ABSTRACT:
Urea is a common non-protein supplement used in ruminant feed; however, excessive consumption may lead to poisoning by NH3. Although the slow release of urea into the rumen has shown to be an essential aspect for ruminant feed, to date only a few studies have addressed this matter. In this study we examined the influence of five different NH3-N slow release systems based on clay-urea nanocomposites on the fiber digestibility of low-quality forage (sugarcane straw) in vitro. Physical properties of nanocomposites were evaluated and their effects on digestibility were tested in vitro using pristine urea as a positive control (level of 1 % of DM of sugarcane straw sample) and sugarcane (with no additives) as a negative control. Ammonia release and digestibility were evaluated at 12, 24, 36, 48, 72 and 96-h. Generally, all nanocomposites increased (p < 0.05) digestibility of fiber over control under all the conditions stipulated, but the samples with hydrogel content were more expressive. We concluded that an ideal release rate and optimum environment for microbial synthesis are necessary to maximize the digestion of sugarcane.
Keywords:
slow-release urea; ruminants feed; non-protein nitrogen
Introduction
A topic of interest in recent years in ruminant feed has been the search for strategies that can optimize nutrient synchrony between N and carbohydrate compounds in the rumen, and promote better nutrient use and energy efficiency as well as reduce the risk of environmental pollution (Holder et al., 2015Holder, V.B.; Tricarico, J.M.; Kim, D.H.; Kristensen, N.B.; Harmon, D.L. 2015. The effects of degradable nitrogen level and slow release urea on nitrogen balance and urea kinetics in Holstein steers. Animal Feed Science and Technology 200: 57-65.; Spanghero et al., 2018Spanghero, M.; Nikulina, A.; Mason, F. 2018. Use of an in vitro gas production procedure to evaluate rumen slow-release urea products. Animal Feed Science and Technology 237: 19-26.; Yan et al., 2018Yan, X.T.; Yan, B.Y.; Ren, Q.M.; Dou, J.J.; Wang, W.W.; Zhang, J.J.; Zhou, J.W.; Long, R.J.; Ding, L.M.; Han, J.; Li, Z.P.; Qiu, Q. 2018. Effect of slow-release urea on the composition of ruminal bacteria and fungi communities in yak. Animal Feed Science and Technology 244: 18-27.). N retention in the rumen is mainly mediated by the degradation rate of N compounds and carbohydrates, and by the energy available for protein synthesis (Calsamiglia et al., 2010Calsamiglia, S.; Ferret, A.; Reynolds, C.K.; Kristensen, N.B.; van Vuuren, A.M. 2010. Strategies for optimizing nitrogen use by ruminants. Animal 4: 1184-1196.). In low-quality forage diets, a source of Non-Protein Nitrogen (or NPN) is needed to improve microbial growth. However, their use is limited because the ruminal degradation rates of fiber (energy) and urea hydrolyses are very different and they can lead to loss of N or even toxicity (Almora et al., 2012Almora, E.G.A.; Huntington, G.B.; Burns, J.C. 2012. Effects of supplemental urea sources and feeding frequency on ruminal fermentation, fiber digestion, and nitrogen balance in beef steers. Anim. Feed Science Technology 171: 136-145.; Holder et al., 2013Holder, V.B.; El-Kadi, S.W.; Tricarico, J.M.; Vanzant, E.S.; McLeod, K.R.; Harmon, D.L. 2013. The effects of crude protein concentration and slow release urea on nitrogen metabolism in Holstein steers. Archives of Animal Nutrition 67: 93-103.; Spanghero et al., 2018Spanghero, M.; Nikulina, A.; Mason, F. 2018. Use of an in vitro gas production procedure to evaluate rumen slow-release urea products. Animal Feed Science and Technology 237: 19-26.). However, urea is still the most used NPN for this purpose due to its low cost, availability, easy handling and application (Kertz, 2010Kertz, A.F. 2010. Review: urea feeding to dairy cattle: a historical perspective and review. The Professional Animal Science 26: 257-272.).
The maintenance of bodily nitrogen concentration at non-toxic levels requires additional expenditure of energy from ruminants (Pearson and Smith, 1943Pearson, R.M.; Smith, J.A.B. 1943. The utilization of urea in the bovine rumen. 2. The conversion of urea to ammonia. Biochemical Journal 37: 148-153.; Paixao et al., 2006Paixao, M.L.; Valadares, S.D.; Leão, M.I.; Valadares, R.F.D.; Paulino, M.F.; Marcondes, M.I.; Fonseca, M.A.; Silva, P.A.; Pina, D.D.S. 2006. Urea in diets of steers: intake, digestibility, performance, carcass traits and microbial yield. Revista Brasileira de Zootecnia 35: 2451-2460 (in Portuguese, with abstract in English).). Furthermore, high urea levels have negative consequences for the environment, as they indicate that more nitrogen is being excreted in manure and urine, thereby increasing problems in the water quality, and odors from gases exhaled from these waste products (Cherdthong and Wanapat, 2010Cherdthong, A.; Wanapat, M. 2010. Development of urea products as rumen slow release feed for ruminant production: a review. Australian Journal of Basic Applied Science 4: 2232-2241.). A slow or controlled release of urea into the rumen is therefore an essential aspect for rational use of these inputs in feed. Earlier studies by our research group (Pereira et al., 2012Pereira, E.I.; Minussi, F.B.; da Cruz, C.C.T.; Bernardi, A.C.C.; Ribeiro, C. 2012. Urea Montmorillonite-Extruded Nanocomposites: A Novel Slow-Release Material. Journal of Agricultural and Food Chemistry 60: 5267-5272.; Pereira et al., 2015Pereira, E.I.; Cruz, C.C.T.; Solomon, A.; Le, A.; Cavigelli, M.A.; Ribeiro, C. 2015. Novel slow-release nanocomposite nitrogen fertilizers: the impact of polymers on nanocomposite properties and function. Industrial and Engineering Chemistry Research 54: 3717-3725.; Yamamoto et al., 2016Yamamoto, C.F.; Pereira, E.I.; Mattoso, L.H.C.; Matsunaka, T.; Ribeiro, C. 2016. Slow release fertilizers based on urea/urea-formaldehyde polymer nanocomposites. Chemical Engineering Journal 287: 390-397.) have seen the development of a new class of nanocomposites intended for slow nitrogen release from the simultaneous extrusion of montmorillonite and urea, with further addition of polymers (hydrogel and paraformaldehyde). These materials were tested under agronomical conditions, showing better performance than commercial polymer-coated urea (Pereira et al., 2017Pereira, E.I.; Nogueira, A.R.A.; Cruz, C.C.T.; Guimaraes, G.G.F.; Foschin, M.M.; Bernardi, A.C.C.; Ribeiro, C. 2017. Controlled urea release employing nanocomposites increases the efficiency of nitrogen use by forage. Sustainable Chemical and Engineering 5: 9993-10001.). To date, only a few studies on controlled urea release strategies in the rumen have been found in the literature, although a significant number of slow urea release materials have been manufactured and tested in the past for other purposes, such as the application of urea as a fertilizer (Ribeiro et al., 2011Ribeiro, S.S.; Vasconcelos, J.T.; Morais, M.G.; Itavo, C.; Franco, G.L. 2011. Effects of ruminal infusion of a slow-release polymer-coated urea or conventional urea on apparent nutrient digestibility, in situ degradability, and rumen parameters in cattle fed low-quality hay. Animal Feed Science and Technology 164: 53-61.; Holder et al., 2013Holder, V.B.; El-Kadi, S.W.; Tricarico, J.M.; Vanzant, E.S.; McLeod, K.R.; Harmon, D.L. 2013. The effects of crude protein concentration and slow release urea on nitrogen metabolism in Holstein steers. Archives of Animal Nutrition 67: 93-103.).
Thus, our main hypothesis is that the regular supply of urea by these novel nanocomposites in a ruminal medium may favor biological activity in the same way by better controlled application of the N source avoiding the toxical effects of high dosage as previously observed under agronomical conditions. Consequently, this study is aimed at analyzing the performance of different urea nanocomposites by an in vitro release approach involving simulated conditions in the rumen. Based on our results, we concluded that, in an effort to reduce ruminal degradation, appropriate rates of urea can be developed using these slow-release technologies.
Materials and Methods
Preparation of nanocomposites
Commercial urea (Ur) had been previously ground with a ball milling apparatus (Servitech CT-241) and then sieved through a 30 mesh sieve. Montmorillonite (MMT) was used without purification. Polyacrylamide hydrogel (HG) was synthesized following the methodology reported by Bortolin et al. (2012)Bortolin, A.; Aouada, F.A.; Moura, M.R.; Ribeiro, C.; Longo, E.; Mattoso, L.H.C. 2012. Application of polysaccharide hydrogels in adsorption and controlled-extended release of fertilizers processes. Journal of Applied Polymer Science 123: 2291-2298.. Paraformaldehyde (Pf - solid) was purchased from Sigma-Aldrich and used as received.
Five different nanocomposites were prepared and divided into two groups. The first group comprised mixtures between montmorillonite and urea at different mass ratios, namely, MMT/Ur 1:1 (50 wt.% urea), MMT/ Ur 1:2 (66 wt.% urea) and MMT/Ur 1:4 (80 wt.% urea). The second group of nanocomposites was prepared on the basis of MMT/Ur 1:4 formulation, but modified with two different components, polyacrylamide hydrogel (2 wt.%) and paraformaldehyde (1:0.5 urea: paraformaldehyde molar ratio). These nanocomposites were re-named as MMT/Ur 1:4/HG and MMT/Ur 1:4/Pf, respectively, and were prepared following the methodology previously reported by Pereira et al. (2015)Pereira, E.I.; Cruz, C.C.T.; Solomon, A.; Le, A.; Cavigelli, M.A.; Ribeiro, C. 2015. Novel slow-release nanocomposite nitrogen fertilizers: the impact of polymers on nanocomposite properties and function. Industrial and Engineering Chemistry Research 54: 3717-3725. and Yamamoto et al. (2016)Yamamoto, C.F.; Pereira, E.I.; Mattoso, L.H.C.; Matsunaka, T.; Ribeiro, C. 2016. Slow release fertilizers based on urea/urea-formaldehyde polymer nanocomposites. Chemical Engineering Journal 287: 390-397.. The components (urea, montmorillonite, paraformaldehyde, and hydrogel) were weighed separately and then mixed until a homogeneous formulation was obtained. Afterwards, water was added to the formulation at a concentration of 13 wt.% based on the total sample mass, homogenized again and then extruded on a twin-screw extruder (Coperion Werner and Pfleiderer) at 40 °C and 150 rpm (Pereira et al., 2012Pereira, E.I.; Minussi, F.B.; da Cruz, C.C.T.; Bernardi, A.C.C.; Ribeiro, C. 2012. Urea Montmorillonite-Extruded Nanocomposites: A Novel Slow-Release Material. Journal of Agricultural and Food Chemistry 60: 5267-5272.). The resulting nanocomposite samples were dried at room temperature for five days, with the exception of the MMT/Ur 1:4 /Pf 0.5 nanocomposite, which was dried at 90 °C in order to formulate a curing process (formation of urea-formaldehyde polymer). Extrusion processing was used in this work due to its feasibility in terms of obtaining a large amount of pelleted mater possibly enabling further commercial application of the MMT/urea nanocomposites.
Characterizations of nanocomposites
X-ray diffraction (XRD) analysis was carried out on samples previously ground in a mortar and pestle. The relative intensity was recorded in the angular range (2θ) from 3 to 40°, using Cu-Kα radiation (λ = 0.1546 nm). The scan speed was 1° min−1, and the voltage and current of the X-ray tube were 30 kV and 30 mA, respectively. The XRD technique is commonly used to determine crystalline phases. Crystalline solids have atoms arranged in ordered crystal planes which are separated from each other by distances of the same order of magnitude as the X-ray radiation wavelength. By focusing an X-ray beam on a crystal, the beam interacts with the atoms present in the solid, resulting in the diffraction phenomenon. The interplanar distance “d” of MMT was calculated using Bragg's Law which establishes a relationship between the diffraction angle and the interplanar distances (characteristic for each crystalline phase):
where n is the order of reflection (n = 1), λ the wavelength of the X-ray, and θ the angle of diffraction.
The morphological and qualitative chemical analysis of the samples were performed by Scanning Electron Microscopy (SEM) coupled to an energy dispersive X-ray (EDX) analysis system. Pellets from the samples were manually broken and fixed on stubs with carbon tapes, so that the cross-sectional surface was examined. Samples were coated with carbon in Sputter Coating equipment complete with a carbon evaporation accessory. The accelerating voltage in the SEM analysis was 15 kV. Composites and their starting materials were subjected to thermogravimetric analysis on Q500 equipment. Samples, approximately 10 mg in size, were placed on a platinum crucible and heated up to 600 °C at a heating rate of 10 °C min−1 under a dynamic atmosphere of synthetic air with flow of 60 mL min−1. Elemental analysis was conducted using 5 mg of sample for each measurement.
Evaluation of urea release in water
The study of urea release to an aqueous medium at 25 °C consisted of adding sample pellets directly to a beaker containing 400 mL of distilled water at neutral pH. Each experimental unity (beaker) was shaken in a thermostated device running at 30 rpm for 120 h. A constant mass of urea per sample was used in all experiments, which were performed with five repetitions for each type of nanocomposite. Pure commercial urea was also tested for comparison purposes.
0.5 mL aliquots were collected at different time intervals up to a maximum sampling time of 120 h. Urea concentration was immediately determined using a methodology proposed by With et al. (1961) in a UV-Vis spectrophotometer. The method is based on mixing aliquots of 0.5 mL of an Ehrlich's reagent and 2 mL of a 10 % trichloroacetic acid solution with a subsequent absorbance reading at a wavelength of λ = 435 nm.
In vitro tests of dry matter digestibility
The in vitro dry matter digestibility experiments were conducted at São Carlos, in the state of São Paulo, Brazil (21°57′31” S, 47°50′32” W, altitude: 837 m). Tests were performed using pure sugarcane straw and sugarcane straw supplemented with 7 materials, 6 non-protein nitrogen sources and pure montmorilonite. The samples tested in this experiment were: (a) MMT/Ur 1:1; (b) MMT/Ur 1:2; (c) MMT/Ur 1:4; (d) MMT/Ur 1:4/HG; (e) MMT/Ur 1:4/Pf; (f) pure MMT, and (g) pure urea.
Composites had been previously dried in a circulating air oven at 90 °C, ground and sieved through a 25 mesh sieve to obtain a powder particle size smaller than 1 mm (Figure 1A and B). Sugarcane was dried at 60 °C for 72 h and milled on a Willey milling machine with a 1 mm sieve (particle size smaller than 1 mm) and its compositional analysis is summarized in Table 1. Sugarcane was chosen because it presents a low protein content, low digestibility, and is thus suitable in non-protein nitrogen supplementation studies. Rumen fluid was collected 1 h before starting the experiment from two animals previously fasted for 12 h and stored in a thermal preheated bottle.
Digestibility tests were conducted in two stages: fermentation with rumen microorganisms and solubilization of fermentation residues with a mild detergent solution. First, apparent digestibility was determined using an automated incubator system. In this step the samples were weighed and stored under heat-sealed F-57 bags in order to obtain 0.05 g of sugarcane and 1 wt.% of urea in each bag. Each digestion jar contained a type of food, totaling 24 bags (18 samples and 6 empty bags as a control), where each bag corresponds to an experimental unit. The samples were incubated at a 4:1 buffer solution volume ratio (MacDougall solution, final pH = 6.69) and 400 mL of rumen fluid. The bags were distributed evenly into jars with the aid of an internal divisor and then CO2 was flushed through the jars for 5 min to adjust the pH. The jars were incubated at 39 °C under mild rotational stirring. The samples were tested in triplicate at 6 different times: 12, 24, 36, 48, 72 and 96 h. The pH was also measured during the tests. For each data collection event, the bag was washed with hot water and dried in an oven for 24 h. The dried bag mass was registered, and then the bag was stored in a desiccator prior to the second step.
In the second stage, bags collected at the different digestion times were subjected to a reflux procedure with a mild detergent solution for approximately 1 h in order to separate the undigested cell wall portion from the microbial material, which enabled determination of true digestibility (Van Soest et al., 1991Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science 74: 3583-3597.). The NDF analysis was performed on a fiber analysis equipment. The reflux treatment was followed by three succesive washing cycles with deionized water for 15 min. After this procedure, the bags were dried at 90 °C and weighed for calculation of dry matter (DM) digestibility (Ankom Technology, 2015Ankom Technology. Method 3: In vitro true digestibility using the DaisyII Incubator. Available at: http://www.ankom.com/media/documents/IVDMD_0805_D200.pdf [Accessed Apr 15, 2015]
http://www.ankom.com/media/documents/IVD...
).
The change in ammonia during the digestibility tests was examined by a spectrophotometric method and flow injection analysis (FIA) in a spectrophotometer coupled to a peristaltic pump. 5 mL of rumen liquid were collected with an automatic pippetor and stored in a falcon tube containing 0.2 mL of sulfuric acid solution (50 % v/v). This method consists of injecting the sample into a carrier stream system driven by a peristaltic pump, whereby the sample reacts with a sufficient quantity of reagents to form an indophenol blue complex whose color intensity is proportional to the ammonia concentration (Nogueira et al., 1996Nogueira, A.R.A.; Souza, G.B.; Batista, L.A.R. 1996. Spectrophotometric determination of nitrogen in plants digested by flow injection system analysis. Quimica Nova 19: 33-36 (in Portuguese, with abstract in English).).
The Orskov and McDonald equation (1979)Orskov, E.R.; McDonald, I. 1979. Estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. Journal of Agricultural Science 92: 499-503. was used for digestibility of kinetic parameter determination:
where p is the DM degradation (%) at time t, a the soluble fraction, b the slowly degradable fraction (%), and c the rate of degradation of the b fraction (% h−1). Non-linear regression was used for parameter estimation and the Durbin Watson test was used to verify autocorrelation of the regression residuals. The parameters of the digestibility kinetics of the different materials were compared by analysis of variance and Duncan's multiple comparison test. All statistical analyses were performed using the R software 3.3.2 package at a significance level of 5 %.
Results and Discussion
Pure MMT and MMT-loaded nanocomposite samples were characterized by XRD to verify the occurrence of MMT exfoliation and/or intercalation within the nanocomposite structures. Based on this equation and the results shown in Figure 2A, MMT presented a basal reflection 001 at 6.84° of 2θ (d001 = 1.30 nm) which is displaced to very small angles in all the nanocomposites – confirming the correct nanocomposite formation. The XRD analysis showed that intensity of this reflexion largely decreased in the nanocomposite patterns, indicating that the MMT particles were exfoliated within the nanocomposite matrixes due to intercalation of urea (Ahmadi et al., 2004Ahmadi, S.J.; Huang, Y.D.; Wei, L. 2004. Synthesis of EPDM/organoclay nanocomposites: effect of the clay exfoliation on structure and physical properties. Iranian Polymer Journal 13: 415-422.; Wang et al., 2010Wang, N.; Zhang, X.X.; Han, N.; Liu, H.H. 2010. A facile method for preparation of thermoplastic starch/urea modified montmorillonite nanocomposites. Journal of Composite Materials 44: 27-39.).
(A) X-ray diffraction (XRD) patterns (2θ = 3-10° angular range) of pure Montmorillonite (MMT) and composites. SEM images of (B) pure MMT, (C) pure urea, (D) MMT/Ur 1:1, (E) MMT/Ur 1:2. (F) MMT/Ur 1:4, (G) MMT/Ur 1:4/HG, and (H) MMT/Ur 1:4/Pf.
Scanning electron microscopy (SEM) was performed in order to verify the samples's microstructure, as shown in Figure 2B-H. The microstructural domains of the nanocomposite samples were confirmed to be urea, polymerized urea or MMT by SEM-EDX. The characteristic lamellar structure of MMT was observed in all nanocomposites, but this feature was less pronounced in the MMT/Ur 1:4 sample, indicating a higher MMT dispersion level with increasing urea content. The particle size of urea crystals decreased in the samples MMT/Ur 1:4, MMT/Ur 1:4/HG and MMT/Ur 1:4/Pf when compared with pure urea. The MMT/Ur 1:4/Pf nanocomposite presented crystals with a needle-like shape, which were identified as being polymerized urea (urea-formaldehyde polymer). These changes in size and shape of crystals are related to recrystallization of solubilized urea. The needle-shaped crystals had N, O, and C as their major elements, while the lamellar forms or the material surrounding the particles were mainly composed of Al, Mg and Si. These are characteristic elements of urea and MMT, respectively.
The thermal decomposition of the composites was studied by thermogravimetric analysis (TG and DTG). DTG spectra differed according to the composition of the nanocomposites and are presented in Figure 3. Pure urea exhibited a thermal profile that corroborates with others reported in previous studies (Brack et al., 2014Brack, W.; Heine, B.; Birkhold, F.; Kruse, M.; Schoch, G.; Tischer, S.; Deutschmann, O. 2014. Kinetic modeling of urea decomposition based on systematic thermogravimetric analyses of urea and its most important by-products. Chemistry Engineering Science 106: 1-8.). It starts before the melting point (132.5 °C) up to approximately 400 °C, where complete oxidation of urea takes place. Pure MMT presented mass loss only at approximately 800 °C, which corresponds to structural water or MMT dehydroxylation, as previously observed by Zhang et al. (2015)Zhang, Y.M.; Liu, Q.F.; Wu, Z.G.; Zhang, Y.F. 2015. Thermal behavior analysis of two bentonite samples selected from China. Journal of Thermal Analysis and Calorimetry 121: 1287-1295.. Pure HG presented 3 mass loss steps: 100-230 °C (elimination of water), 300-430 °C (oxidation of side amide groups and crosslinking agent), and finally at 430-560 °C (degradation of polyacrylamide chains). Pure Pf depolymerizes at temperatures above 115 °C; formaldehyde is rapidly volatilized above this temperature range (Yamamoto et al., 2016Yamamoto, C.F.; Pereira, E.I.; Mattoso, L.H.C.; Matsunaka, T.; Ribeiro, C. 2016. Slow release fertilizers based on urea/urea-formaldehyde polymer nanocomposites. Chemical Engineering Journal 287: 390-397.).
DTG curves of nanocomposites and their neat precursor materials. The % weight losss of main thermal events are indicated on the curves.
The MMT/Ur nanocomposites exhibited weight loss stages analogous to those of pure urea, but the first loss stage was shifted towards lower temperatures probably due to cohesion of the materials. In contrast, the MMT/Ur 1:4/Pf nanocomposite presented shifts in the first and second stages towards higher degradation temperatures, indicating that urea polymerization had taken place in this sample. Accordingly, losses were observed at approximately 115 °C, which noticeably suggests that all paraformaldehyde molecules were primarily converted into a polymer with urea. The degradation stages of the MMT/Ur 1:4/HG nanocomposite were exposed to higher temperatures, suggesting interaction between urea and hydrogel. The peak at 350 °C is indicative of this interaction and may be attributed to urea fractions strongly linked to the hydrogel. The final residue content of all samples, showed by TG curves, was proportional to the MMT amount, since MMT does not suffer thermal decomposition in this temperature range.
In order to examine the chemical composition of the formulations, the samples were studied by elemental analysis (CHN), Table 2. As MMT is not composed of nitrogen, the N content of the nanocomposites was expected to be proportional only to the amount of urea present in each formulation. CHN analysis of all formulations showed the correct levels of nitrogen, indicating that there was no loss of nitrogen through volatilization during the extrusion process. Urea also contains carbon in its molecular structure; thus, the content of carbon of the nanocomposites was seen to be proportional to their urea content. The C contents were in excess of the expected values, which may be due to the presence of organic matter in the MMT. The H percentage corresponds to the portion of urea in the formulations, but certain values were slightly higher than expected, which may be attributed to the presence of hydration water molecules.
The urea release in water was studied in order to examine whether there was a delay in the urea release time for nanocomposites in comparison with pure urea. Figure 4 reports that pure urea was totally solubilized in less than 5 h. All nanocomposites exhibited slower release behavior compared with pure urea. The MMT/Ur 1:1 and MMT/Ur 1:2 samples reached 100 % of urea released over 24 h and 48 h, respectively. The MMT/Ur 1:4 nanocomposite did not release its total urea content even after 120 h, reaching approximately 84 %. The MMT/Ur 1:4/HG nanocomposite displayed urea release behavior slower than those of the MMT/Ur nanocomposites, releasing 80 % and 100 % of its urea content over 96 h and 120, respectively. The MMT/Ur 1:4/Pf nanocomposite exhibited the highest urea retention capacity, and released about 70 % of its urea content over 96 h.
Slower urea release from nanocomposites indicates that the MMT showed interaction with the urea derived from the extrusion process (Pereira et al., 2017Pereira, E.I.; Nogueira, A.R.A.; Cruz, C.C.T.; Guimaraes, G.G.F.; Foschin, M.M.; Bernardi, A.C.C.; Ribeiro, C. 2017. Controlled urea release employing nanocomposites increases the efficiency of nitrogen use by forage. Sustainable Chemical and Engineering 5: 9993-10001.). The hydrophilic polymer (HG) increases the urea release control. HG competes against the water molecules and hinders urea diffusion through the nanocomposite structure (Bortolin et al., 2012Bortolin, A.; Aouada, F.A.; Moura, M.R.; Ribeiro, C.; Longo, E.; Mattoso, L.H.C. 2012. Application of polysaccharide hydrogels in adsorption and controlled-extended release of fertilizers processes. Journal of Applied Polymer Science 123: 2291-2298.). On the other hand, the remarkable delayed release is due to the urea-formaldehyde polymer structure, which needs to be disrupted to further solubilize urea. However, urea-formaldehyde polymer displays hydrophobic characteristics and may have acted as a structural barrier against diffusion of water molecules, consequently resulting in an expressively slower urea release, according to Giroto et al. (2018)Giroto, A.S.; Guimarães, G.G.F.; Ribeiro, C. 2018. A novel, simple route to produce urea: urea–formaldehyde composites for controlled release of fertilizers. Journal of Polymeres and the Environment 26: 2448-2458..
The results revealed gains in the digestion percentages when the composite was used (Table 3). The maximum sugarcane digestion over 96 h was 60 %. The MMT/Ur 1:1, MMT/Ur 1:2, MMT/Ur 1:4, MMT/Ur 1:4/HG and MMT/Ur 1:4/Pf samples increased the digestibility gains by 3, 2, 3, 4 and 3 %, respectively. Pure urea did not influence on the digestibility value, while pure MMT presented a gain of 2 % at maximum digestibility. Beta results did not change significantly (p > 0.05), indicating that the digestion rates of the nanocomposites were similar. As regards the constant, which represents the level of the beginning of digestion, there were statistical differences between the samples (p < 0.05). Sugarcane and urea presented the lowest and highest constant values, respectively, whereas the values among the nanocomposites were similar.
Results (in %) of "in vitro" sugarcane digestibility. Means ± standard deviations within a column followed by the same letter do not differ significantly (Duncan test; p < 0.05).
The increase of dry matter digestibility in the presence of nanocomposites may be correlated with the cation exchange capacity of MMT, which favors stabilization of the NH4+ concentration. The results demonstrate that the slow release nanocomposites are capable of improving the dry matter digestibility of sugarcane. Duncan statistical tests showed that the digestibility was higher for the nanocomposites in all the measured time periods: even at 12 h, the nanocomposites exerted, statistically, more influence than urea, which presented almost the same digestibility as sugar cane and MMT. At 24 h, urea showed a better effect, however, the nanocomposites maintained better performance – noteably, MMT/Ur1:4/HG material was the best material for these times, and this trend was sustained until 96 h.
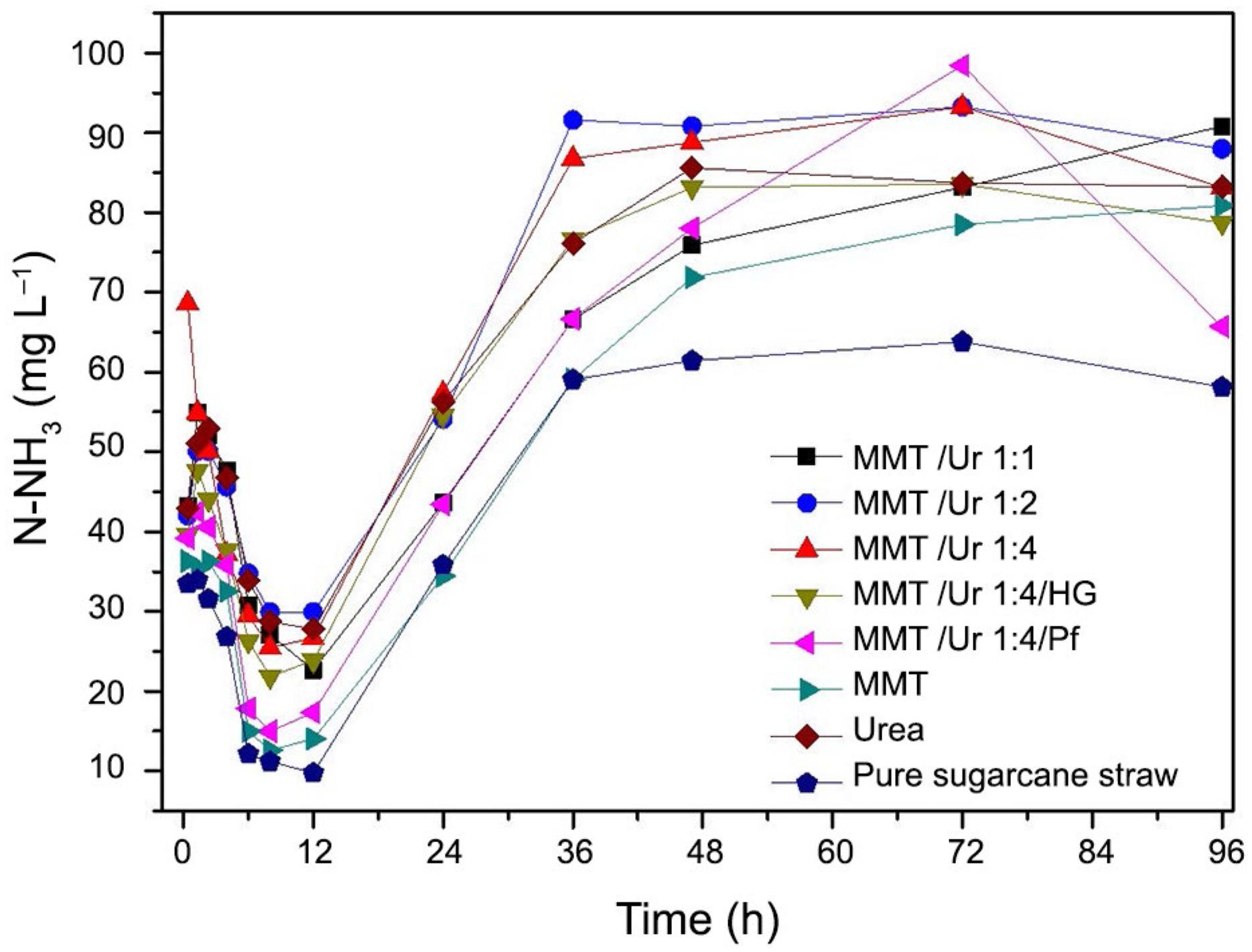
Table 4 shows ruminal kinetic parameters during in vitro digestion test. The a parameter represents the soluble fraction of DM, ranging from 27 for urea to 34.4 for MMT/Ur 1:4/Pf. In general, the presence of MMT increased the DM soluble fraction. The result also showed a small decrease in the slowly degradable fraction of DM (b) in the presence of MMT. On the other hand, the degradation rate of the slowly degradable fraction (represented by c) remained similar, only the urea and MMT/Ur1:4/HG treatments showed higher rate values. Figure 5 shows that all samples imparted an increase in ammonia concentration for up to approximately 2 h of digestion. The average ammonia concentration was 50 mg L−1 NH3 for all samples with the exception of pure sugarcane. After 2 h, there was a decrease in the ammonia concentration until 12 h of experiment. Pure urea showed maximum concentration of NH3 after 48 h, and the MMT/Ur 1-4/Pf, MMT/Ur 1-4 and MMT/Ur 1-2 samples presented higher NH3 concentrations after longer times. The pH evolution during the in vitro digestibility test is shown in Figure 6. Its profile is a consequence of the concentration of NH3 in the rumen fluid.
In vitro ruminal kinetic parameters. Means ± standard deviations within a column followed by the same letter do not differ significantly (Duncan test; p < 0.05).
The decrease in ammonia concentration until 12 h of digestibility in this experiment may be explained by the minimum NH3 level in rumen fluid required for maximum microbial synthesis, which consumes the NH3 excess to synthesize proteins (Hoover, 1986Hoover, W.H. 1986. Chemical factors involved in ruminal fiber digestion. Journal of Dairy Science 69: 2755-2766.). Different NH3 levels for maximum microbial synthesis have been suggested in other studies; for instance, Leng and Nolan (1984)Leng, R.A.; Nolan, J.V. 1984. Nitrogen-metabolism in the rumen. Journal of Dairy Science 67: 1072-1089. suggested a range of N 150-200 mg L−1 and Satter and Slyter (1974)Satter, L.D.; Slyter, L.L. 1974. Effect of ammonia concentration on rumen microbial protein production in vitro. British Journal of Nutrition 32: 199-208. reported a range of N 50-80 mg L−1. Another explanation for the reduced ammonia concentration is related to the cation exchange capacity of MMT, which may have scavenged the NH3 molecules present in the rumen fluid.
As expected, sugarcane and MMT displayed NH3 levels lower than those of nanocomposites and pure urea. The concentrations of NH3 found for these two samples are most probably related to the inherent nitrogen content of the rumen fluid and sugarcane. Dietary nitrogen sources include nucleic acids, amino acids, proteins, peptides, amines, amides, nitrates, nitrites, urea and ammonia. Endogenous sources include discarded cells and urea re-entering the rumen through the epithelium or saliva. With the exception of certain proteins and N associated with adenosine diphosphate, other sources of N are readily soluble and susceptible to degradation in the rumen (Huntington and Archibeque, 1999Huntington, G.B.; Archibeque, S.L. 1999. Practical aspects of urea and ammonia metabolism in ruminants. Journal of Animal Science 78: 1-11.). The NH3 concentrations for sugarcane and MMT up to 36 h were very similar, after this time the NH3 concentration for MMT was observed to increase. It is suggested that at this point the clay started to release the NH4+ ions, as the pure sugar concentration remained constant. The MMT/Ur 1:4/Pf nanocomposite, which exhibited the slowest urea release kinetics, may have released urea after the maximum microbial synthesis phase, resulting in an accumulation of volatile NH3 molecules. In fact, there was an abnormally low ammonia concentration in the time range 72 – 96 h, which may be related to NH3 volatilization.
It is worthy mentioning that even after 96 h the ammonia levels in the rumen fluid were much lower than the amount considered to be toxic, 1,000 mg L−1 (Owens and Bergen, 1983Owens, F.N.; Bergen, W.G. 1983. Nitrogen-metabolism of ruminant animals: historical perspective, current understanding and future implications. Journal of Animal Science 57: 498-518.). According to the literature, there is no a standardized minimum toxic NH3 concentration. For example, Lewis (1970) admitted toxicity when NH3 levels exceeded 1,760 mg L−1 NH3/Liquid rumen. Other studies have reported that the toxicity levels will depend on pH and the time over which ammonia will be released into the rumen, Helmer and Bartley (1971)Helmer, L.G.; Bartley, E.E. 1971. Progress in the utilization of urea as a protein replacer for ruminants. a review. Journal of Dairy Science 54: 25-51..
The initial drop in pH after 12 h can be attributed to the consumption of NH3 for protein synthesis and thus the release of acids by fermentation (Goularte et al., 2011Goularte, S.R.; Itavo, L.C.V.; Santos, G.T.; Itavo, C.; Oliveira, L.C.S.; Favaro, S.P.; Dias, A.M.; Torres, R.A.A.; Bittar, C.M.M. 2011. Volatile fatty acids in rumen of cows fed different concentrate level diets. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 63: 1479-1486 (in Portuguese, with abstract in English).). All samples had a maximum pH value of approximately 7, with the exception of MMT (pH = 6.9), where the lower pH may have corresponded to the adsorption capacity of MMT. It is important to point out that pH was maintained within an optimal range during the experiment, since the urease activity is highest in the pH range 7-9 (Pearson and Smith, 1943Pearson, R.M.; Smith, J.A.B. 1943. The utilization of urea in the bovine rumen. 2. The conversion of urea to ammonia. Biochemical Journal 37: 148-153.). Bacterial activity is also influenced by pH, Davis and Stallcup (1964)Davis, G.V.; Stallcup, O.T. 1964. Influence of dietary nitrogen on nitrogen metabolism in the rumem. Journal of Dairy Science 47: 1237-1242. having reported that the maximum activity of bacteria occurs in the pH range 5.5-7.
In summary, different MMT/Urea nanocomposite structures intended for slow release of nitrogen exhibited a further beneficial effect on digestibility of sugarcane straw, which is broadly known as improper fodder. Generally, all nanocomposites increased (p < 0.05) digestibility of fiber over control under all the conditions, but the samples with hydrogel content were more expressive. The nanocomposite included with 2 wt.% hydrogel was found to be the most effective for animal nutrition purposes, probably due to the adsorption of NH4+ ions during the microbial synthesis process – which is beneficial mainly because of reduction of NH3 toxicity and maintenance of a more constant N content in the rumen. Despite the increases in sugarcane, digestibility was in agreement with the urea release tests; the gain in digestibility was not very expressive for nanocomposites displaying very slow urea release kinetics. We concluded that an ideal release rate and optimum environment for microbial synthesis are necessary to maximize digestion of sugarcane. This research study demonstrates that designed nanostructures are powerful tools for incrementing digestibility of conventional fodders and will serve as a foundation for upcoming in vivo tests.
Acknowledgments
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Financiadora de Estudos e Projetos (FINEP) and Rede Agronano-Embrapa for their financial support.
References
- Ahmadi, S.J.; Huang, Y.D.; Wei, L. 2004. Synthesis of EPDM/organoclay nanocomposites: effect of the clay exfoliation on structure and physical properties. Iranian Polymer Journal 13: 415-422.
- Almora, E.G.A.; Huntington, G.B.; Burns, J.C. 2012. Effects of supplemental urea sources and feeding frequency on ruminal fermentation, fiber digestion, and nitrogen balance in beef steers. Anim. Feed Science Technology 171: 136-145.
- Ankom Technology. Method 3: In vitro true digestibility using the DaisyII Incubator. Available at: http://www.ankom.com/media/documents/IVDMD_0805_D200.pdf [Accessed Apr 15, 2015]
» http://www.ankom.com/media/documents/IVDMD_0805_D200.pdf - Bortolin, A.; Aouada, F.A.; Moura, M.R.; Ribeiro, C.; Longo, E.; Mattoso, L.H.C. 2012. Application of polysaccharide hydrogels in adsorption and controlled-extended release of fertilizers processes. Journal of Applied Polymer Science 123: 2291-2298.
- Brack, W.; Heine, B.; Birkhold, F.; Kruse, M.; Schoch, G.; Tischer, S.; Deutschmann, O. 2014. Kinetic modeling of urea decomposition based on systematic thermogravimetric analyses of urea and its most important by-products. Chemistry Engineering Science 106: 1-8.
- Calsamiglia, S.; Ferret, A.; Reynolds, C.K.; Kristensen, N.B.; van Vuuren, A.M. 2010. Strategies for optimizing nitrogen use by ruminants. Animal 4: 1184-1196.
- Cherdthong, A.; Wanapat, M. 2010. Development of urea products as rumen slow release feed for ruminant production: a review. Australian Journal of Basic Applied Science 4: 2232-2241.
- Davis, G.V.; Stallcup, O.T. 1964. Influence of dietary nitrogen on nitrogen metabolism in the rumem. Journal of Dairy Science 47: 1237-1242.
- Giroto, A.S.; Guimarães, G.G.F.; Ribeiro, C. 2018. A novel, simple route to produce urea: urea–formaldehyde composites for controlled release of fertilizers. Journal of Polymeres and the Environment 26: 2448-2458.
- Goularte, S.R.; Itavo, L.C.V.; Santos, G.T.; Itavo, C.; Oliveira, L.C.S.; Favaro, S.P.; Dias, A.M.; Torres, R.A.A.; Bittar, C.M.M. 2011. Volatile fatty acids in rumen of cows fed different concentrate level diets. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 63: 1479-1486 (in Portuguese, with abstract in English).
- Helmer, L.G.; Bartley, E.E. 1971. Progress in the utilization of urea as a protein replacer for ruminants. a review. Journal of Dairy Science 54: 25-51.
- Holder, V.B.; El-Kadi, S.W.; Tricarico, J.M.; Vanzant, E.S.; McLeod, K.R.; Harmon, D.L. 2013. The effects of crude protein concentration and slow release urea on nitrogen metabolism in Holstein steers. Archives of Animal Nutrition 67: 93-103.
- Holder, V.B.; Tricarico, J.M.; Kim, D.H.; Kristensen, N.B.; Harmon, D.L. 2015. The effects of degradable nitrogen level and slow release urea on nitrogen balance and urea kinetics in Holstein steers. Animal Feed Science and Technology 200: 57-65.
- Hoover, W.H. 1986. Chemical factors involved in ruminal fiber digestion. Journal of Dairy Science 69: 2755-2766.
- Huntington, G.B.; Archibeque, S.L. 1999. Practical aspects of urea and ammonia metabolism in ruminants. Journal of Animal Science 78: 1-11.
- Kertz, A.F. 2010. Review: urea feeding to dairy cattle: a historical perspective and review. The Professional Animal Science 26: 257-272.
- Leng, R.A.; Nolan, J.V. 1984. Nitrogen-metabolism in the rumen. Journal of Dairy Science 67: 1072-1089.
- Lewis, D. 1960. Ammonia toxicity in the ruminant. Journal of Agricutural Science 55: 111-117.
- Nogueira, A.R.A.; Souza, G.B.; Batista, L.A.R. 1996. Spectrophotometric determination of nitrogen in plants digested by flow injection system analysis. Quimica Nova 19: 33-36 (in Portuguese, with abstract in English).
- Orskov, E.R.; McDonald, I. 1979. Estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. Journal of Agricultural Science 92: 499-503.
- Owens, F.N.; Bergen, W.G. 1983. Nitrogen-metabolism of ruminant animals: historical perspective, current understanding and future implications. Journal of Animal Science 57: 498-518.
- Paixao, M.L.; Valadares, S.D.; Leão, M.I.; Valadares, R.F.D.; Paulino, M.F.; Marcondes, M.I.; Fonseca, M.A.; Silva, P.A.; Pina, D.D.S. 2006. Urea in diets of steers: intake, digestibility, performance, carcass traits and microbial yield. Revista Brasileira de Zootecnia 35: 2451-2460 (in Portuguese, with abstract in English).
- Pearson, R.M.; Smith, J.A.B. 1943. The utilization of urea in the bovine rumen. 2. The conversion of urea to ammonia. Biochemical Journal 37: 148-153.
- Pereira, E.I.; Cruz, C.C.T.; Solomon, A.; Le, A.; Cavigelli, M.A.; Ribeiro, C. 2015. Novel slow-release nanocomposite nitrogen fertilizers: the impact of polymers on nanocomposite properties and function. Industrial and Engineering Chemistry Research 54: 3717-3725.
- Pereira, E.I.; Minussi, F.B.; da Cruz, C.C.T.; Bernardi, A.C.C.; Ribeiro, C. 2012. Urea Montmorillonite-Extruded Nanocomposites: A Novel Slow-Release Material. Journal of Agricultural and Food Chemistry 60: 5267-5272.
- Pereira, E.I.; Nogueira, A.R.A.; Cruz, C.C.T.; Guimaraes, G.G.F.; Foschin, M.M.; Bernardi, A.C.C.; Ribeiro, C. 2017. Controlled urea release employing nanocomposites increases the efficiency of nitrogen use by forage. Sustainable Chemical and Engineering 5: 9993-10001.
- Ribeiro, S.S.; Vasconcelos, J.T.; Morais, M.G.; Itavo, C.; Franco, G.L. 2011. Effects of ruminal infusion of a slow-release polymer-coated urea or conventional urea on apparent nutrient digestibility, in situ degradability, and rumen parameters in cattle fed low-quality hay. Animal Feed Science and Technology 164: 53-61.
- Satter, L.D.; Slyter, L.L. 1974. Effect of ammonia concentration on rumen microbial protein production in vitro. British Journal of Nutrition 32: 199-208.
- Spanghero, M.; Nikulina, A.; Mason, F. 2018. Use of an in vitro gas production procedure to evaluate rumen slow-release urea products. Animal Feed Science and Technology 237: 19-26.
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science 74: 3583-3597.
- Wang, N.; Zhang, X.X.; Han, N.; Liu, H.H. 2010. A facile method for preparation of thermoplastic starch/urea modified montmorillonite nanocomposites. Journal of Composite Materials 44: 27-39.
- Yamamoto, C.F.; Pereira, E.I.; Mattoso, L.H.C.; Matsunaka, T.; Ribeiro, C. 2016. Slow release fertilizers based on urea/urea-formaldehyde polymer nanocomposites. Chemical Engineering Journal 287: 390-397.
- Yan, X.T.; Yan, B.Y.; Ren, Q.M.; Dou, J.J.; Wang, W.W.; Zhang, J.J.; Zhou, J.W.; Long, R.J.; Ding, L.M.; Han, J.; Li, Z.P.; Qiu, Q. 2018. Effect of slow-release urea on the composition of ruminal bacteria and fungi communities in yak. Animal Feed Science and Technology 244: 18-27.
- Zhang, Y.M.; Liu, Q.F.; Wu, Z.G.; Zhang, Y.F. 2015. Thermal behavior analysis of two bentonite samples selected from China. Journal of Thermal Analysis and Calorimetry 121: 1287-1295.
Edited by
Publication Dates
-
Publication in this collection
20 Dec 2019 -
Date of issue
2020
History
-
Received
22 Nov 2018 -
Accepted
13 Mar 2019