Abstracts
Brazilian Ultra High Temperature (UHT) milk consumption has increased during the last decade from 187 to 4,200 million liters. In the continuous UHT process, milk is submitted for 2-4 s to 130-150ºC, in a continuous flow system with immediate refrigeration and aseptical packing in hermetic packages. This research had the purpose to verify the incidence of B. cereus species from the B. cereus group, in UHT milk. In 1998 high indexes of these organisms were reported, reaching 34.14% of the analyzed samples. Beyond this fact, there was the need to establish methods and processes adjusted for correct identification of B. cereus. Thus, commercial sterility tests of 6,500 UHT milk packages were investigated in two assays, after ten days incubation at 37ºC and 7ºC to germinate all possible spores and/or to recuperate injured vegetative cells followed by pH measurement. Samples (1,300 packages each) from five Brazilian UHT plants of whole UHT milk processed by direct steam injection, packaged in carton were investigated for the presence of Bacillus cereus through phenotypic and genetic (PCR) tests. Values of pH were different for the samples, ranging between 6.57 and 6.73. After storage of the samples, only four packages with pH measurement below the lower limit of 6.5 were found and analyzed for the presence of B. cereus. This organism was not detected in any of the samples indicating that the five Brazilian UHT milk processors control pathogenic microorganisms and it can be said that the consumption of UHT milk does not present safety problems to consumers. Fourier Transform Infrared Spectroscopy (FTIR) and PCR tests were efficient and must be adopted to confirm the biochemical series for B. cereus.
FTIR; PCR; commercial sterility; statistical analyses
O consumo de leite ultra-alta temperatura (UHT) brasileiro aumentou, durante a última década, de 187 milhões de litros para 4,200 milhões de litros. No processo contínuo de leite UHT o leite é submetido por 2-4 seg a 130-150ºC, em sistemas de escoamento contínuo com refrigeração imediata e envase asséptico em embalagens herméticas. Esta pesquisa teve a finalidade de verificar a incidência da espécie B. cereus, em leite UHT. Em 1998, foi reportado alto índice destes organismos neste produto atingindo 34,14% das amostras analisadas. Além deste fato, existia a necessidade de se estabelece métodos e processos adequados para correta identificação de B. cereus. Assim, a esterilidade comercial de 6.500 embalagens de leite UHT foi estudada, em dois ensaios, após incubação das amostras a 37ºC e 7ºC por dez dias para germinar todos os possíveis esporos e/ou recuperar células vegetativas injuriadas. Após a incubação, foi realizada a medida de pH. Neste sentido, hum mil e trezentas amostras de cada uma de cinco plantas Brasileiras de processo UHT, que operam com injeção direta de vapor produzindo leite integral em embalagens cartonadas, foram investigadas em relação à presença de Bacillus cereus, através de ensaios fenotípicos e genéticos (PCR). Os valores de pH foram diferentes para as amostras analisadas oscilando entre 6,57 e 6,73. Após a estocagem das amostras, somente quatro embalagens suspeitas, com medida de pH abaixo de 6,5 foram analisadas na procura por B. cereus. Não foi detectado B. cereus em nenhuma amostra indicando que o leite integral UHT Brasileiro, das cinco plantas processadoras, não apresenta o microrganismo patogênico. Somente microrganismos deteriorantes foram isolados indicando que dentro do espaço amostral, o leite UHT analisado não representa riscos e problemas de saúde aos consumidores. A Espectroscopia Infravermelha por Transformada de Fourier (FTIR) e a Reação em cadeia da polimerase (PCR) foram consideradas eficientes e devem ser adotadas para confirmar a série bioquímica de B. cereus.
FTIR; PCR; esterilidade comercial; análises estatísticas
FOOD SCIENCE AND TECHNOLOGY
Bacillus cereus in Brazilian Ultra High Temperature milk
Bacillus cereus em leite UHT brasileiro
Cristiana de Paula Pacheco-Sanchez; Pilar Rodriguez de Massaguer* * Corresponding author < esteril@unicamp.br>
UNICAMP/FEA - Depto. de Ciência de Alimentos, C.P. 6121 - 13083-862 - Campinas, SP - Brasil
ABSTRACT
Brazilian Ultra High Temperature (UHT) milk consumption has increased during the last decade from 187 to 4,200 million liters. In the continuous UHT process, milk is submitted for 2-4 s to 130-150ºC, in a continuous flow system with immediate refrigeration and aseptical packing in hermetic packages. This research had the purpose to verify the incidence of B. cereus species from the B. cereus group, in UHT milk. In 1998 high indexes of these organisms were reported, reaching 34.14% of the analyzed samples. Beyond this fact, there was the need to establish methods and processes adjusted for correct identification of B. cereus. Thus, commercial sterility tests of 6,500 UHT milk packages were investigated in two assays, after ten days incubation at 37ºC and 7ºC to germinate all possible spores and/or to recuperate injured vegetative cells followed by pH measurement. Samples (1,300 packages each) from five Brazilian UHT plants of whole UHT milk processed by direct steam injection, packaged in carton were investigated for the presence of Bacillus cereus through phenotypic and genetic (PCR) tests. Values of pH were different for the samples, ranging between 6.57 and 6.73. After storage of the samples, only four packages with pH measurement below the lower limit of 6.5 were found and analyzed for the presence of B. cereus. This organism was not detected in any of the samples indicating that the five Brazilian UHT milk processors control pathogenic microorganisms and it can be said that the consumption of UHT milk does not present safety problems to consumers. Fourier Transform Infrared Spectroscopy (FTIR) and PCR tests were efficient and must be adopted to confirm the biochemical series for B. cereus.
Key words: FTIR, PCR, commercial sterility, statistical analyses
RESUMO
O consumo de leite ultra-alta temperatura (UHT) brasileiro aumentou, durante a última década, de 187 milhões de litros para 4,200 milhões de litros. No processo contínuo de leite UHT o leite é submetido por 2-4 seg a 130-150ºC, em sistemas de escoamento contínuo com refrigeração imediata e envase asséptico em embalagens herméticas. Esta pesquisa teve a finalidade de verificar a incidência da espécie B. cereus, em leite UHT. Em 1998, foi reportado alto índice destes organismos neste produto atingindo 34,14% das amostras analisadas. Além deste fato, existia a necessidade de se estabelece métodos e processos adequados para correta identificação de B. cereus. Assim, a esterilidade comercial de 6.500 embalagens de leite UHT foi estudada, em dois ensaios, após incubação das amostras a 37ºC e 7ºC por dez dias para germinar todos os possíveis esporos e/ou recuperar células vegetativas injuriadas. Após a incubação, foi realizada a medida de pH. Neste sentido, hum mil e trezentas amostras de cada uma de cinco plantas Brasileiras de processo UHT, que operam com injeção direta de vapor produzindo leite integral em embalagens cartonadas, foram investigadas em relação à presença de Bacillus cereus, através de ensaios fenotípicos e genéticos (PCR). Os valores de pH foram diferentes para as amostras analisadas oscilando entre 6,57 e 6,73. Após a estocagem das amostras, somente quatro embalagens suspeitas, com medida de pH abaixo de 6,5 foram analisadas na procura por B. cereus. Não foi detectado B. cereus em nenhuma amostra indicando que o leite integral UHT Brasileiro, das cinco plantas processadoras, não apresenta o microrganismo patogênico. Somente microrganismos deteriorantes foram isolados indicando que dentro do espaço amostral, o leite UHT analisado não representa riscos e problemas de saúde aos consumidores. A Espectroscopia Infravermelha por Transformada de Fourier (FTIR) e a Reação em cadeia da polimerase (PCR) foram consideradas eficientes e devem ser adotadas para confirmar a série bioquímica de B. cereus.
Palavras-chave: FTIR, PCR, esterilidade comercial, análises estatísticas
INTRODUCTION
The level of failure expected by the limitation on conducting a reasonable amount of microbiological tests is one contaminated unit in 1,000 processed units (Franklin, 1970). A simple method to determine the proportion of defective units in a lot of Ultra High Temperature (UHT) milk is to measure the pH, gas formation and sensorial evaluation after incubation since non sterility is an undesirable attribute; but for those microorganisms which do not change the pH a microbiological test is recommended to detect non sterile packages. It is preferable to check a large number of samples with simple methods rather than to check a limited number of samples by sophisticated methods (Von Bockelmann, 1982).
One criterion for evaluating UHT milk stability, during its shelf life, considers that fluctuations in pH should be lower than 0.2 units (Von Bockelmann, 1989). Assuming UHT milk average pH as 6.70, with a range between 6.60 and 6.80 (Von Bockelmann, 1982), a minimum limit of 6.50 may be established as a simple criterion to identify non sterile samples with safe margin.
In 1998, 34.17% of Brazilian analyzed UHT milk samples were reported positive for members of the cereus group (De Rezende, 1998). It became relevant to establish the real incidence of B. cereus, because in the ANVISA (2001) standard, the absence of pathogenic bacteria must be verified after the commercial sterility test (ANVISA, 2001). Since phenotypic series are not discriminative, the identification of B. cereus by methods as Polymerize Chain Reaction and Fourier Transform Infrared Spectroscopy has been recommended for dairy processing lines (Svensson et al., 1998; Beattie et al., 1998).
Since the Brazilian UHT milk consumption has increased during the last decade from 187 million liters to 4,200 million liters (ABLV, 2003), the main objective of this study was to investigate the presence of the Bacillus cereus through a microbiological survey on 6,500 cartons packed whole processed UHT milk samples.
MATERIAL AND METHODS
Commercial sterility test for B. cereus
An initial survey was conducted to establish the commercial sterility of 500 hermetic packages of whole UHT milk (one-two months of shelf life) from five Brazilian UHT milk industries. Sampling comprised 100 packages of each brand (A, B, C, D and E). Fifty of them were incubated 10 days at 37ºC and the others ten days at 7ºC in order to isolate vegetative cells of mesophilic and psychrotrophic B. cereus strains. After the incubation period, packages were checked visually for defects and aseptically opened under laminar flow. After this procedure, gas formation, milk coagulation, phase separation and organoleptic characteristics as determined by the analyst, as well as pH measurement, using a pH meter model mPA-210 (MS Tecnopon) were analysed. For each sample with pH less than 6.50 and/or positive to gas formation, smell, color, coagulation and phase separation, 50ml were added to 450ml Butterfield's phosphate-buffered (pH 7.20) and blended for 2 min at 18,000-20,000 rpm, following the FDA (1998) procedure for B. cereus enumeration.
According to the FDA method, typical B. cereus colonies on Mannitol Egg Yolk Agar (MYP-Difco) supplemented with Polimixin B sulfate 0.1% (Sigma), are surrounded by a precipitated zone which indicates lecithinase activity and a pink color is observed because mannitol is not fermented. Furthermore, 3 mm loopful of milk were transferred to DTA (Dextrose Tryptone Agar - Difco) and BHI (Brain Heart Infusion - Difco) searching for flat sour sporoformers and B. sporothermodurans, respectively, in order to recuperate all possible contaminants with 48h incubation at 37ºC. All typical and atypical colonies on MYP were isolated, codified, transferred to NA (Nutrient Agar - Difco) slants and incubated at 30ºC or 7ºC for 24h for biochemical tests of B. cereus. For DTA and BHI isolates, incubation was made at 37ºC for 48h. The second survey evaluated the commercial sterility of 1,200 milk samples of each brand (A, B, C, D and E). Six hundred packages, from each brand, were incubated at 37ºC and 7ºC in order to detect one non sterile unit, in 93% of the cases, with 95% of confidence (Ahvenainen, 1988).
Confirmatory test for B. cereus
All isolates from NA, after incubation for 24h, were examined by Gram and spore stain for morphologic characterization and catalase/benzidine test. Other confirmatory tests with the same cultures, after suspension in phosphate-buffered dilution water (pH 7.20), were carried out: anaerobic growth in phenol red glucose broth, reduction of nitrate to nitrite; modified VP (Voges-Proskauer) test for production of acetyl methyl carbinol; decomposition of tyrosine and resistance to lysozyme (FDA, 1998). The isolates that presented negative catalase were tested for growth on Man, Rogosa and Sharpe (MRS- Oxoid) and skim-milk broth.
Differential test for B. cereus
Motility test, rhizoid growth on NA, test for hemolytic activity and test for protein toxin crystal production were applied to differentiate typical strains of B. cereus from other members of the cereus group (FDA, 1998).
FTIR (Fourier Transform Infrared Spectroscopy)
All colonies obtained were transferred, in duplicate, to plates on the same culture media and sent to external analysts for complete identification by FTIR (Beattie et al., 1998; Schraft & Griffiths, 1998).
Genetic test for B. cereus
PCR test was applied for genotypic confirmation of B. cereus. Specific primers, from Invitrogen Brazil, for B. cereus were used (Yamada et al., 1999). The reaction procedure was based on the recommendations of above mentioned authors, but some modifications were included (Kaji et al., 1994). Thus, the final reaction (25 µL) consisted of: 3 µL of the isolated cells in NB (Nutrient Broth - DIFCO) incubated at 32C for 18 h; 4 µL of primer; 2.5 µL of reaction buffer PCR 10X - Tris-HCl, 200 mM; MgCl2, 2 nM; KCl, 500 mM; pH 8.4; 2.5 µL of deoxynucleoside triphosphates; 0.2 µL of Taq DNA Polymerase and 13 µL of sterile distilled water. A PTC-100 Peltier-Effect Cycling (M.J. Research, Inc.) was programmed with the following cycles in order to amplify the DNA: 1 cycle for 3 min at 94ºC; 30 cycles, each consisting of 1 min at 94ºC, 1.5 min at 58ºC and 2.5 min at 72ºC, with a final extension step for 7 min at 72ºC (Yamada et al., 1999). Two typical strains of B. cereus ATCC 11778 and ATCC 14579 and one strain of B. thuringiensis ATCC 10972 were tested after the standardization of the method. After the amplification of the DNA, the PCR fragments were analyzed by submarine gel electrophoresis (Power Pac 3000, Bio-Rad) and visualized under UV illumination (Electronic UV Transluminator, Ultra-Lum). Suitable molecular size markers (100bp) were included to guide the size of the amplification band.
Statistical analyses
pH data was organized by brand. Descriptive analyses for average pH, standard deviation, confidence interval of average, minimum and maximum pH, amplitude of the pH distribution, and confidence interval of whole data was applied. ANOVA was used to determine the significance of brand type and temperature (7ºC and 37ºC) on pH using STATISTICA (v. 5.0). Frequency of pH and accumulated frequency distribution for each brand were also calculated for 7ºC and 37ºC.
RESULTS AND DISCUSSION
Commercial sterility test in Brazilian milk
All samples of the initial survey showed no visual physical alteration (gas formation), no pH measurement below 6.5 neither sensorial alteration (organoleptic characterization and phase separation); therefore, no non-sterile package was found to reproduce the rate reported by De Rezende (1998). In addition, the occurrence of B. cereus or any other microorganisms were not verified in 500 samples. The sampling criterion was modified to increase the number of samples in order to detect low defective rates. According to Von Bockelmann (1989), it would be necessary to incubate 3,000 samples for each incubation temperature and brand, in order to discriminate 1:1,000 defective packages (Franklin, 1970) with a probability confidence of 95% assured for aseptic lines (Ahvenainen, 1988). Thus, assuming a proportion of defective units in the production of 0.005, 600 samples were incubated, for each temperature and brand, with the probability of 93% that at least one non defective unit will be found in the samples with 95% safety conditions (Ahvenainen, 1988). The expected number of defective units in the sample was three. For the second commercial sterility test, the reason of defects was calculated as the number of defective experimental samples per total number of analyzed samples in each brand, considering 600 samples as the complete batch taken from the local supermarket.
Brands A, C and D had one package with pH below the limit of 6.50 with positive result for viable bacterial cells Table 1. Gas formation (one sample) and change in color (one sample) were verified for brand D, and gas formation and change in smell for samples from brand A and C.
The pH of UHT milk samples incubated at 37ºC and at 7ºC, respectively, are presented in Table 2 and 3, and the distributions are shown in Figures 2 to 6. An extensive statistical evaluation of the pH measurement is included since no data are available in Brazil of such measurement, and it is an excellent indicative tool to begin microbiological examination of fermentative B. cereus. Of course other spoilage by food safety relevant microorganisms cannot necessarily be detected by pH measurements.
The lowest pH measurement, after ten days of incubation at 37ºC, occurred in samples of brands: A (6.13), C (6.04) and D (5.62) and the highest in brand B (6.79). The largest pH variability (standard deviation) was observed for samples from brand A followed, in decreasing order, by samples from brands B, D, E and C. The large amplitude of the pH distribution was observed in brand D (1.11 pH units). The largest pH average occurred in brand B (6.68) and the lowest in brand C (6.57). Brand E presented the greatest homogeneity in the lot, since its pH amplitude distribution was the lowest (0.19).
The results from the second commercial sterility test after incubation at 7ºC presented only one defective package from brand E, in 600 samples, with physical and sensorial alteration and pH measured below the limit of 6.50. In this case the reason of defects was 0.00167.
The highest pH measurement was 6.87 for brand A. The largest variability occurred in brand E followed in decreasing order by brands, A, B, C and D. At 7ºC, the highest average pH was 6.73 for brand A and the lowest, 6.63, for brand C. Brand C and D presented greater homogeneity in the lot, since the respective pH amplitude distribution was 0.14 and 0.15 and the standard deviation 0.022 and 0.025, respectively.
Effects of incubation temperature (37ºC and 7ºC) in the pH distribution
Comparing both incubation temperatures it was observed that, except for brand B, the average pH was higher after incubation at 7ºC, indicating a possibility of larger mesophilic bacterial activity at 37ºC than psychrotrophic activity at 7ºC. But this fact alone is not the only reason for low pH values, once at 37ºC protein denaturation could occur along ten days of incubation. This fact could lead to an oxidation reaction that would lower the pH of the product slightly at that temperature (Fox, 1992). Brand type and incubation temperature were different (P < 0.001) and there was an interaction between both variables in relation to pH. Figure 1 presents the comparison between pH measurements at 7ºC and 37ºC.
For brand A, the difference between the measurement at 7ºC and 37ºC was 0.083 and for brand D, C, E and B 0.071, 0.054, 0.047 and 0.020, respectively.
Brand A presented 0.167% of the samples with pH below 6.50. Only this brand presented 48 measurements between 6.50 and 6.55 (Figure 2).
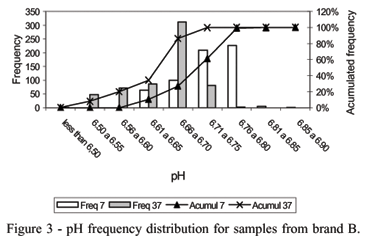
Most of the pH values for samples from brand B (69.67%) after incubation at 37ºC were concentrated between 6.71 the 6.75. No measurements below 6.50 had occurred for both temperatures. Thus, brand B showed a very good stability since all packages had pH above 6.5 (Figure 3).
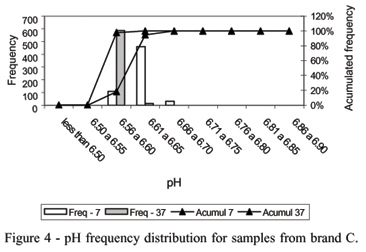
Milk pH measurements (97%) from samples of the brand C, after incubation at 37ºC, were between 6.56 and 6.60, showing a large number of samples close to the mean value. At this temperature one measurement occurred below the limit (6.04). This brand showed the most homogeneous milk samples (Figure 4).
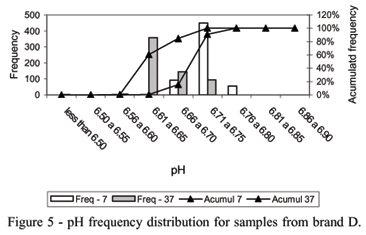
Milk pH measurements (75%) from brand D, after incubation at 7ºC, were between 6.71 and 6.75. At 37ºC occurred 0.167% of measurements below 6.50. Approximately 76% of the pH measurements from samples of the brand E after incubation at 37ºC, were found between 6.56 and 6.60. For this last brand, only one package presented a pH measurement of 5.98, at 7ºC, indicating the presence of psychrotrophic activity. In all cases, when pH measurements are analyzed, the physico-chemical changes in UHT treated milk during storage must be considered (Figures 5 and 6).
Akhtar et al. (2003) studied the changes in pH, acidity and viscosity of UHT milk after 0, 30, 60 and 90 days of storage at room temperature. They indicated an increase in acidity and viscosity with a decrease in pH. After 60 days, the decrease in pH value was 0.12, with a 6.62 measurement. This value is still greater than the limit 6.5 adopted in this research, even though, the incubation temperature was higher (37ºC). The reason to choose this lower limit was to increase the challenge and the safety margin. The decrease in pH of milk samples, during storage, may be due to a number of reasons. It is well known that pH involves the negative logarithm of hydrogen ion concentration, which means if the acidity increases pH will decrease. Storage time and temperature have great effect on pH values of the stored samples as reported by Kocak & Zadow (1985).
Reddy at al. (1991) studied the effect of storage temperature on UHT whole milk stored at room temperature (20-22ºC), 37ºC and at 4-6ºC for six months. During storage pH, heat and alcohol stability, and reflectance of all samples decreased. The greatest decrease rate was observed in samples stored at 37ºC followed by those stored at room temperature. Rise in temperature favored the growth of bacteria resulting in the production of lactic acid and eventually lowering the pH. Milk pH is more dependent on temperature than on inorganic buffer solutions, therefore, the pH of refrigerated milk will not be the same as that of the same milk at room temperature. This effect is probably due to changes in the solubility of calcium phosphate as reported by Sherbon (1988). The International Dairy Federation (1981) stated the reason for this drop in pH as a result of the interaction between lactose and milk protein.
Characterization of non-sterile milk samples
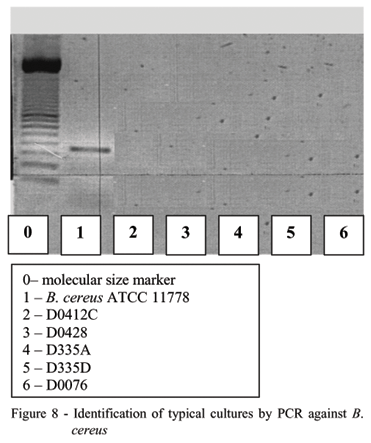
Under random sampling, pH was a normally distributed variable. Deviations from the normal distribution may occur probably due to lot, conditions of the raw material and thermal process binomium, which had not been randomized correctly in the distribution of packages as a function of temperature. Even without typical characterization on MYP, Table 4 presents pH and temperature of each non sterile sample, the color and phase separation of milk, growth in BHI and DTA and the seven isolates that were submitted as confirmatory samples and differential tests for the cereus group (Table 5) as well as identification by FTIR (Table 6) and PCR (Figures 7 and 8) for all Bacillus strains.
All brands presented one non sterile package in the 600 samples that have been analyzed (0.167% of contamination). These packages presented gas formation (flipper) and UHT typical smell.
Identification of the isolates from non-sterile milk samples
Isolate D0412B, a non sporeforming aerobic Gram positive rod presented good growth in MRS with white pin head colonies and in Skim-milk Broth and the catalase and benzidine tests, as negative, confirmed Lactobacillus sp Table 5. The proliferation of these organisms was probably limited by the competition with the other two contaminants D0412A and D0412C, identified as Coryneform bacteria and Bacillus oleronius, not reducing intensively the final pH and not promoting sensoric changes.
Our results differ from those obtained by De Rezende (1998). This author found 34.17% of samples, from four different brands, contaminated by Bacillus from the cereus group, which are not necessarily B. cereus, while no isolate of the respective group was verified in our study. Packages were opened for analysis, closed with adhesive ribbon and incubated again under refrigeration for 48h in order to be analyzed again in the De Rezende study. This procedure could promote the enrichment of the contamination and the counts had obviously exceeded the original value. On the other hand, De Rezende (1998) did not describe both, the psychrotrophic biochemical characterization of the positive isolates or the confirmatory tests for the cereus group (decomposition of the tyrosine and resistance of lysozyme). Results of the motility test, rhizoid growth and hemolytic activity test were not presented as well. Therefore, no clear differentiation could be determined. De Rezende (1998) examined the results in the same sample before and after the refrigeration and the final reason of defects of 34.17% represented the accumulative results of both tests.
The non-sterile sample from brand D presented the lowest pH as compared with the other non sterile samples. This sample presented fermentation and proteolysis of the milk denoting the hydrolysis of the casein that causes sudden pH decline as a result of secondary acid metabolisms. Gordon et al. (1973) mentioned that Bacillus licheniformis causes decomposition of casein and four strains tested presented resistance of lysozyme. In addition, Sneath (1986) affirmed that fifty percent of Bacillus licheniformis strains might ferment lactose causing and justifying this kind of spoilage. The non sterile sample from brand C presented an intermediated pH value (6.04) because B. sporothermodurans did not produce acid from sugars (Hammer, 2000).
Some of the seven isolates from UHT milk, shown in Table 6, are reported as contaminants of dairy plants, environment and distribution centers by Gilmour & Rowe (1985) who provided a reference dossier containing organisms associated with milk and milk products, including the Coryneform group of bacteria, Lactobacillaceae and Bacillaceae as members found by this approach. The presence of Lactobacillus sp. and Coryneform bacteria could be explained as isolated cases of cross or re-contamination with milk products, like yogurt and/or cheese, in the same processing plant and/or in the cleaning procedures after thermal processes or may be also due to improper storage conditions in the distribution centers because the samples presented mixed flora and both microorganisms did not present thermal resistance in the UHT region. After the commercial sterility test in 169 UHT milk samples, Mostert et al. (1979) isolated 110 cultures identified as being from the Bacillus group. Thirty-eight of these strains were subjected to UHT treatments (135ºC/10sec) and the following strains survived (number of strains in brackets): B. subtilis (4), B. licheniformis (2), and B. pumilus (1). Of course, this treatment represents a lower processing condition than the one applied on the common Brazilian dairy plants. Finally, in Brazil, Zacarchenco et al. (2000) found 45% of non sterile packages and identified 24 B.sporothermodurans strains in 300 isolates. From this study the 24 isolates came from 5 different dairy plants, with various processing conditions, as indicated by the authors: plant IV and VIII (137-140ºC/3sec indirect tubular heating), plants V (150/3sec steam injection), plant VI A (138-139/4sec indirect plate heat exchanger), plant VI B (150/3sec steam injection) and plant VII (thermal process, data not available).
In 2001, the identification of 100 selected isolates from six feed concentrate samples for dairy cattle was obtained by a combination of fatty acid methyl ester analysis, amplified ribosomal DNA restriction analysis and 16S rDNA sequencing. The concentrates were originated from five different farms. This research aims to explain the nature and the origin of aerobic spores in milk. Ninety-seven isolates could be identified to the species level or assigned to a phylogenetic species group. Most isolates obtained after a heat treatment of 10 min at 80ºC were identified as members of the B. subtilis group (32 isolates), B. pumilus (25 isolates), B. clausii (eight isolates) and B. licheniformis (eight isolates). The isolates with very heat-resistant spores, obtained after a heat treatment of 30 min at 100ºC, were identified as members of the B. subtilis group (five isolates), B. sporothermodurans (three isolates), B. amyloliquefaciens (one isolate), B. oleronius (one isolate) and B. pallidus (one isolate). Bacillus cereus was present in each feed concentrate sample and was isolated using a selective mannitol egg yolk polymyxin agar medium (Vaerewijck at al., 2001). The survival and germination of spores after a heat treatment of 30min at 100ºC is of particular importance because these spores may be considered as very heat-resistant as compared with the spores obtained after a conventional heat shock of 10min at 80C. The 11 isolates produced very heat-resistant spores. The heat resistance of B. subtilis spores was shown by Condon et al. (1992), who found D108 C values between 0·7 and 3·5 min when spores were suspended in buffer. Bacillus oleronius is a species isolated for the first time from the hindgut of the termite Reticulitermes santonensis (Kuhnigk et al., 1995). This observation indicates the possibility that feed concentrates may harbour species which might be derived from tropical ingredients and which are transferred to raw milk through feeding and faecal contamination of the udder. Bacillus oleronius is the closest phylogenetic neighbor of B. sporothermodurans (De Silva et al., 1998). The latter species is the only one presently known to produce highly heat-resistant spores, surviving UHT and industrial sterilization processes. So far, it has only been occasionally isolated from UHT and sterilized milk and milk products which do not attend to the sterility criteria for these products (Pettersson et al., 1996).
Chopra et al. (1980) and Ahmed et al. (1983) had reported Bacillus cereus incidence in pasteurized milk and milk products. Both authors justify the low incidence of the microorganism (<1/mL de yoghurt and 3.0E+01 CFU mL-1 of milk) due to the severity of the applied thermal treatment. Such explanation is compatible with the results obtained in this research, since the applied thermal treatment on the milk samples was around 140ºC/2-4 sec. In this context, Gillis et al. (1985) evaluated the effect of the raw milk quality on the UHT milk, processed at 149ºC for 3 sec. They concluded that there were no survivors even with inoculation levels of 1+E06 UFC mL-1 of mesophilic and psychrotrophic bacteria. Rangasamy et al. (1993) found B. cereus in samples of raw and pasteurized milk, however in contrast, the UHT milk was considered free of this microorganism, although there is no report in the literature recuperating thermal resistant Bacillus, except for B. sporothermodurans, after UHT milk process.
Heat resistance of bacterial spores is genetically determined but is also influenced by environmental conditions during sporulation (Mazas et al., 1995). So soil/feed composition, in which spores are formed, may be considered as a variable for spore resistance.
In 2002, the PCR method was used as a screening method for strains with very heat resistant endospores, isolated at the dairy farm level after heat treatment for 30 min at 100ºC, and under these conditions 17 strains were identified as B. sporothermodurans (Scheldeman et al., 2002).
Figure 7 shows the primer standardization and the reaction (in duplicate) of two typical strains of B. cereus (ATCC 11778 and ATCC 14579) and one typical strain of B. thuringiensis (ATCC 10972). A fragment of approximately 350bp was amplified by PCR in both B. cereus strains as expected and no fragment was detected in the B. thuringiensis strain showing the specificity of the used primer. Another PCR reaction with the five strains isolated in this work was also carried out and no fragment was obtained, confirming the earlier analysis that these isolates are not B. cereus.
None of the five Bacilli isolates had the electrophoretic profile of Bacillus cereus.
CONCLUSIONS
Brazilian UHT milk does not present B. cereus contamination. The presence of the aerobic contaminants found in this research is possible in commercial dairy plants, but their occurrence is low as compared to the amount of UHT milk produced today, and its thermal resistance in the literature is low considering the thermal process applied for milk. Its incidence could be attributed to poor handling conditions, storage procedures and cross contamination. Only B. sporothermodurans presents kinetic parameters that may lead to its survival in processed Brazilian UHT milk, but its presence in a commercial sterile product is tolerable. The phenotypic series for B. cereus must be complemented by identification with PCR and/or FITR for best confirmation.
ACKNOWLEDGEMENTS
The authors acknowledge the financial support from CNPq and Dr.Yoko Rosato for the guidance in the PCR procedures.
Received January 09, 2006
Accepted March 02, 2007
- AGÊNCIA NACIONAL DE VIGILÂNCIA SANITÁRIA - ANVISA. Resolução RDC nº 12, de 02 de janeiro de 2001 que aprova o Regulamento Técnico sobre padrões microbiológicos para alimentos. D.O.U. - Diário Oficial da União Poder Executivo, de 10 de janeiro de 2001. 2001.
- AHMED, D.A-H.; MOUSTAFA, M.F.; MARTH, E.H. Incidence of Bacillus cereus in milk and some milk products. Journal of Food Protection, v.46, p.126-128, 1983.
- AHVENAINEN, R. Quality assurance and quality control of aseptic packaging. Food Reviews International, v.4, p.45-76, 1988.
- AKHTAR, S.; ZAHOOR, T.; HASHMI, A.M. Physical-chemical changes in UHT treated and whole milk powder during storage at ambient temperature. Journal of Research (Science), v.14, p.97-101, 2003.
- ASSOCIAÇÃO BRASILEIRA DE LEITE LONGA VIDA - ABLV. Disponível em: <http://www.ablv.org.br/ index.cfm? fuseaction=longavida> Accessed at: July 2003.
- BEATTIE, S.H.; HOLT, C.; HIRST, D.; WILLIAMS, A.G. Discrimination among Bacillus cereus, B. mycoides and B. thuringiensis and some other species of the genus Bacillus by Fourier transform infrared spectroscopy. FEMS Microbiology Letters, v.164, p.201-206. 1998.
- CHOPRA, P.; SINGH, A.; KALRA M.S. Occurrence of Bacillus cereus in Milk and Milk Products. Indian Journal Dairy Science, v.33, p.248-252, 1980.
- CONDON, S.; BAYARTE, M.; SALA, F.J. Influence of the sporulation temperature upon the heat resistance of Bacillus subtilis Journal of Applied Bacteriology, v. 73, p.251-256, 1992.
- DE REZENDE, N.C.M. Ocorrência de bactérias do grupo B. cereus e de microrganismos indicadores em leite UHT integral. Jaboticabal: UNESP/FCAV, 1998.
- DE SILVA, S.; PETTERSSON, B.; AQUINO DE MURO, M.; PRIEST, F.G. A DNA probe for the detection and identification of Bacillus sporothermodurans using the 16S23S rDNA spacer region and phylogenetic analysis of some field isolates of Bacillus which form highly heat resistant spores. Systematic and Applied Microbiology, v.21, p.398407, 1998.
- FOOD DRUG ADMINISTRATION - FDA. .Bacteriological analytical manual. 8.ed. Arlington: FDA; AOAC, 1998. chap. 14.
- FOX, P.F. Advanced dairy chemistry Dordrecht: Kluwer Academic, 1992. v.1.
- FRANKLIN, J.G. Spores in milk: Problems associated with UHT processing. Journal of. Applied Bacteriology, v.33, p.180-191, 1970.
- GILLIS, W.T.; CARTLEDGE, M.F.; RODRIGUEZ, I.R.; SUAREZ, E.J. Effect of raw milk quality on ultra-high temperature processed milk. Journal of Dairy Science, v.68, p.2875-2879, 1985.
- GILMOUR, A.; ROWE, M.T. Microorganisms associated with milk. In: ROBINSON, R.K. Dairy microbiology London: Elsevier Applied Science, 1985. v.1, cap.2, p.35-76.
- GORDON, R.E.; HAYNES, W.C.; HOR-NAY PANG, C. The genus Bacillus Washington, D.C.: USDA, 1973. (Agriculture Handbook, 427).
- HAMMER, P. Raw materials: Quality and pricing. In: INTERNATIONAL DAIRY FEDERATION IDF. Bacillus sporothermodurans A Bacillus forming highly heat-resistant spores. Brussels, 2000. chap.2, p.5-8. (Bulletin, 357).
- INTERNATIONAL DAIRY FEDERATION. New monograph on UHT milk Brussels, 1981. (Bulletin, 133).
- KAJI, D.A.; PRIEST, F.; CANHOS, V.P.; ROSATO, Y.B. Characterization of whole cell proteins of some bacillus Thuringiensis subs. Israelensis isolated in Brazil by Plyacrilamide gel electrophoresis. Systematic and Applied Microbiology, v.17, p.104-107, 1994.
- KOCAK, H.R.; ZADOW, J.G. Age gelatin of UHT whole milk as influenced by storage temperature. Australian Journal of Dairy Technology, v.40, p.14-21, 1985.
- KUHNIGK, T.; BORST, E.M.; BREUNIG, A.; KONIG, H.; COLLINS, M.D.; HUDSON, R.A.; KAMPFER, P. Bacillus oleronius sp.nov., a member of the hindgut flora of the termite Reticulitermes santonensis Canadian Journal of Microbiology, v.41, p.699-706, 1995.
- MAZAS, M.; GONZÁLES, I.; LÓPES, M.; GONZÁLES, J.; SARMIENTO, R.M. Effects of sporulation media and strain on thermal resistance of Bacillus cereus spores. International Journal of Food Science and Technology, v.30, p.71-78, 1995.
- MOSTERT, J.F.; LÜCK, H.; HUSMANN, R.A.; Isolation, identification and practical properties of Bacillus species from UHT and sterilized milk. South African Journal of Dairy Technology, v.11, p.125-132, 1979.
- PETTERSSON, B.; LEMBKE, F.; HAMMER, P.; STACKEBRANDT, E.; PRIEST, F.G. Bacillus sporothermodurans, a new species producing highly heat-resistant endospores. International Journal of Systematic Bacteriology, v.46, p.759764, 1996.
- RANGASAMY, P.N.; IYER, M.; ROGINSKI H. Isolation and characterization of Bacillus cereus in milk and dairy products manufactured in Victoria. The Australian Journal of Dairy Technology, v.48, p.93-95, 1993.
- REDDY, K.K.; NGYEN, M.H.; KAILASAPATHY, K.; ZADOW, J.G. The effect of some treatments and storage temperature on UHT whole milk. Australian Journal of Dairy Technology, v.46, p.57-63, 1991.
- SCHELDEMAN, P.; HERMAN, L.; GORIS, J.; De VOS, P.; HEYNDRICKX M. Polymerase chain reaction identification of Bacillus sporothermodurans from dairy sources. Journal of Applied Microbiology, v.92, p.983-991, 2002.
- SCHRAFT, S.F.L.H.; GRIFFITHS, M.W. Identification of Bacillus cereus by Fourier Transform Infrared Spectroscopy (FTIR). Journal of Food Protection, v.61, p.921-923, 1998.
- SHERBON, J.W. Physical properties of milk. In: WONG, N.P. (Ed.). Fundamentals of dairy chemistry 3.ed. New York: Van Nostrand Reinhold, 1988. p.409-460.
- SNEATH, P.H.A.; MAIR, N.S.; SHARPE, N.E.; HOLT, J.G. Bergey's manual of systematic bacteriology Baltimore: Williams & Wilkins, 1986. 1599p.
- SVENSSON, B.; CHRISTIANSSON, A.; ENEROTH, A.; BRENDEHAUGT, S. Propagation of B. cereus in the dairy processing line. In: INTERNATIONAL CONGRESS OF DAIRY PRODUCTS, 25., Aarhus Denmark, 1998.
- VAEREWIJCK, M.J.M.; DE VOS, P.; LEBBE , L.; SCHELDEMAN, P.; HOSTE, B.; HEYNDRICK, M. Occurrence of Bacillus sporothermodurans and other aerobic spore-forming species in feed concentrate for dairy cattle. Journal of Applied Bacteriology, v.91, p.1074-1084, 2001.
- VON BOCKELMANN, B. Aseptic packaging processing Lausanne: Tetra Pak International, 1982.
- VON BOCKELMANN, B. Quality control of aseptically packaged food products. In: REUTER, H. Aseptic packaging of food. Lancaster: Technomic Publications, 1989.
- YAMADA, S.; OHASHI, E.; AGATA, N.; VENKATESWARAN, K. Cloning and nucleotide sequence analysis of gyrB of Bacillus cereus, B. thuringiensis, B. mycoides and B. anthracis and their application to the detection of B. cereus in rice. Applied and Environmental Microbiology, v.65, p.1483-1490, 1999.
- ZACARCHENCO, P.B.; LEITÃO, M.F.F.; DESTRO, M.T.; ANDRIGUETO, C. Ocorrência de B. sporothermodurans em leite UAT/UHT brasileiro e a influência do tratamento térmico. Ciência e Tecnologia de Alimentos, v.20, p.363-368, 2000.
Publication Dates
-
Publication in this collection
09 May 2007 -
Date of issue
2007
History
-
Accepted
02 Mar 2007 -
Received
09 Jan 2006