Abstracts
OBJECTIVE: To analyze the diagnostic and therapeutic approach of pregnant women with positive IgM test for toxoplasmosis and the follow-up of their children in a public hospital of Rio de Janeiro, Brazil. METHODS: This cross-sectional retrospective study from 2003 to 2006 enrolled 98 pregnant women with positive IgM test for toxoplasmosis and 99 children. The follow-up of the children with or without congenital infection was reviewed, as well as the clinical presentation of those with congenital infection and the laboratory tests used to diagnose the infection by Toxoplasma gondii during pregnancy. RESULTS: Toxoplasmosis was diagnosed in the second and third trimesters of pregnancy in 76 patients. In 36 pregnant women, determination of the serum levels of IgM was the only laboratory method used to diagnose the infection. Low IgM levels analyzed by ELFA were detected in 49 pregnant women. IgG avidity test was performed in 62 patients and in 13% of them the exam was carried out during the first trimester of pregnancy. Specific treatment for toxoplasmosis was applied in 93 women. Vertical transmission rate was 4%. Clinical manifestation of congenital toxoplasmosis was found in all infected children. All non-infected children showed a decrease in IgG serum levels for toxoplasmosis during the follow-up. The mean age for negativation of IgG serum levels in these children was 5.4 months. CONCLUSIONS: Our results suggest that the use of a positive IgM test to toxoplasmosis as the only antibody marker to detect recent infection has a limited value.
toxoplasmosis; prenatal diagnosis; toxoplasmosis, congenital; infant, newborn
OBJETIVO:Avaliar a abordagem diagnóstica e terapêutica da toxoplasmose de gestantes que apresentaram IgM positiva para a doença e o acompanhamento de seus filhos em um hospital público no Rio de Janeiro, RJ. MÉTODOS: Estudo transversal retrospectivo de 2003 a 2006, realizado por meio da análise dos prontuários de 98 gestantes com sorologia IgM positiva para toxoplasmose e seus filhos (99 crianças). O seguimento das crianças com e sem infecção congênita foram analisados, assim como a apresentação clínica daquelas com infecção congênita e os testes diagnósticos utilizados para identificar a infecção pelo Toxoplasma gondii durante a gestação. RESULTADOS: O diagnóstico sorológico foi realizado em 76 pacientes no segundo e terceiro trimestre gestacional. Em 36 gestantes, a determinação dos níveis séricos de IgM foi o único teste diagnóstico realizado para infecção pelo toxoplasma. Em 49 gestantes, os índices de IgM, pela técnica ELFA, foram baixos. O teste de avidez de IgG foi realizado em 62 gestantes e somente 13 o realizaram no primeiro trimestre gestacional. O tratamento específico para toxoplasmose foi empregado em 93 gestantes. A taxa de transmissão vertical foi de 4%. Manifestações clínicas de toxoplasmose congênita foram encontradas em todas as crianças infectadas. Todas as crianças não infectadas apresentaram declínio de IgG específica para toxoplasmose ao longo do acompanhamento ambulatorial; a idade média de IgG comprovadamente negativa nessas crianças foi de 5,4 meses. CONCLUSÕES: Os resultados sugerem que uma sorologia positiva para IgM, como um único marcador sorológico para detectar infecção recente, tem um valor limitado.
toxoplasmose; diagnóstico pré-natal; toxoplasmose congênita; recém-nascido
ORIGINAL ARTICLE
Diagnostic and therapeutic management of toxoplasmosis in pregnancy and the effect in the newborn
Abordaje diagnóstico y terapéutico de la toxoplasmosis en gestantes y las repercusiones en el recién nacido
Tatiana Melino PessanhaI; Manoel de CarvalhoII; Marcos Vinícius S. PoneIII; Saint Clair Gomes JúniorIV
Instituição: Universidade Federal Fluminense (UFF), Rio de Janeiro, RJ, Brasil
IMestre em Saúde da Criança e do Adolescente pela UFF, Rio de Janeiro, RJ, Brasil
IIDoutor em Saúde da Mulher e da Criança pela Fundação Oswaldo Cruz (Fiocruz); Professor Adjunto da UFF, Rio de Janeiro, RJ, Brasil
IIIMestre em Saúde da Criança e do Adolescente pela UFF; Tecnologista Pleno II do Instituto Fernandes Figueira da Fiocruz, Rio de Janeiro, RJ, Brasil
IVDoutor em Engenharia Biomédica pelo Instituto Alberto Luiz Coimbra de Pós-graduação e Pesquisa de Engenharia da Universidade Federal do Rio de Janeiro; Pesquisador em Saúde Pública do Departamento de Neonatologia do Instituto Fernandes Figueira da Fiocruz, Rio de Janeiro, RJ, Brasil
Endereço para correspondência
ABSTRACT
OBJECTIVE: To analyze the diagnostic and therapeutic approach of pregnant women with positive IgM test for toxoplasmosis and the follow-up of their children in a public hospital of Rio de Janeiro, Brazil.
METHODS: This cross-sectional retrospective study from 2003 to 2006 enrolled 98 pregnant women with positive IgM test for toxoplasmosis and 99 children. The follow-up of the children with or without congenital infection was reviewed, as well as the clinical presentation of those with congenital infection and the laboratory tests used to diagnose the infection by Toxoplasma gondii during pregnancy.
RESULTS: Toxoplasmosis was diagnosed in the second and third trimesters of pregnancy in 76 patients. In 36 pregnant women, determination of the serum levels of IgM was the only laboratory method used to diagnose the infection. Low IgM levels analyzed by ELFA were detected in 49 pregnant women. IgG avidity test was performed in 62 patients and in 13% of them the exam was carried out during the first trimester of pregnancy. Specific treatment for toxoplasmosis was applied in 93 women. Vertical transmission rate was 4%. Clinical manifestation of congenital toxoplasmosis was found in all infected children. All non-infected children showed a decrease in IgG serum levels for toxoplasmosis during the follow-up. The mean age for negativation of IgG serum levels in these children was 5.4 months.
CONCLUSIONS: Our results suggest that the use of a positive IgM test to toxoplasmosis as the only antibody marker to detect recent infection has a limited value.
Key-words: toxoplasmosis; prenatal diagnosis; toxoplasmosis, congenital; infant, newborn.
RESUMEN
OBJETIVO: Evaluar el abordaje diagnóstico y terapéutico de la toxoplasmosis de gestantes que presentaron IgM positiva para toxoplasmosis y el seguimiento de sus hijos en un hospital público de Rio de Janeiro, Brasil.
MÉTODOS: Estudio transversal retrospectivo de 2003 a 2006, realizado mediante el análisis de los prontuarios de 98 gestantes con serología IgM positiva para toxoplasmosis y sus hijos (99 niños). El seguimiento de los niños con y sin infección congénita fue analizado, así como la presentación clínica de aquellos con infección congénita y las pruebas diagnósticas utilizadas para identificar la infección por el Toxoplasma gondii durante la gestación.
RESULTADOS: El diagnóstico serológico fue realizado en 76 pacientes (77%) en el 2º y 3er trimestres gestacionales. En 36 gestantes (37%), la determinación de los niveles séricos de IgM fue la única prueba diagnóstica realizada para infección por el toxoplasma. En 49 gestantes (50%), los índices de IgM, por la técnica ELFA, fueron bajos. La prueba de avidez de IgG fue realizada en 62 gestantes (63%) y solamente 13 la realizaron durante el primer trimestre gestacional. El tratamiento específico para toxoplasmosis fue empleado en 93 gestantes (95%). La tasa de transmisión vertical fue de 4%. Manifestaciones clínicas de toxoplasmosis congénita fueron encontradas en todos los niños infectados. Todos los niños no infectados presentaron reducción del IgG específico para toxoplasmosis y, a lo largo del seguimiento ambulatorial, el promedio de edad de IgG comprobadamente negativa en estos niños fue de 5,4 meses.
CONCLUSIONES: Los resultados sugieren que una serología positiva para IgM como único marcador serológico para detectar la infección reciente tiene un valor limitado en detectar infección reciente.
Palabras clave: toxoplasmosis; diagnóstico prenatal; toxoplasmosis congénita; recién nacido.
Introduction
If pregnant women become infected by Toxoplasma gondii, there can be serious consequences for the fetus, including miscarriage, restricted intrauterine growth, prematurity and neurological and ophthalmic disorders(1). Several studies conducted in Brazil have found seroprevalence rates varying from 42 to 90%(2). Once an expectant mother has become infected, the global risk of fetal infection is 40%. However, the level of risk varies depending on the gestational age at which the mother acquires the infection and is lowest in the first trimester and greatest in the third(3).
It is crucial to diagnosis infections during pregnancy while they are still acute, since it is generally during this period that an expectant mother runs the risk of transmitting the disease to her fetus(4). Since the majority of infections of both pregnant women and newborn infants are asymptomatic, laboratory tests should be used to diagnose gestational acute toxoplasmosis and congenital toxoplasmosis so that they can be treated promptly(5).
The most widely-used method for diagnosing acute infections is by detecting IgM antibodies specific for toxoplasmosis. The value of this test is, however, limited, since it can be positive for a long time after the acute infection(6). It is therefore necessary to employ other methods to diagnose the acute infection in pregnant women, such as pairing IgM and IgG serology with a 3-week interval(4) and the IgG avidity test at the start of the gestation(7). In cases of acute maternal infections or when serological tests lead to a high degree of suspicion that an infection has been acquired during gestation, amniocentesis should be performed and polymerase chain reaction (PCR) used to test an amniotic fluid sample(8). This test has good accuracy and has become the procedure of choice for diagnosing fetal infections(9). Real-time PCR analysis offers sensitivity of 92.2%(10). Additionally, fetal morphology should be monitored with ultrasound throughout pregnancy(11). Placental examination aids with diagnosis of congenital toxoplasmosis if T. gondii has been isolated from specimens or if there are histopathological findings suggestive of the infection(12).
The recommended treatment for expectant mothers is with spiramycin or with sulfadiazine, pyrimethamine and folinic acid, depending on the stage of gestation and whether fetal infection has been confirmed(13). Questions have been raised about the efficacy of spiramycin for preventing congenital infections(14,15). Notwithstanding, diagnosis of congenital toxoplasmosis during the neonatal period can be difficult, which is why it is necessary to conduct both serological and clinical follow-up in order to rule out or confirm infections(16). IgG antibodies detected in the newborn maybe a manifestation of a maternal infection caused by passive antibody transfer. This is why tests are generally used to detect both IgA and IgM in order to diagnose infection in a child(17). The efficacy of treating children with congenital infections for 1 year using sulfadiazine, pyrimethamine and folinic acid has been confirmed and this treatment is associated with a reduction in childhood sequelae, primarily neurological and ophthalmic(15).
The objectives of this paper are to describe the diagnostic and therapeutic management of toxoplasmosis during the prenatal care of 98 mothers who tested IgM positive for toxoplasmosis and to analyze the vertical transmission rate and clinical and laboratory data for infected and uninfected children and compare the infected and uninfected children who were followed up at the Instituto Fernandes Figueira (IFF - FIOCRUZ, RJ, Brazil).
Method
This was a cross-sectional, descriptive and retrospective study based on an analysis of medical records at the Instituto Fernandes Figueira. The study population comprised expectant mothers who had IgM serology results positive for toxoplasmosis during pregnancy and their children, who were monitored for 4 years (January 1, 2003 to December 31, 2006) at a pediatric infectious diseases clinic for suspected congenital toxoplasmosis.
The study enrolled all expectant mothers who had specific IgM serology positive for toxoplasmosis during gestation and who received their prenatal care at the IFF clinic and whose children were followed-up at the IFF pediatric infectious diseases clinic until a diagnosis of congenital Toxoplasma gondii infection was confirmed or ruled out.
All of the expectant mothers and children studied had tests for toxoplasmosis-specific serology, which were performed by the IFF immunology laboratory. Tests were conducted using enzyme-linked fluorescent assay with a BioMérieux Vidas system. The cutoff for a positive IgM result is an index greater than or equal to 0.65, and IgG is positive when greater than or equal to 8UI/ml(18). Expectant mothers who are IgG negative are tested serologically for toxoplasmosis (IgG and IgM) every trimester in order to detect seroconversion. Expectant mothers with positive IgM serology undergo the IgG avidity test in order to try to identify the time of infection. An expectant mother who has a high avidity test result within the first 3 months probably did not acquire the infection during the previous 3 months and, if the infection was acquired before pregnancy, the fetus essentially runs no risk of congenital infection(19). The combination of testing for specific IgM and IgG avidity has shown specificity of 99% and sensitivity of 95% for diagnosis of acute infections. Serial fetal ultrasound scans are also conducted and the findings suggestive of congenital toxoplasmosis are dilatation of cerebral ventricles and an enlarged placenta(3). The finding most often described in infected fetuses is dilatation of ventricles in isolation(20). When possible, a PCR test is run on amniotic fluid and the placenta undergoes histopathology. Examination of the placenta helps to confirm a diagnosis of congenital toxoplasmosis if Toxoplasma gondii can be isolated or if there are histological findings suggestive of infection, such as a chronic inflammatory reaction (lymphocyte infiltration) in the decidua and focal reactions in the villa(12).
During follow-up of the children at the clinic, IgM and IgG serology is tested during the first days of life, again during the first month of life and every 2 to 3 months depending on the case, until IgG tests return negative.
The criteria used in this study to confirm diagnoses of congenital toxoplasmosis were those that have been adopted in the literature: a) toxoplasmosis-specific IgM positive and/or b) toxoplasmosis-specific IgG that does not decline or is still increasing after 3 months of life and/or c) persistently positive IgG after the child has reached 12 months, without prior reductions and/or d) signs and symptoms suggestive of congenital infection by T. gondii (chorioretinitis, hydrocephalus, cerebral calcification)(3).
As part of their prenatal care at the IFF, expectant mothers with positive IgM serology are treated with spiramycin during the first trimester. After the first trimester, sulfadiazine, pyrimethamine and folinic acid are alternated with the spiramycin in 3-week cycles, up until delivery.
The exclusion criteria were as follows: the mother did not receive prenatal care at the IFF, the mother had a history of positive IgM serology prior to the pregnancy or the child was not followed-up until congenital infection had been confirmed or ruled out.
Maternal variables analyzed were: chronological age, gestational age when IgM positive, IgM and IgG serology results (in figures), IgG avidity test, amniocentesis/PCR, obstetric ultrasound, placental histopathology results, time of start of toxoplasmosis treatment and type of treatment given. Variables relating to the infants and children were as follows: sex, birth weight, relationship of weight to gestational age, clinical findings, fundoscopy, transfontanellar ultrasound, cranial tomography, IgM and IgG serology results (in figures), age at which uninfected child was confirmed negative, number of consultations and follow-up time for uninfected children and toxoplasmosis treatment given.
Data were analyzed using Epi Info version 3.3.2 and SPSS version 13.0. Statistical analyses were conducted using simple measures of frequency (means) and measures of central tendency (medians). Student's t test and Fisher's exact test were used to compare means and medians to a statistical significance of p<0.05. The study was approved by the Human Research Ethics Committee at IFF/FIOCRUZ.
Results
A total of 155 newborn infants and 152 expectant mothers were analyzed during the study period and 56 newborn infants and 54 expectant mothers were excluded. Four of the expectant mothers who were excluded had had positive IgM serology confirmed before becoming pregnant, 20 had received their prenatal at institutions other than the Instituto Fernandes Figueira and 30 were excluded because their children did not meet the criteria. Thirty-one of the newborn infants who were excluded were lost to follow-up at the pediatric infectious diseases clinic before confirmation of infection status and 25 were excluded because their mothers had been excluded. The final sample therefore comprised 99 infants and 98 mothers.
With regard to the mothers, 22.4% began prenatal care during the first trimester, 49% in the second trimester and 28.6% in the third trimester. For 76 of the 98 mothers (77.6%), serological diagnosis was made in the second or third trimesters. For 36 mothers (36.7%), serology alone was used to diagnose T. gondii infection.
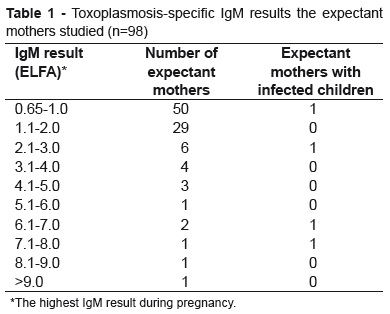
For 49 of the mothers (50.1%) IgM levels were low (<1), while two whose children acquired congenital infection had results greater than 7. There was a great deal of variation in IgG titers among these mothers: eight had IgG levels below 100UI/ml, 30 between 101 and 300UI/ml, 38 between 301 and 1,200UI/ml and 21 had concentrations greater than 1,200UI/ml (Table 1).
The IgG avidity test was conducted for 62 mothers (63.3%), 13 during the first trimester. Amniotic fluid was tested by PCR for 7 mothers (7%) and the result was negative in all cases.
Four of the mothers studied had children with congenital infection. The vertical transmission rate was therefore 4%. Fetal ultrasonography findings suggestive of congenital toxoplasmosis were present in 3 of the 4 mothers whose children acquired congenital infections and in one mother whose child was not infected (Table 2). These findings included dilatation of cerebral ventricles, cerebral calcification, splenomegaly and placental thickening.
Ninety-three (95%) of the mothers were given specific treatment for toxoplasmosis, 76 of whom were given the full treatment with sulfadiazine, pyrimethamine and folinic acid alternated with spiramycin every 3 weeks and 17 of whom were only given spiramycin. None of the 5 mothers who began treatment during the first trimester had a child with congenital infection; two of the 45 mothers who started treatment in the second trimester had children with congenital infections and one of the 43 who started treatment in the third trimester had a child with congenital infection. One of the five mothers who did not receive treatment had a child with congenital infection.
The mean age of the mothers whose children contracted congenital infections was significantly younger than the mothers of uninfected newborn infants (18 vs 25 years, p<0.01). There were no differences between newborn infants with and without congenital infections in terms of birth weight, gestational age or Apgar scores (Table 3).
The mean time to confirm negative IgG status in the group of children without congenital infections was 5.4 months. The mean number of serological tests run on these children was 3.3 tests per child. All of the children with congenital infections had visual and central nervous system involvement (Table 4).
Discussion
It is crucial that acute T. gondii infections be diagnosed during pregnancy in order to determine the risk of fetal infection(4). The ideal is that the gestational T. gondii infection be diagnosed by serology for toxoplasmosis at the start of the gestation(21), but this is very often not possible, particularly in Brazil where few expectant mothers begin their prenatal care in the first trimester(22,23). The most common method used to diagnose acute T. gondii infection in the mothers studied here was serology and in 36 of them (36.7%), this was the only method used for diagnosis.
Many authors have shown that a positive IgM result is not enough by itself to support a diagnosis of acute infection, since the test can return positive results a long time after the primary infection(3). This casts doubt on the value of IgM for diagnosis of recent infections in expectant mothers and suggests that serial serological tests, the IgG avidity test and diagnostic methods for identifying fetal infection are required.
Few of the mothers in our study underwent the IgG avidity test during the first trimester, which is probably because of their late entry to prenatal care. If performed at the end of gestation, this test is of limited value for determining the time when the infection occurred and is probably no longer indicated at that point. Although amniotic fluid PCR is a confirmatory test for diagnosis of fetal infection(3), it was also little-employed in this study (7.1%). The low percentage for the number of mothers tested with amniocentesis and amniotic fluid PCR is the result of late diagnosis in some cases combined with the fact that the test must be paid for by the patient, since it is not available at the institution where the study was conducted.
The serological method used to assay IgG and IgM was enzyme linked fluorescent assay (VIDAS machine), which offers a high sensitivity of 93.5 to 100%(24). Using this method, the IgM cutoff point is very low, (positive above 0.65). This means that the test may detect a greater number of patients with false-positive serology and contribute to increasing the number of patients diagnosed with T gondii infection. Pujol-Riqué et al. suggested a new cutoff point for the test: results below 1.05 would only be linked to infection if they persist for more than 12 weeks(25). Other authors have found that lower values are more likely to be related to residual infections than to acute infections(26), suggesting that the cutoff point for detecting recent infections should be changed. The majority of the mothers analyzed had low IgM results (51% below 1) which may not indicate an acute infection, only 13 expectant mothers (13.2%) had IgM greater than 3 and two of these had children with congenital infections. These data suggest that acute infections are more closely linked with higher IgM results.
There are reports that early diagnosis and treatment during gestation prevent sequelae in infected children or minimize their frequency and intensity(3) In our study, the diagnosis and treatment employed did not reduce the harm suffered by the infected infants. This may be the result of these mothers having started treatment later on in the gestation when the infection had probably occurred during the initial phase of pregnancy.
The mother-fetus transmission rate in this sample was 4%. This rate is lower than reported by studies of women who were not given specific treatment during pregnancy(3,20). Similar rates have been observed in some studies of expectant mothers given treatment(2). An assessment of recently-delivered Brazilian mothers found a 1.7% prevalence of congenital toxoplasmosis(27).
It was observed that the age of the subset of expectant mothers whose children acquired congenital infections was significantly lower younger than the age of the group of mothers whose children did not contract congenital infections. This has also been demonstrated by Vidigal et al in a study conducted in Belo Horizonte, in 2002. It is possible that the expectant mothers whose children did not have congenital infections had contracted the primary infection before becoming pregnant, since first infections are more common in younger age groups(28), particularly in regions where T gondii seroprevalence is elevated, as is the case in Rio de Janeiro(29).
The majority of the infected newborn infants were symptomatic, probably because their mothers had been infected at the start of gestation. The greater proportion of children having symptomatic congenital toxoplasmosis observed here is in contrast with the results of other studies which have reported that 70 to 80% of children with congenital toxoplasmosis are born with no obvious signs on routine clinical examination of the neonate(30). This could be explained by a failure to detect mothers who were infected during the last trimester, since when infection takes place in the last trimester of pregnancy the infant is generally born asymptomatic(3). Monthly serology testing is not routine for expectant mothers with negative IgG serology at the institution where the study was carried out.
The mean time taken to confirm negative IgG status in the uninfected children in this sample was 5.4 months, which is the time taken to eliminate antibodies transmitted by the mother. With uninfected children, IgG levels generally decline gradually during the first months of life(3). A mean of 3.3 serological tests were conducted per child with suspected congenital toxoplasmosis. It is likely that a large proportion of these children did not need such a large number of screening serology tests, particularly not those who had already exhibited falling IgG levels over the first 3 months and were free from clinical signs suggestive of congenital toxoplasmosis.
In conclusion, the results of this study suggest that the IgG avidity test should always be considered when attempting to diagnose acute T. gondii infection during the first trimester in expectant mothers with positive IgM serology. The presence of positive IgM serology alone has limited value for detecting recent infections and should be employed in combination with other supplementary tests to diagnose acute infections. Such a strategy should reduce the number of expectant mothers treated and the number of children in outpatients follow-up because of possible infection.
References
- 1. Jones J, Lopes A, Wilson M. Congenital toxoplasmosis. Am Fam Physician 2003;67:2131-8.
- 2. Figueiró-Filho EA, Lopes AH, Senefonte FR, Souza Júnior VG, Botelho CA, Figueiredo MS et al Toxoplasmose aguda: estudo da freqüência, taxa de transmissão vertical e relação entre os testes diagnósticos materno-fetais em gestantes em estado da Região Centro-Oeste do Brasil. Rev Bras Ginecol Obstet 2005;27:442-9.
- 3. Remington JS, Mcleod R, Thulliez P, Desmonts G. Toxoplasmosis. In: Remington JS, Klein JO, Baker C, Wilson C, editors. Infectious diseases of the fetus and the newborn infant. 6th ed. Philadelphia: WB Saunders; 2006. p. 974-1105.
- 4. Montoya JG, Rosso F. Diagnosis and management of toxoplasmosis. Clin Perinatol 2005;32:705-26.
- 5. Andrade GM, Carvalho AL, Carvalho IR, Mello BF, Tibúrcio FR, Castro FC. Toxoplasmose na gestante e no recém-nascido. Estudo de 86 pares de mãe filho atendidos no período de 1996-99 no ambulatório de infectologia pediátrica do HC-UFMG. Rev Med Minas Gerais 2001;11:202-7.
- 6. Bobić B, Sibalić D, Djurković-Djaković O. High levels of IgM antibodies specific for Toxoplasma gondii in pregnancy 12 years after primary toxoplasma infection. Case report. Gynecol Obstet Invest 1991;31:182-4.
- 7. Lappalainen M, Koskiniemi M, Hiilesmaa V, Amälä P, Teramo K, Koskela P et al Outcome of children after maternal primary Toxoplasma infection during pregnancy with emphasis on avidity of specific IgG. The Study Group. Pediatr Infect Dis J 1995;14:354-61.
- 8. Hohlfeld P, Daffos F, Costa JM, Thulliez P, Forestier F, Vidaud M. Prenatal diagnosis of congenital toxoplasmosis with a polymerase-chain-reaction test on amniotic fluid. N Engl J Med 1994;331:695-9.
- 9. Beazley DM, Egerman RS. Toxoplasmosis. Semin Perinatol 1998;22:332-8.
- 10. Wallon M, Franck J, Thulliez P, Huissoud C, Peyron F, Garcia-Meric P et al Accuracy of real-time polymerase chain reaction for Toxoplasma gondii in amniotic fluid. Obstet Gynecol 2010;115:727-33.
- 11. Pratlong F, Boulot P, Villena I, Issert E, Tamby I, Cazenave J et al Antenatal diagnosis of congenital toxoplasmosis: evaluation of the biological parameters in a cohort of 286 patients. Br J Obstet Gynaecol 1996;103:552-7.
- 12. Garcia AG, Coutinho SG, Amendoeira MR, Assumpção MR, Albano N. Placental morphology of newborns at risk for congenital toxoplasmosis. J Trop Pediatr 1983;29:95-103.
- 13. Montoya JG, Remington JS. Management of Toxoplasma gondii infection during pregnancy. Clin Infect Dis 2008;47:554-66.
- 14. SYROCOT (Systematic Review on Congenital Toxoplasmosis) study group, Thiébaut R, Leproust S, Chêne G, Gilbert R. Effectiveness of prenatal treatment for congenital toxoplasmosis: a meta-analysis of individual patient's data. Lancet 2007;369:115-22.
- 15. McLeod R, Boyer K, Karrison T, Kasza K, Swisher C, Roizen N, et al. Outcome of treatment for congenital toxoplasmosis, 1981-2004: the National Collaborative Chicago-Based, Congenital Toxoplasmosis Study. Clin Infect Dis 2006;42:1383-94.
- 16. Lecomte B, Patural H, Flori P, Bellete B, Paricio C, Jaziri F et al Atypical neurological form of congenital toxoplasmosis after maternal seroconversion in the first trimester of pregnancy: severe manifestation at 2 months of age. Eur J Obstet Gynecol Reprod Biol 2006;124:255-6.
- 17. Montoya JG. Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J Infect Dis 2002;185 (Suppl 1):S73-82.
-
18Biomerieux Brasil S/A. Manual VIDAS TOXO. São Paulo: Biomerieux Brasil S/A; 2006.
- 19. Pelloux H, Brun E, Vernet G, Marcillat S, Jolivet M, Guergour D et al Determination of anti-Toxoplasma gondii immunoglobulin G avidity: adaptation to the Vidas system (bioMérieux). Diagn Microbiol Infect Dis 1998;32:69-73.
- 20. Virkola K, Lappalainen M, Valanne L, Koskiniemi M. Radiological signs in newborns exposed to primary Toxoplasma infection in utero. Pediatr Radiol 1997;27:133-8.
- 21. Jenum PA, Stray-Pedersen B. Development of specific immunoglobulins G, M, and A following primary Toxoplasma gondii infection in pregnant women. J Clin Microbiol 1998;36:2907-13.
- 22. Maranhão AQ, Joaquim MM, Siu C. A mortalidade perinatal e neonatal no Brasil. Brasília: Ministério da Saúde, UNICEF; 1998.
- 23. Puccini RF, Pedroso GC, Silva EM, Araújo NS, Silva NN. Eqüidade na atenção pré-natal e ao parto em área da região metropolitana de São Paulo, 1996. Cad Saude Publica 2003;19:35-45.
- 24. Wilson M, Remington JS, Clavet C, Varney G, Press C, Ware D. Evaluation of six commercial kits for detection of human immunoglobulin M antibodies to Toxoplasma gondii. The FDA Toxoplasmosis Ad Hoc Working Group. J Clin Microbiol 1997;35:3112-5.
- 25. Pujol-Riqué M, Quintó L, Danés C, Valls ME, Coll O, Moreno A et al Datación de IgM anti-Toxoplasma en el embarazo con métodos VIDAS-ELFA. Enferm Infecc Microbiol Clin 2000;18:274-8.
- 26. Wilson M, Schantz PM, Tsang VC. Clinical immunoparasitology. In: Rose NR, Macario EC, Folds JJ. Manual of clinical laboratory immunology. Washington: American Society for Microbiology; 1997. p. 575-84.
- 27. Madi JM, Souza Rda S, Araújo BF, Oliveira Filho PF, Rombaldi RL, Mitchell C et al Prevalence of toxoplasmosis, HIV, syphilis and rubella in a population of puerperal women using Whartman 903 filter paper. Braz J Infect Dis 2010;14:24-9.
- 28. Bahia-Oliveira LM, Abreu AM, Azevedo-Silva J, Oréfice F. Toxoplasmosis in southeastern Brazil: an alarming situation of highty endemic acquired and congenital infection. In: Petersen E, Pollak A, Reiter-Owona I, editors. Recents trends in research on congenital toxoplasmosis. Int J Parasitol 2001;31:115-44.
- 29. Souza WJ, Coutinho SG, Lopes CW, dos Santos CS, Neves NM, Cruz AM. Epidemiological aspects of toxoplasmosis in schoolchildren residing in localities with urban or rural characteristics within the city of Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 1987;82:475-82.
- 30. Guerina NG, Hsu HW, Meissner HC, Maguire JH, Lynfield R, Stechenberg B et al Neonatal serologic screening and early treatment for congenital Toxoplasma gondii infection. N Engl J Med 1994;330:1858-63.
Publication Dates
-
Publication in this collection
14 Oct 2011 -
Date of issue
Sept 2011
History
-
Received
22 June 2010 -
Accepted
17 Dec 2010