Abstracts
Trace elements are potentially toxic to the environment. Their toxicity in soils relies on their type of chemical associations. Hence it is important to determine the chemical form they occur in the soils in order to assess their quantities. The objective the present work was to evaluate the possibility of using the concentrations of Cu and Zn in different soil fractions and the total concentration as predictors of their quantification by comparing the classical extractants DTPA, Mehlich-1 and HCl. Comparisons were made also with the concentrations of absorbed by rice and soybean in contaminated soil. Seven soil samples with a different degree of contamination were studied using a randomized experimental design, with four replicates. By using an ICP-OES we analyzed the concentrations of Cu and Zn in the diagnostic leafs, at the end of the cycle (LCE) and several of its content in the soil: available extracted with the DTPA, Mehlich-1 and HCl extractants, soluble+exchangeable contents, bound to organic matter, to oxides and the total content. For both, soybean and rice, the concentrations of Cu and Zn extracted from the sequential extraction was found to be correlated with the concentration in LCE. For soybean, Zn concentration extracted with DTPA was correlated with the total concentration, while Cu concentration extracted with three extractors, DTPA, Mehlich-1 and HCl, correlated with the total concentration, probably due to the high concentration of Cu and Zn in this soil. For rice, both Cu and Zn concentration as extracted by the three extracting solutions correlated with the concentration of all the fractions and with the total concentration.
DTPA; HCl; crops; Mehlich-1; sequential extraction
Os elementos traços são potencialmente tóxicos para o meio ambiente. Estas toxicidades dependem de suas associações químicas. Por isso a determinação da forma química destes elementos no solo é importante para quantificá-lo. O objetivo do presente trabalho foi avaliar a possibilidade de se utilizar teores de Cu e Zn nas diferentes frações do solo para prognosticar suas disponibilidades. Para isso, compararam-se as mesmas com soluções extratoras clássicas, como DTPA, Mehlich-1 e HCl, e com teores absorvidos por plantas de arroz e soja em materiais de solos contaminados. Foram estudadas sete amostras de solos com diferentes graus de contaminação empregando delineamento experimental inteiramente casualizado, com quatro repetições. Utilizando a técnica de ICP-OES, foram avaliados teores de Cu e Zn em folha diagnóstico e folha em final de ciclo (LCE) e no solo: teores disponíveis utilizando as soluções extratoras DTPA, Mehlich-1 e HCl, teores nas frações de solo solúvel+trocável, ligados à matéria orgânica e ligados a óxidos e o teor total. Para a soja e arroz, os teores de Cu e Zn extraídos do fracionamento correlacionaram significativamente com os teores obtidos na LCE. Para a soja, os teores de Zn extraídos com DTPA apresentaram alta correlação com os teores totais, enquanto que os teores de Cu extraídos pelos três extratores, DTPA, Mehlich-1 e HCl, correlacionaram com o teor total, provavelmente devido aos altos teores de Cu e Zn neste solo. Para o arroz, os teores de Cu e Zn extraídos pelas três soluções correlacionaram com teores em todas as frações e com o teor total.
DTPA; HCl; culturas; Mehlich-1; extração seqüencial
SOILS AND PLANT NUTRITION
Copper and zinc quantification in contaminated soil as evaluated by chemical extractants
Quantificação de cobre e zinco avaliados por extratores químicos em solo contaminado
Maria Ligia de Souza SilvaI; Anderson Ricardo TrevizamII; Godofredo César VittiIII* * Corresponding author < gcvitti@esalq.usp.br>
IUSP/ESALQ - Programa de Pós-Graduação em Solos e Nutrição de Plantas
IIUSP/CENA - Programa de Pós-Graduação em Ciências, C.P. 96 - 13400-970 Piracicaba, SP - Brasil
IIIUSP/ESALQ - Depto. Ciência do Solo, C.P. 09 - 13418-900 - Piracicaba, SP - Brasil
ABSTRACT
Trace elements are potentially toxic to the environment. Their toxicity in soils relies on their type of chemical associations. Hence it is important to determine the chemical form they occur in the soils in order to assess their quantities. The objective the present work was to evaluate the possibility of using the concentrations of Cu and Zn in different soil fractions and the total concentration as predictors of their quantification by comparing the classical extractants DTPA, Mehlich-1 and HCl. Comparisons were made also with the concentrations of absorbed by rice and soybean in contaminated soil. Seven soil samples with a different degree of contamination were studied using a randomized experimental design, with four replicates. By using an ICP-OES we analyzed the concentrations of Cu and Zn in the diagnostic leafs, at the end of the cycle (LCE) and several of its content in the soil: available extracted with the DTPA, Mehlich-1 and HCl extractants, soluble+exchangeable contents, bound to organic matter, to oxides and the total content. For both, soybean and rice, the concentrations of Cu and Zn extracted from the sequential extraction was found to be correlated with the concentration in LCE. For soybean, Zn concentration extracted with DTPA was correlated with the total concentration, while Cu concentration extracted with three extractors, DTPA, Mehlich-1 and HCl, correlated with the total concentration, probably due to the high concentration of Cu and Zn in this soil. For rice, both Cu and Zn concentration as extracted by the three extracting solutions correlated with the concentration of all the fractions and with the total concentration
Key words: DTPA, HCl, crops, Mehlich-1, sequential extraction
RESUMO
Os elementos traços são potencialmente tóxicos para o meio ambiente. Estas toxicidades dependem de suas associações químicas. Por isso a determinação da forma química destes elementos no solo é importante para quantificá-lo. O objetivo do presente trabalho foi avaliar a possibilidade de se utilizar teores de Cu e Zn nas diferentes frações do solo para prognosticar suas disponibilidades. Para isso, compararam-se as mesmas com soluções extratoras clássicas, como DTPA, Mehlich-1 e HCl, e com teores absorvidos por plantas de arroz e soja em materiais de solos contaminados. Foram estudadas sete amostras de solos com diferentes graus de contaminação empregando delineamento experimental inteiramente casualizado, com quatro repetições. Utilizando a técnica de ICP-OES, foram avaliados teores de Cu e Zn em folha diagnóstico e folha em final de ciclo (LCE) e no solo: teores disponíveis utilizando as soluções extratoras DTPA, Mehlich-1 e HCl, teores nas frações de solo solúvel+trocável, ligados à matéria orgânica e ligados a óxidos e o teor total. Para a soja e arroz, os teores de Cu e Zn extraídos do fracionamento correlacionaram significativamente com os teores obtidos na LCE. Para a soja, os teores de Zn extraídos com DTPA apresentaram alta correlação com os teores totais, enquanto que os teores de Cu extraídos pelos três extratores, DTPA, Mehlich-1 e HCl, correlacionaram com o teor total, provavelmente devido aos altos teores de Cu e Zn neste solo. Para o arroz, os teores de Cu e Zn extraídos pelas três soluções correlacionaram com teores em todas as frações e com o teor total.
Palavras-chave: DTPA, HCl, culturas, Mehlich-1, extração seqüencial
INTRODUCTION
Soil contamination by trace elements is a problem frequently observed in developing countries (Mendes et al., 2006). Contaminated soils can hinder the development of many plant, species as well as soil microorganisms (Paiva et al., 2002).
Trace elements in soils may be parted according to their geochemical forms, which can be selectively extracted using proper reagents. Each method employs a group of reagents that should specifically interact with the geochemical forms (Tessier et al., 1979; Amaral Sobrinho et al., 1997). The information thus gained will be useful for the evaluation of the phytoavailability, phytotoxicity, dynamics and transformations of the trace elements among their chemical forms as found in contaminated soils (Costa et al., 2002). The sequential extraction (SE) methods have been used in order to identify the structures in which those elements are associated (Amaral Sobrinho et al., 1997).
In spite of the long time required to perform a SE, these methods have the advantage of being useful to make inferences on the origin, occurrence forms, bioavailability, flow, mobility and transport of trace elements (Tessier et al., 1979). By the use of SE methods it is possible to detect the trace elements both in their relatively more labile chemical forms (soluble, exchangeable and linked to the carbonates), and in their more stable forms and also the ones with smaller mobility and/or bioavailability (linked to oxides of Fe or of Mn, linked to the organic matter of the soil; Silva, 1999a).
The availability of trace elements can be evaluated by means of chemical extraction followed by correlation analysis with the amount of the element accumulated (or concentrated) in the plant tissues (Abreu et al., 2002).
Complexant agents allow removing elements linked to organic radicals and carbonates, easily extracting the labile forms without dissolving the non-labile (Abreu et al., 1997). Acid extractors extract amounts close to the total due to their dissolving power of the mineral structures retaining trace elements in the soil (Taylor et al., 1993; Roca & Pomares, 1991). Among the extractors normally used to study the phytodisponibility, stands out the complexant DTPA and the acid solutions HCl 0.1 mol L-1 and Mehlich-1 (Anjos & Mattiazzo, 2001; Oliveira, 2000).
The objective the present work was to evaluate the possibility of using the concentrations of Cu and Zn in different soil fractions and their total concentration in the soil as predictors of their quantification by comparing them with classical extractors and with the concentrations absorved by rice and soybean in contaminated soil.
MATERIAL AND METHODS
The soil used had been accidentally contaminated presenting high levels of trace elements (Table 1). Samples of a Typic Hapludox were colleted in Paulínia, São Paulo state, Brazil (22°45' S, 47°09' W) (Table 2). Samples were taken at a 020 cm depth in seven different points following a 100 m interval, starting from the source of pollution. They were air dried sieved (5 mm) and stored. Soil samples were placed in 5 dm-3 capacity pots. Irrigation was done in a way not to exceed the maximum of 70% and minimum of 40% of the maximum water retention capacity, by daily weighting the pots.
The experiments were conducted in a greenhouse, in Piracicaba, SP, Brazil from November, 2004 to April, 2005. The plant species used were rice (Oriza sativa L. cv. IAC 202) and soybean (Glycine max L. cv. BRS 133). Fertilization with NPK was done according to each crop need A randomized experimental design was used, in a factorial scheme 7 x2 (seven soil samples and two plants species), with four replicates, performing a total of 56 experimental units.
Ten seeds were sown in each pot on November 25, 2004, both for rice and soybeans. Germination began on the 28th day. Plants were thinned to four per pot on December 11, 2004. The diagnostic leaves were collected following Malavolta et al. (1997). For rice, the leaf Y (immediately below the newest uncoiled leaf) was collected in the middle of the tilling period, 50 days after sowing. For soybean, the first ripe leaf starting from the tip of the branch was collected, at the end of the flowering period (on the 69th day after sowing). The samples were stored in paper bags and dried. Thereafter, the plants were grown until maturation and cut close to the soil surface. The colleted material was washed in running water and rinsed in distilled water. Afterwards, they were dried in an aerated stove (temperature maintained between 6070ºC), weighed and crushed in a Wiley mill. Digestion was done in an open system, using a slight modification of the method described by Oliva et al. (2003).
The available concentrations of Cu and Zn in soil samples were extracted by solutions of Mehlich-1 (HCl 0.05 mol L-1 + H2SO4 0.0125 mol L-1; Silva, 1999b), HCl 0.1 mol L-1 (Wear & Sommer, 1947) and DTPA at pH 7.3 (Lindsay & Norvell, 1978).
The sequential extraction for Cu and Zn in each soil fraction after plant cultivation was performed according to Ahnstrom & Parker (1999), except for the carbonate phase, since carbonates is known to be present in very small concentrations in this soil. 2 g samples of were crushed in a porcelain mortar with the aim of mixing the components and make easy the extractors action. All extractions were carried out in 50 mL centrifuge tubes. To extract Cu and Zn from the fraction 1 (soluble + exchangeable (S/Ex)), 15 mL of Sr(NO3)2 0.1 mol L-1 was added to the tubes, agitating it for 2 h at low speed. Afterwards, samples were centrifuged (2,500 rpm for 10 minutes). The supernatant was filtered (with quantitative blue strip paper) to 50 mL volumetric flasks. This procedure was repeated three times. For the extraction from fraction 2 (linked the organic matter (OM)), 5 mL of 5% NaOCl was used (with pH adjusted to 8.5 with concentrated HNO3). Tubes were placed in a water bath (9095 °C) for 30 minutes under slow agitation. After this time, the samples were centrifuged (2,500 rpm during 10 minutes) and the supernatant filtered (quantitative blue strip paper) and collected in 50 mL volumetric flasks. This procedure was repeated until the organic matter was destroyed, that is, when foam was no longer present.
For the extraction from fraction 3 ( oxide tied (Ox)), 20 mL of a solution containing 0.2 mol L-1 oxalic acid + 0.2 mol L-1 NH4 oxalate + 0.1 mol L-1 ascorbic acid (adjusted to pH 3.0 with NH4OH) was added. The tubes were placed in a water bath (9095°C) for 30 minutes under slow agitation. Afterwards, the samples were centrifuged (2,500 rpm for 10 minutes) and the supernatant was again filtered with quantitative blue strip paper filter and collected in 100 mL volumetric balloon. This procedure was repeated until the color of the soil turned gray (without iron) and the supernatant turned yellowish. At the end of each extraction phase (fractions 1, 2 and 3), the soil samples were washed with 5 mL of NaCl 0.1 mol L-1, centrifuged at 2,500 rpm for 10 minutes, and the supernatant added to the one already got from the respective fraction. Afterwards, 1 mL of concentrated HNO3 was added, and the volume was completed with distilled water to 50 mL, 50 mL and 100 mL for the extracts from the fractions 1, 2 and 3, respectively.
The extraction of the total (T) concentration of Cu and Zn from the soil samples was done with aqua regia (HCl/HNO3 3:1) in a microwave oven, according to Nieuwenhuize et al. (1991). The concentrations of Cu and Zn in the extracts were analyzed by an inductively coupled plasma optical emission spectrometer (ICP-OES).
The data on the concentration of Cu and Zn extracted form the fractions S/Ex, OM, Ox, and the T concentration were submitted to linear correlation analysis with the data obtained from the Mehlich-1, HCl 0.1 mol L-1 and DTPA, extraction methods, as well as with the data obtained from the extractions in the leaf diagnosis (DL) and in the leaf end of cycle (LCE) separately from both rice and soybean. The linear correlation was calculated using SAS for Windows version 6.12 (SAS, 1985).
RESULTS AND DISCUSSION
Soybean
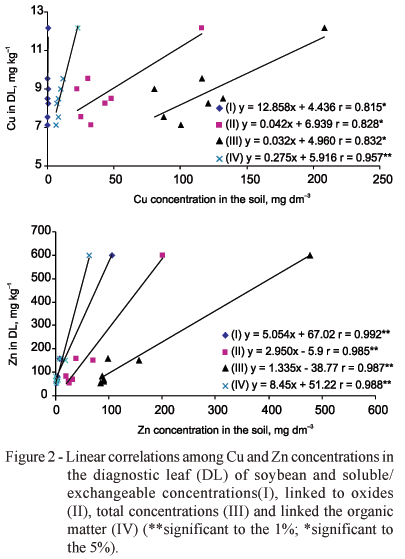
The sequential extraction method has been used in an attempt to evaluate the relationship between the bioavailable fraction of the metal in the soil and the metal content in the plant (Zhang et al., 1998; Buanuam et al., 2005). The concentrations Cu and Zn extracted from the fraction S/Ex, linked to OM and OX, and the T concentration were highly and positively correlated with the concentrations obtained in DL and LCE (Figures 1 and 2). Only the concentration S/Ex of Cu was not correlated with the concentration in LCE. Possibly, this was due to presence of Zn in high concentrations. High activities of Zn+2 in the soil solution may inhibit the Cu+2 uptake (Alloway, 1995). The T concentration in the soil is not considered to be a reliable parameter to predict phytoavailability (Zhang et al., 1998). However, it can be noticed in this study when the soil is contaminated with high concentrations of trace elements, the total concentration is well correlated with the concentrations in plants (DL and LCE). Both Cu and Zn have high affinity for the organic matter. Gomes et al. (1997) reported that Cu reacts with the groups COOH and OH - phenol, simultaneously, forming highly stable chemical complexes.
The concentrations obtained in the sequential extraction (from the fractions S/Ex, linked to OM and Ox) and the T concentration were highly correlated with the available concentrations extracted by the methods Mehlich-1, DTPA and HCl 0.1 mol L-1, both for Cu and Zn (p < 0.01; Figures 3 and 4). Such a result can be explained by the elevated concentrations of Cu and Zn in the studied soil. It is possible that in a soil with low concentrations of Cu and Zn, for example 0.8 mg dm3 to Cu and 1.2 mg dm3 to Zn extracts by DTPA (Raij et al., 1997), the same results will not be obtained. Zhang et al. (1998) did not obtain significant correlations among the concentrations uptake by the plants and the concentrations obtained in the fractions in soils with standard concentrations of Cu and Zn.
The acid extractors such as Mehlich-1 and HCl 0.1 mol L-1 extract the trace elements mainly by dissolving the clay minerals. The amount extracted will depend on the acid solution concentration, on the time of extraction and on the solution/soil ratio. The acids extract amounts close to the total concentration due to their dissolving power, (even when partially) (Taylor et al., 1993; Roca & Pomares, 1991). Chelant solutions act by combining with metallic ions thereby forming soluble complexes, and reducing the ionic activity in soil solution. Consequently, the ions are desorbed from the surface of the soil solids to reestablish the solution ionic equilibrium (Abreu et al., 2002). Ure (1991) considers that the sequential extraction acts in the soil through chemical reagents, or solvents, denominated extractors, specifically to extract elements linked or associated with the clay fraction. Perhaps, for that reason, there was a positive correlation among the concentrations obtained for the fractions of the soil with the obtained with the extractors, Mehlich-1 and HCl 0.1 mol L-1 (acids) and DTPA (chelating agents).
Rice
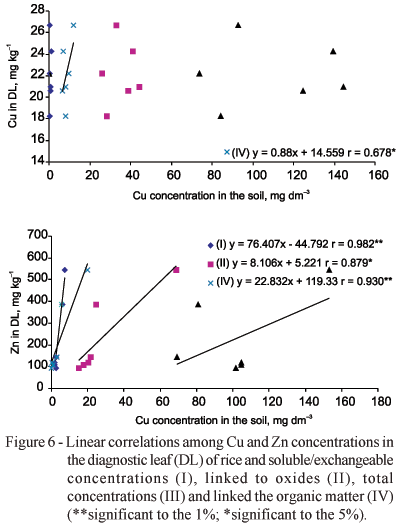
The concentration of Cu and Zn in LCE were correlated to the ones extracted by the sequential extraction method (Figure 5), except for Cu extracted from the S/Ex fraction. Such correlation was not obtained being probably influenced by Zn concentrations in the soil or unavailable forms of Cu in the soil. The amount of metals absorbed is affected by many factors and it is difficult to determine the relationship between plant uptake and the metal fractions in the soil with simple correlation analysis (Zhang et al., 1998). Cu concentrations in DL were correlated only with the concentration obtained in OM. Zn concentration in DL were significantly correlated with the concentrations obtained from all the fractions, but not with T concentration (Figure 6). Perhaps the adsorption is the main process to be considered to understand the availability of Cu and Zn, being indicative of the solubility and mobility of those elements and the consequent availability for the plants (Nascimento & Fontes, 2004).
Zhang et al. (1998) correlating the concentrations of Cu and Zn obtained in the different parts of the corn with the concentrations of Cu and Zn in the fractions of the soil (exchangeable, bound to oxide or organic matter) did not obtain significant correlation. The concentrations of Zn in the corn roots presented significant correlation with the fraction bound to oxides of Fe-Mn.
Usually, the total amount of trace elements is a parameter used for to control soil pollution (Camargo et al., 2000). As already mentioned, the T concentration of traces elements in the soil is not a reliable parameter to express their plant availability. Contrary to what was observed for soybean, there was no correlation between the concentrations in DL and T for rice. Obata (1995) observed Cu concentrations in rice from 5 to 20 mg kg-1. The excess of Cu in the soil cause damage to the plant only when the soluble concentration of this element goes beyond 125 mg dm3. For Zn, the normal concentration in rice varies from 30 to 100 mg kg-1, indicating that this species is relatively tolerant to its excess (Obata, 1995). If toxicity occurs, it appears in the form of a decrease in the height of the plant and in the tiller number (Obata, 1995).
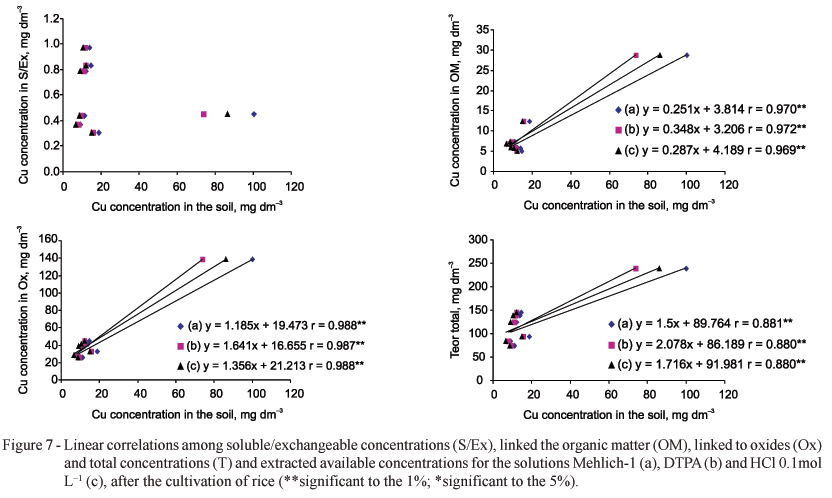
The absorption of Cu by the plants do not correlate very well with the concentrations of Cu in the soil and/or with amounts extracted by the several extractors due to its inconstant chemical activity in soil solutions (Alloway, 1995). Both for Cu and Zn the "available" concentrations extracted by Mehlich-1, HCl and DTPA had highly significant correlations (p < 0.01) with the concentrations extracted by sequential extractors in all the fractions (S/EX, linked to OM and Ox) and the T concentration, except for the Cu concentration from the S/Ex fraction (Figures 7 and 8).
A great number of factors influence the availability and sorption of trace elements in the soil, such as the characteristics of the colloids (clay minerals, oxides and organic matter), pH, ionic concentration of the solution, presence of cations and organic and inorganic ligands (Ross, 1994). Considering that in this study the soil has high concentrations of Cu and Zn, it is plausible that concentrations linked to Ox, the T concentrations correlated with the "available" concentrations extracted by the solutions, since great part of Cu and Zn is soluble or quickly available in the soil. Furthermore, the concentrations of Zn accessed by the extractors Mehlich-1, DTPA and HCl 0.1 mol L-1 had highly significant correlations with the concentrations in the fractions S/EX and linked to OM, which are potential reservoirs of those elements for the plants. These extractors possess appropriate capacity of extraction of the most available forms (Shuman, 1988). Trace elements linked to Ox are not available to plants (Shuman, 1988). However, when a soil exhibits high levels of trace elements, this could not be observed, probably because the reactions of adsorption-desorption tend to be faster, caused by the competition among ions for the surfaces adsorbents (Harter, 1991).
In trace elements bioavailability studies of contaminated soil, is generally accepted that trace elements bioavailability in soils depends on many factors. These factors are not completely understood and simple relationships are seldom found in natural soil systems between the plant trace elements levels and the total amount of the trace elements concentrations in soil. In some investigations, correlations between specific trace elements fractions and plant trace elements contents were found. For example, in soils that had been amended with composted or liquid sewage sludge it was found that Zn in exchangeable and oxide fractions had a strong correlation with Zn in the leaves of barley (Hordeum vulgar). A simple relationship existed between Co in the exchangeable fraction and the Co concentration in winter wheat (Triticum aestivum L.) and alfalfa (Medicago sativa L.) while the adsorption of Ni, Cu and Pb by plants could be predicted by a stepwise multiple regression procedure. These studies show the possibility for using sequential extraction data to evaluate the correlation between trace elements in soil and plant uptake. Therefore, the relationship between plant uptake of trace elements and the trace elements concentration in some fractions was also investigated (Buanuam et al., 2005).
CONCLUSIONS
For the soybean, both Cu and Zn concentrations in LCE and DL are derived from the fractions bound to the OM, to Ox and the T contents. However, for rice, Zn concentrations in LCE and DL seems to be derived from the S/Ex, OM and Ox fractions, while Cu concentration in LEC were derived from the OM, Ox fractions and T contents.
The DTPA, HCl 0.1 mol L-1 and Mehlich-1 extractors, under this soil condition, after the cultivation of both cultures, extracted Cu and Zn from the OM, Ox fractions and T concentration.
Received December 21, 2006
Accepted June 23, 2008
- ABREU, C.A.; ABREU, M.F.; SOARES, L.H.; ANDRADE, J.C. The effects of the DTPA extraction conditions on the determination of micronutrients in Brazilian soils. Communications in Soil Science and Plant Analysis, v.28, p.1-11, 1997.
- ABREU, C.A.; ABREU, M.F.; BERTON, R.S. Análise química de solos para metais pesados. Tópicos Ciência do Solo, v.2, p.1-48, 2002.
- ABREU, C.A.; ANDRADE, J.C. Determinação de cobre, ferro, manganês, zinco, cádmio, cromo, níquel e chumbo em solos usando a solução de DTPA em pH 7,3. In: RAIJ, B. van; ANDRADE, J.C.; CANTARELLA, H.; QUAGGIO, J.A. (Ed.) Análise química para avaliação da fertilidade de solos tropicais. Campinas: Instituto Agronômico, 2001. cap.16, p.240-250.
- AHNSTROM, Z.S.; PARKER, D.R. Development and assessment of a sequential extraction procedure for the fractionation of soil cadmium. Soil Science Society of America Journal, v.63, p.1650-1658, 1999.
- ALLOWAY, B.J. Heavy metals in soils 2 ed. London: Blackie Academic & Professional, 1995. 368p.
- AMARAL SOBRINHO, N.M.B.; VELLOSO, A.C.X.; OLIVEIRA, C. Solubilidade de metais pesados em solo tratado com resíduo siderúrgico. Revista Brasileira de Ciência do Solo, v.21, p.9-16, 1997.
- ANJOS, A.R.M.; MATTIAZZO, M.E. Extratores para Cd, Cu, Cr, Mn, Ni, Pb e Zn em Latossolos tratados com biossólido e cultivados com milho. Scientia Agricola, v.58, p.337-344, 2001.
- BUANUAM, J.; SHIOWATANA, J.; PONGSAKUL, P. Fractionation and elemental association of Zn, Cd and Pb in soils contaminated by Zn minings using a continuous-flow sequential extraction. Journal of Environmental Monitoring, v.7, p.778-784, 2005.
- CAMARGO, O.A.; MONIZ, A.C.; JORGE, J.A.; VALADARES, J.M.S. Métodos de análise química, mineralógica e física de solos do Instituto Agronômico Campinas: Instituto Agronômico, 1986. 94p.
- CAMARGO, M.S.; ANJOS, A.R.M.; ROSSI, C.; MALAVOLTA, E. Adubação fosfatada e metais pesados em latossolo cultivado com arroz. Scientia Agricola, v.57, p.513-518, 2000.
- COSTA, A.C.S.; ALMEIDA, V.C.; LENZI, E.; NOVAKI, J. Determinação de cobre, alumínio e ferro em solos derivados do basalto através de extrações seqüenciais. Química Nova, v.25, p.548-552, 2002.
- GOMES, P.C.; FONTES, M.P.F.; COSTA, L.M.; MENDONÇA, E.S. Extração fracionada de metais pesados em Latossolo vermelho-amarelo. Revista Brasileira de Ciência do Solo, v.21, p.543-551, 1997.
- HARTER, R.D. Micronutrient adsorption-desorption reactions in soils. In: MORTVERDT, J.J.; COX, F.R.; SHUMAN, L.M.; WELCH, R.M. (Ed.) Micronutrients in the agriculture Madison: Soil Science Society of America, 1991. p.59-88.
- LINDSAY, W.L.; NORVELL, W.A. Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Science Society of American Journal, v.42, p.421-428, 1978.
- MALAVOLTA, E.; VITTI, G.C.; OLIVEIRA, S.A. Avaliação do estado nutricional das plantas: princípios e aplicações 2 ed. Piracicaba: POTAFOS, 1997. 319p.
- MENDES, A.M.S.; DUDA, G.P.; NASCIMENTO, C.W.A.; SILVA, M.O. Bioavailability of cadmium and lead in a soil amended with phosphorus fertilizers. Scientia Agricola, v.63, p.328-332, 2006.
- NASCIMENTO, C.W.A; FONTES, R.L.F. Correlação entre características de Latossolos e parâmetros de equações de adsorção de cobre e zinco. Revista Brasileira de Ciencia do Solo, v.28, p.965-971, 2004.
- NIEUWENHUIZE, J.; POLEY-VOS, C.H.; AKKER, A.H. van den; DELFT, W. van. Comparison of microwave and conventional extraction techniques for the determinations of metals in soil, sediment and sludge sample by atomic spectrometry. The Analyst, v.116, p.347-351, 1991.
- OBATA, H. Micro essential elements. In: MATSUO, T.; KUMAZAWA, K.; ISHII, R.; ISHIHARA, K.; HIRATA, H. (Ed.) Science of the rice plant Tokyo: Food and Agriculture Policy Research Center, 1995. cap.2, p.420-433.
- OLIVA, S.R.; RAITIO, H.; MINGORANCE, M.D. Comparison of two wet digestion procedures for multi-element analysis of plant samples. Communications in Soil Science and Plant Analysis, v.34, p.2913-2923, 2003.
- OLIVEIRA, F.C. Disposição de lodo de esgoto e composto de lixo num Latossolo Vermelho-amarelo cultivado com cana-de-açúcar. Piracicaba: USP/ESALQ, 2000. 247p. (Doutorado).
- PAIVA, H.N.; CARVALHO, J.G.; SIQUEIRA, J.D.; CORRÊA, J.B.D. Teor, conteúdo e índice de translocação de nutrientes em mudas de cedro (Cedrela fissilis vell.) submetidas a doses crescentes de zinco. Ciência Florestal, v.13, p.1-10, 2002.
- RAIJ, B. van; QUAGGIO, J.A.; CANTARELLA, H.; ABREU, C.A. Os métodos de análise química do sistema IAC de análise de solo no contexto nacional. In: RAIJ, B. van; ANDRADE, J.C.; CANTARELLA, H.; QUAGGIO, J.A. (Ed.) Análise química para avaliação da fertilidade de solos tropicais. Campinas: Instituto Agronômico, 2001. cap.17, p.251-261.
- RAIJ, B. van; CANTARELA, H.; QUAGGIO, J.A.; FURLANI, A.M.C. (Ed.) Recomendações de adubação e calagem para o Estado de São Paulo 2. ed. Campinas: Instituto Agronômico, 1997. 285p.
- ROCA, J.; POMARES, F. Prediction of available heavy metals by six chemical extractants in a sewage sludge-amended soil. Communications in Soil Science and Plant Analysis, v.22, p.2119-2136, 1991.
- ROSS, S.M. Retention, transformation and mobility of toxic metals in soil-plant systems. In: ROSS, S.M. (Ed.) Toxic metals in soil-plant systems Chichester: John Wiley, p.63-152, 1994.
- SAS INSTITUTE. SAS user's guide: statistics. 5 ed. Carey; SAS Institute, 1985.
- SHUMAN, L.M. Effect of phosphorus level on extractable micronutrients and their distribution among soil fractions. Soil Science of America Journal, v.52, p.136-141, 1988.
- SILVA, F.A.M. Fracionamento e biodisponibilidade de metais para o feijoeiro (Pahseolus vulgaris L.) em solos tratados com pó de forno de aciaria elétrica. Lavras: UFL, 1999a. 80p. (Mestrado).
- SILVA, F.C. Manual de análises químicas de solos, plantas e fertilizantes Brasília: Embrapa, 1999b. 370p.
- TAYLOR, R.W.; IBEABUCHI, I.O.; SISTANI, K.R.; SHUFORD, J.W. Heavy metal concentration in forage and extractabilility from some acid mine spoils. Water, Air and Soil Pollution, v.68, p.363-372, 1993.
- TESSIER, A.; CAMPBELL, P.G.C.; BISSON, M. Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, v.51, p.844-851, 1979.
- URE, A.M. Trace elements in soil, soils extracts in solution. Microchymica Acta, v.2, p.49-57, 1991.
- ZHANG, T.; SHAN, X.; LI, F. Comparison of two sequential extraction procedures for speciation analysis of metals in soils and plant availability. Communication in Soil Science and Plant Analysis, v.29, p.1023-1034, 1998.
- WEAR, J.I.; SOMMER, A.L. Acid-extractable zinc of soils in relation to the occurrence of zinc deficiency symptoms of corns: a method of analysis. Soil Science Society of America Proceedings, v.12, p.143-144, 1947.
Publication Dates
-
Publication in this collection
14 Nov 2008 -
Date of issue
Dec 2008
History
-
Accepted
23 June 2008 -
Received
21 Dec 2006