decreased levels of growth hormone (Figure 2)88.
Other researchers have identified higher levels of CRH, arginine vasopressin (AVP), ACTH and cortisol in studies of schizophrenia and other psychiatric conditions91-93. Functional magnetic resonance imaging (fMRI) studies have also identified larger pituitary volumes in first episode schizophrenia patients and pituitaries in chronic sufferers have been shown to be smaller, which may indicate adaptation, medication effects or desensitization to HPA axis hyperactivity94.
Many hormones are affected by ultradian and circadian rhythms. Therefore, it is likely that the molecules identified in the above studies are co-regulated in a feedforward-feedback loop between the endocrine pancreas, pituitary and other tissues of the HPA and hypothalamic-pituitary-gonadal (HPG) systems95. As a case in point, increased insulin levels have been associated with increased prolactin secretion96 and decreased growth hormone secretion pulses97. Furthermore, studies using a diet-induced insulin resistance rat model have found increased insulin and progesterone levels98. Also, we have previously reported the co-regulated expression levels of insulin, growth hormone, leptin and cortisol in first onset schizophrenia patients, using a targeted analyte cluster method which detects patterned behavior99. The finding of increased cortisol levels using this method is consistent with other studies100.
We also analyzed post-mortem pituitaries from schizophrenia patients using a combination of liquid chromatography tandem mass spectrometry (LC-MS), two dimensional difference in-gel electrophoresis (2D-DIGE) and multiplex immunoassays88. Using these diverse methods we identified changes in cortisol, ACTH, AVP, agouti-related protein, growth hormone, prolactin and secretagogin. The finding of increased ACTH and cortisol supports the hypothesis that HPA axis hyperactivity may be involved in the pathophysiology of the disease101. Previous studies have linked abnormal AVP levels to changes in mood and behaviour102, and to psychotic disorders103,104. In addition, AVP may affect HPA axis sensitivity since there appears to be a positive correlation between the circulating levels of AVP, ACTH and cortisol in schizophrenia patients101. The finding that some of these changes were also detected in the circulation of living schizophrenia subjects (see above) suggests that they may play a role in the pathophysiology of the disease and could also lead to translation of these molecules as peripheral biomarkers for schizophrenia.
Therapeutic implications
The discovery that hyperinsulinaemia might play a role in late onset disorders in individuals with prenatally-stressed mothers, suggests that drugs which improve insulin signalling and glucose handling, may represent a potential novel treatment strategy. In the case of psychiatric disorders, such as schizophrenia, therapeutic strategies that target the underlying metabolic dysfunction could provide an effective alternative to traditional antipsychotic medications87. This possibility is supported by the finding that the insulin-sensitizing agents metformin and rosiglitazone can correct the insulin resistance that seems to come hand in hand with antipsychotic treatment without inhibiting the antipsychotic drug efficacy in treating the disease105.
Similar strategies are already proving fruitful for treatment of memory deficits in Alzheimer's disease. Clinical trials are focusing on the use of gamma peroxisome proliferator-activated receptor (PPARγ) agonists such as rosiglitazone and pioglitazone as an alternative therapy to enhance cognition106. PPARγ agonists induce the transcription of specific genes leading to the increase in the body's sensitivity to insulin, amongst other effects, and were first used in clinical trials with the aim of treating diabetes mellitus and atherosclerosis (http://www.diabetesselfmanagement.com/Articles/Diabetes-Definitions/ppar_agonists/). One research group conducted a 6-month, randomized controlled trial in patients suffering from mild Alzheimer's disease and type II diabetes107. Patients were assigned randomly to one of two groups. One group was treated with 15-30 mg pioglitazone once a day and the other was used as a control. The pioglitazone group showed improved cognitive function and increased blood flow in the parietal lobe of the brain and the control group showed no improvements. It was interesting that the effects of pioglitazone were accompanied by an increase in cerebral blood flow as this could potentially lead to an increase in the amount of glucose available in the brain.
The adrenal steroid dehydroepiandrosterone (DHEA) has antiglucocorticoid properties that may prove useful regulating high cortisol levels and glucocorticoid action in the brains of psychiatric patients108. Studies using DHEA in addition to antipsychotic medication in already-medicated schizophrenic patients found a significant improvement in the negative, depressive and anxiety symptoms of the disease109. DHEA treatment has also produced improvement in extrapyramidal side-effects such as involuntary tremors associated with antipsychotic treatment110.
Conclusions and future prospects
Prenatal stress has been linked with many psychological and behavioral problems such as schizophrenia, ADHD, autism, and depression. Changes are seen in the brains of a variety of animal models in response to prenatal stress. In particular, changes are seen in the hippocampus, amygdala, corpus callosum, cerebral cortex, cerebellum and hypothalamus. These areas of the brain are responsible for the control of behavior and their alteration could explain the psychological problems witnessed, although this has not been proven to occur in humans. The mechanisms by which these changes in the brain are achieved most likely surround the maternal and fetal HPA axes and the effect of the intrauterine environment on fetal brain development. Regardless of the effect of environment on the developing fetus, it is likely that there is also a genetic element to the contraction of such problems.
It appears that the timing and severity of the stress experienced by mothers has an impact on the development of psychological and behavioural problems in the fetus. However, further research is needed before the exact effects of timing and severity of this can be determined. There is an ever-increasing amount of evidence for metabolic and hormonal components in conditions such as schizophrenia, which in some cases may be associated with prenatal stress. Abnormalities in the metabolism of glucose, insulin signalling and the HPA axis appear to be present in the early stages of these disorders and may provide the basis for the development of much-needed biomarkers for psychiatric conditions. The use of such biomarkers could lead to improved diagnosis and patient orientated personalized medicine strategies (the right drug for the right patient at the right time) as well as providing the possibility of pre-emptive treatment. Given the potential of this line of research to improve diagnosis and create alternative treatment strategies, more research is warranted.
It is clear however that the environmental trigger of stress in the intrauterine environment is not the only catalyst for development of the disease. It is likely that there is also a genetic predisposition that when combined with an environmental trigger leads to disease development. This is in accordance with the two hit hypothesis. We have the tendency to dismiss our "emotional" responses like sadness or stress as they are not tangible. However, everything that we feel is due to the movement of chemicals within us. These chemicals, neurotransmitters and hormones, produce the feeling of stress but also lead to a cascade of other reactions in the body, which can have very real and tangible outcomes.
Acknowledgements
This research was supported by the Stanley Medical Research Institute (SMRI) and the European Union FP7 SchizDX research programme (grant reference 223427).
References
- 1. Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577-80.
- 2. Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595-601.
- 3. Owen MJ, O'Donovan MC, Thapar A, Craddock N. Neurodevelopmental hypothesis of schizophrenia. Br J Psychiatry. 2011;198:173-5.
- 4. Barker DJ. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. 1997;13:807.
- 5. Lovejoy DA. Neuroendocrinology, an integrated approach. Chichester: John Wiley & Sons Ltd.; 2005. p. 243-56.
- 6. Perry BD, Pollard R. Homeostasis, stress, trauma, and adaptation. A neurodevelopmental view of childhood trauma. Child Adolesc Psychiatr Clin N Am. 1998;7:33-51, viii.
- 7. Vitaliano PP, Maiuro RD, Mitchell E, Russo J. Perceived stress in medical school: resistors, persistors, adaptors and maladaptors. Soc Sci Med. 1989;28:1321-9.
- 8. Vaz RF, Mbajiorgu EF, Acuda SW. A preliminary study of stress levels among first year medical students at the University of Zimbabwe. Cent Afr J Med. 1998;44:214-9.
- 9. Stuber ML. Psychiatric sequelae in seriously ill children and their families. Psychiatr Clin North Am. 1996;19:481-93.
- 10. Simmons BL, Nelson DL. Eustress at work: the relationship between hope and health in hospital nurses. Health Care Manage Rev. 2001;26:7-18.
- 11. Seyle H. Stress and distress. Compr Ther. 1975;1:9-13.
- 12. Arun CP. Fight or flight, forbearance and fortitude: the spectrum of actions of the catecholamines and their cousins. Ann N Y Acad Sci. 2004;1018:137-40.
- 13. Stratakis CA, Chrousos GP. Neuroendocrinology and pathophysiology of stress. Ann NY Acad Sci. 1995;771:1-18.
- 14. Vaccaro KK, Liang BT, Sheard BE, Perlman RL. Monensin inhibits catecholamine synthesis in pheochromocytoma cells. J Pharmacol Exp Ther. 1982;221:536-40.
- 15. Halter JB, Beard JC, Porte D Jr. Islet function and stress hyperglycemia: plasma glucose and epinephrine interaction. Am J Physiol. 1984;247(Part 1):E47-52.
- 16. Charil A, Laplante DP, Vaillancourt C, King S. Prenatal stress and brain development. Brain Res Rev. 2010;65:56-79.
- 17. Ng PC. Effect of stress on the hypothalamic-pituitary-adrenal axis in the fetus and newborn. J Pediatr. 2011;158(2 Suppl):e41-3.
- 18. Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal 'programming' of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 2007;3:479-88.
- 19. Wadhwa PD, Porto M, Chicz-DeMet A, Sandman CA. Maternal CRH levels in early third trimester predict length of gestation in human pregnancy. Am J Obstet Gynecol. 1998;179:1079-85.
- 20. Kastin AJ, Akerstrom V. Differential interactions of urocortin/corticotropin-releasing hormone peptides with the blood-brain barrier. Neuroendocrinology. 2002;75:367-74.
- 21. Wadhwa PD, Sandman CA, Garite TJ. The neurobiology of stress in human pregnancy: implications for prematurity and development of the fetal central nervous system. Prog Brain Res. 2001;133:131-42.
- 22. Sandman CA, Wadhwa PD, Glynn L, Chicz-Demet A, Porto M, Garite TJ. Corticotrophin-releasing hormone and fetal responses in human pregnancy. Ann NY Acad Sci. 1999;897:66-75.
- 23. Schneider ML. Delayed object permanence development in prenatally stressed rhesus monkey infants (Macaca mulatta). Occup Ther J Res. 1992;12:96-110.
- 24. Schneider ML, Coe CL. Repeated social stress during pregnancy impairs neuromotor development in the primate infant. J Dev Behav Pediatr. 1993;14:81-7.
- 25. Clarke AS, Schneider ML. Prenatal stress has long-term effects on behavioral responses to stress in juvenile rhesus monkeys. Dev Psychobiol. 1993;26:293-304.
- 26. Clarke AS, Wittwer DJ, Abbott DH, Schneider ML. Long-term effects of prenatal stress on HPA axis activity in juvenile rhesus monkeys. Dev Psychobiol. 1994;27:257-69.
- 27. Li J, Olsen J, Vestergaard M, Obel C. Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: a nationwide follow-up study in Denmark. Eur Child Adolesc Psychiatry. 2010;19:747-53.
- 28. Grizenko N, Shayan YR, Polotskaia A, Ter-Stepanian M, Joober R. Relation of maternal stress during pregnancy to symptom severity and response to treatment in children with ADHD. J Psychiatry Neurosci. 2008;33:10-6.
- 29. Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry. 2003;160:1028-40.
- 30. Van Os J, Selten JP. Prenatal exposure to maternal stress and subsequent schizophrenia. The invasion of The Netherlands. Br J Psychiatry. 1998;172:324-6.
- 31. Susser E, Neugebauer R, Hoek HW, Brown AS, Lin S, Labovitz D, et al. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry. 1996;53:25-31.
- 32. Brown AS, Susser ES. Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophr Bull. 2008;34:1054-63.
- 33. Hoek HW, Brown AS, Susser. The Dutch famine and schizophrenia spectrum disorders. Soc Psychiatry Psychiatr Epidemiol. 1998;33:373-9.
- 34. Kahn HS, Graff M, Stein AD, Lumey LH. A fingerprint marker from early gestation associated with diabetes in middle age: the Dutch Hunger Winter Families Study. Int J Epidemiol. 2009;38:101-9.
- 35. St Clair D, Xu M, Wang P, Yu Y, Fang Y, Zhang F, et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959-1961. JAMA. 2005;294:557-62.
- 36. Huttenen MO, Niskanen P. Prenatal loss of father and psychiatric disorders. Arch Gen Psychiatry. 1978;35:429-31.
- 37. Kinney DK, Hyman W, Greetham C, Tramer S. Increased relative risk for schizophrenia and prenatal exposure to a severe tornado. Schizophr Res. 1999;36:45.
- 38. Cripps RL, Martin-Gronert MS, Archer ZA, Hales CN, Mercer JG, Ozanne SE. Programming of hypothalamic neuropeptide gene expression in rats by maternal dietary protein content during pregnancy and lactation. Clin Sci (Lond). 2009;117:85-93.
- 39. Torres N, Bautista CJ, Tovar AR, Ordáz G, Rodríguez-Cruz M, Ortiz V, et al. Protein restriction during pregnancy affects maternal liver lipid metabolism and fetal brain lipid composition in the rat. Am J Physiol Endocrinol Metab. 2010;298:E270-7.
- 40. Palmer AA, Printz DJ, Butler PD, Dulawa SC, Printz MP. Prenatal protein deprivation in rats induces changes in prepulse inhibition and NMDA receptor binding. Brain Res. 2004;996:193-201.
- 41. Palmer AA, Brown AS, Keegan D, Siska LD, Susser E, Rotrosen J, et al. Prenatal protein deprivation alters dopamine-mediated behaviors and dopaminergic and glutamatergic receptor binding. Brain Res. 2008;1237:62-74.
- 42. Csomor PA, Yee BK, Feldon J, Theodoridou A, Studerus E, Vollenweider VX. Impaired prepulse inhibition and prepulse-elicited reactivity but intact reflex circuit excitability in unmedicated schizophrenia patients: a comparison with healthy subjects and medicated schizophrenia patients. Schizophr Bull. 2009;35:244-55.
- 43. Watson JB, Mednick SA, Huttunen M, Wang X. Prenatal teratogens and the development of adult mental illness. Dev Psychopathol. 1999;11:457-66.
- 44. Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev. 2008;32:1073-86.
- 45. Kinney DK, Miller AM, Crowley DJ, Huang E, Gerber E. Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. J Autism Dev Disord. 2008;38:481-8.
- 46. Beversdorf DQ, Manning SE, Hillier A, Anderson SL, Nordgren RE, Walters SE, et al. Timing of prenatal stressors and autism. J Autism Dev Disord. 2005;35:471-8.
- 47. King S, Laplante DP. The effects of prenatal maternal stress on children's cognitive development: Project Ice Storm. Stress. 2005;8:35-45.
- 48. King S, Mancini-Marie A, Brunet A, Walker E, Meaney MJ, Laplante DP. Prenatal maternal stress from a natural disaster predicts dermatoglyphic asymmetry in humans. Dev Psychopathol. 2009;21:343-53.
- 49. Laplante DP, Barr RG, Brunet A, Galbaud du Fort G, Meaney ML, Saucier JF, et al. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatr Res. 2004;56:400-10.
- 50. Laplante DP, Brunet A, Schmitz N, Ciampi A, King S. Project Ice Storm: prenatal maternal stress affects cognitive and linguistic functioning in 5 1/2-year-old children. J Am Acad Child Adolesc Psychiatry. 2008;47:1063-72.
- 51. Talge NM, Neal C, Glover V. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol Psychiatry. 2007;48:245-61.
- 52. Deminière JM, Piazza PV, Guegan G, Abrous N, Maccari S, Le Moal M, et al. Increased locomotor response to novelty and propensity to intravenous amphetamine self-administration in adult offspring of stressed mothers. Brain Res. 1992;586:135-9.
- 53. Uno H, Tarara R, Else J, Sulemen M, Sapolsky RM. Hippocampal dama-ge associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705-11.
- 54. Uno H, Lohmiller L, Thieme C, Kemnitz JW, Engle MJ, Roecker EB, et al. Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques, 1. Hippocampus Dev Brain Res. 1990;53:157-67.
- 55. Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, et al. Neurotoxici-ty of glucocorticoids in the primate brain. Horm Behav. 1994;28:336-48.
- 56. Schmitz C, Rhodes ME, Bludau M, Kaplan S, Ong P, Ueffing I, et al. Depression: reduced number of granule cells in the hippocampus of female, but not male, rats due to prenatal restraint stress. Mol Psychiatry. 2002;7:810-3.
- 57. Coe CL, Kramer M, Czéh B, Gould E, Reeves AJ, Kirschbaum C, et al. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry. 2003;54:1025-34.
- 58. Salm AK, Pavelko M, Krouse EM, Webster W, Kraszpulski M, Birkle DL. Lateral amygdaloid nucleus expansion in adult rats is associated with exposure to prenatal stress. Brain Res Dev Brain Res. 2004;148:159-67.
- 59. Kraszpulski M, Dickerson PA, Salm AK. Prenatal stress affects the deve-lo-p-mental trajectory of the rat amygdala. Stress. 2006;9:85-95.
- 60. Kawamura T, Chen J, Takahashi T, Ichitani Y, Nakahara D. Prenatal stress suppresses cell proliferation in the early developing brain. Neuroreport. 2006;17:1515-8.
- 61. Coe CL, Lulbach GR, Schneider ML. Prenatal disturbance alters the size of the corpus callosum in young monkeys. Dev Psychobiol. 2002;41:178-85.
- 62. Poland RE, Cloak C, Lutchmansingh PJ, McCracken JT, Chang L, Ernst T. Brain N-acetyl aspartate concentrations measured by H MRS are reduced in adult male rats subjected to perinatal stress: preliminary observations and hypothetical implications for neurodevelopmental disorders. J Psychiatr Res. 1999;33:41-51.
- 63. Barros VG, Duhalde-Vega M, Caltana L, Brusco A, Antonelli MC. Astrocyte-neuron vulnerability to prenatal stress in the adult rat brain. J Neurosci Res. 2006;83:787-800.
- 64. Ulupinar E, Yucel F. Prenatal stress reduces interneuronal connectivity in the rat cerebellar granular layer. Neurotoxicol Teratol. 2005;27:475-84.
- 65. Ulupinar E, Yucel F, Ortug G. The effects of prenatal stress on the Purkinje cell neurogenesis. Neurotoxicol Teratol. 2006;28:86-94.
- 66. Anderson DK, Rhees RW, Fleming DE. Effects of prenatal stress on differentiation of the sexually dimorphic nucleus of the preoptic area (SDN-POA) of the rat brain. Brain Res. 1995;332:113-8.
- 67. Kerchner M, Ward IL. SDN-MPOA volume in male rats is decreased by prenatal stress, but is not related to ejaculatory behavior. Brain Res. 1992;581:244-51.
- 68. Egaas B, Courchesne E, Saitoh O. Reduced size of corpus callosum in autism. Arch Neurol. 1995;52:794-801.
- 69. Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1263-72.
- 70. Innocenti GM, Ansermet F, Parnas J. Schizophrenia, neurodevelopment and corpus callosum. Mol Psychiatry. 2003;8:261-74.
- 71. Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97:11032-7.
- 72. Ward HE, Johnson EA, Salm AK, Birkle DL. Effects of prenatal stress on defensive withdrawal behavior and corticotropin releasing factor systems in rat brain. Physiol Behav. 2000;70:359-66.
- 73. Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83-144.
- 74. Van Oel CJ, Baare WF, Hulshoff Pol HE, Haag J, Balazs J, Dingemans A, et al. Differentiating between low and high susceptibility to schizophrenia in twins: the significance of dermatoglyphic indices in relation to other determinants of brain development. Schizophr Res. 2001;52:181-93.
- 75. Reul JM, Chandramohan Y. Epigenetic mechanisms in stress-related memory formation. Psychoneuroendocrinology. 2007;32(Suppl 1):S21-5.
- 76. Conrad CD, Galea LAM, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine treatment. Behav Neurosci. 1996;110:1321-34.
- 77. Marin MF, Lord C, Andrews J, Juster RP, Sindi S, Arsenault-Lapierre G, et al. Chronic stress, cognitive functioning and mental health. Neurobiol Learn Mem. 2011;96(4):583-95.
- 78. Newsome CA, Shiell AW, Fall CH, Phillips DI, Shier R, Law CM. Is birth weight related to later glucose and insulin metabolism? A systematic review. Diabet Med. 2003;20:339-48.
- 79. Entringer S, Wüst S, Kumsta R, Layes IM, Nelson EL, Hellhammer DH, et al. Prenatal psychosocial stress exposure is associated with insulin resistance in young adults. Am J Obstet Gynecol. 2008;199:498.e1-7
- 80. Entringer S, Kumsta R, Hellhammer DH, Wadhwa PD, Wüst S. Prenatal exposure to maternal psychosocial stress and HPA axis regulation in young adults. Horm Behav. 2009;55:292-8.
- 81. Lesage J, Del-Favero F, Leonhardt M, Louvart H, Maccari S, Vieau D, et al. Prenatal stress induces intrauterine growth restriction and programmes glucose intolerance and feeding behaviour disturbances in the aged rat. J Endocrinol. 2004;181:291-6.
- 82. De Blasio MJ, Dodic M, Jefferies AJ, Moritz KM, Wintour EM, Owens JA. Maternal exposure to dexamethasone or cortisol in early pregnancy differentially alters insulin secretion and glucose homeostasis in adult male sheep offspring. Am J Physiol Endocrinol Metab. 2007;293:E75-82.
- 83. Biedermann F, Fleischhacker WW. Antipsychotics in the early stage of development. Curr Opin Psychiatry. 2009;22:326-30.
- 84. Hasnain M, Fredrickson SK, Vieweg WV, Pandurangi AK. Metabolic syndrome associated with schizophrenia and atypical antipsychotics. Curr Diab Rep. 2010;10:209-16.
- 85. Ryan MC, Collins P, Thakore JH. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am J Psychiatry. 2003;160:284-9.
- 86. Spelman LM, Walsh PI, Sharifi N, Collins P, Thakore JH. Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia. Diabet Med. 2007;24:481-5.
- 87. Guest PC, Wang L, Harris LW, Burling K, Levin Y, Ernst A, et al. Increased levels of circulating insulin-related peptides in first-onset, antipsychotic naïve schizophrenia patients. Mol Psychiatry. 2010;15:118-9.
- 88. Guest PC, Schwarz E, Krishnamurthy D, Harris LW, Leweke FM, Rothermundt M, et al. Altered levels of circulating insulin and other neuroendocrine hormones associated with the onset of schizophrenia. Psychoneuroendocrinology. 2011;36:1092-6.
- 89. Sobey WJ, Beer SF, Carrington CA, Clark PM, Frank BH, Gray IP, et al. Sensitive and specific two-site immunoradiometric assays for human insulin, proinsulin, 65-66 split and 32-33 split proinsulins. Biochem J. 1989;260:535-41.
- 90. Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369-72.
- 91. Banki CM, Bissette G, Arato M, O'Connor L, Nemeroff CB. CSF corticotropin-releasing factor-like immunoreactivity in depression and schizophrenia. Am J Psychiatry. 1987;144:873-7.
- 92. Brunelin J, D'Amato T, Van Os J, Cochet A, Suaud-Chagny MF, Saoud M. Effects of acute metabolic stress on the dopaminergic and pituitary-adrenal axis activity in patients with schizophrenia, their unaffected siblings and controls. Schizophr Res. 2008;100:206-11.
- 93. Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53;865-71.
- 94. Pariante CM, Vassilopoulou K, Velakoulis D, Phillips L, Soulsby B, Wood SJ, et al. Pituitary volume in psychosis. Br J Psychiatry. 2004;185:5-10.
- 95. Walker JJ, Terry JR, Lightman SL. Origin of ultradian pulsatility in the hypothalamic-pituitary-adrenal axis. Proc Biol Sci. 2010;277:1627-33.
- 96. Ben-Jonathan N, Hugo ER, Brandebourg TD, LaPensee CR. Focus on prolactin as a metabolic hormone. Trends Endocrinol Metab. 2006;17:110-6.
- 97. Tannenbaum GS, Martin JB, Colle E. Ultradian growth hormone rhythm in the rat: effects of feeding, hyperglycemia, and insulin-induced hypoglycemia. Endocrinology. 1976;99:720-7.
- 98. Akamine EH, Marcal AC, Camporez JP, Hoshida MS, Caperuto LC, Bevilacqua E, et al. Obesity induced by high-fat diet promotes insulin resistance in the ovary. J Endocrinol. 2010;206:65-74.
- 99. Cheng TM, Lu YE, Guest PC, Rahmoune H, Harris LW, Wang L, et al. Identification of targeted analyte clusters for studies of schizophrenia. Mol Cell Proteomics. 2010;9:510-22.
- 100. Meltzer HY, Lee MA, Jayathilake K. The blunted plasma cortisol response to apomorphine and its relationship to treatment response in patients with schizophrenia. Neuropsychopharmacology. 2001;24:278-90.
- 101. Ryan MC, Sharifi N, Condren R, Thakore JH. Evidence of basal pituitary-adrenal overactivity in first episode, drug naive patients with schizophrenia. Psychoneuroendocrinology. 2004;29:1065-70.
- 102. Heinrichs M, Von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548-57.
- 103. Goldman MB, Robertson GL, Luchins DJ, Hedeker D. The influence of polydipsia on water excretion in hyponatremic, polydipsic, schizophrenic patients. J Clin Endocrinol Metab. 1996;81:1465-70.
- 104. Mai JK, Berger K, Sofroniew MV. Morphometric evaluation of neurophysin-immunoreactivity in the human brain: pronounced inter-individual variability and evidence for altered staining patterns in schizophrenia. J Hirnforsch. 1993;34:133-54.
- 105. Bahtiyar G, Weiss K, Sacerdote AS. Novel endocrine disrupter effects of classic and atypical antipsychotic agents and divalproex: induction of adrenal hyperandrogenism, reversible with metformin or rosiglitazone. Endocr Pract. 2007;13:601-8.
- 106. Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Efficacy of PPAR-gamma agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging. 2011;32(9):1626-33.
- 107. Landreth G, Jiang QG, Mandrekar S, Heneka M. PPAR gamma agonists as therapeutics for the treatment of Alzheimer's disease. Neurotherapeutics. 2008;5:481-9.
- 108. Strous RD, Maayan R, Lapidus R, Stryjer R, Lustig M, Kotler M, et al. Dehydroepiandrosterone augmentation in the management of negative, depressive, and anxiety symptoms in schizophrenia. Arch Gen Psychiatry. 2003;60:133-41.
- 109. Nachshoni T, Ebert T, Abramovitch Y, Assael-Amir M, Kotler M, Maayan R, et al. Improvement of extrapyramidal symptoms following dehydroepiandrosterone (DHEA) administration in antipsychotic treated schizophrenia patients: a randomized, double-blind placebo controlled trial, Schizophr Res. 2005;79:251-6.
- 110. Hunt PJ, Gurnell EM, Huppert FA, Richards C, Prevost AT, Wass JA, et al. Improvement in mood and fatigue after dehydroepiandrosterone replacement in Addison's disease in a randomized, double blind trial. J Clin Endocrinol. 2000;85:4650-6.
Endereço para correspondência:
Publication Dates
-
Publication in this collection
11 Dec 2012 -
Date of issue
2013
History
-
Received
23 Sept 2012 -
Accepted
07 Nov 2012