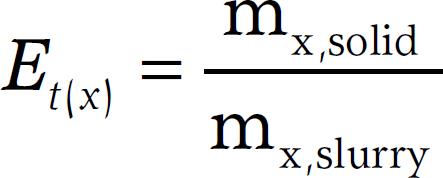ABSTRACT
Biogas digestates contain valuable nutrients but also have high water contents. Di-gestates were sampled from two different biogas facilities before and after solid-liquid separation and were analyzed with regard to their composition and phosphorus (P) fractions. Additionally, to investigate the P fertilizer effects of these digestates in comparison with undigested slurry or TripleSuper-P (TSP), they were applied in a pot experiment (6 kg soil per pot) in an amount corresponding to 200 mg P per pot in combination with various crops (amaranth, maize, maize + beans mixed cropping, sorghum). A separation of digestates resulted in higher P concentrations of the solid fraction in comparison with the liquid fraction. The proportion of the readily soluble P fractions (H2O-P, NaHCO3-P) to the total P was higher than 70 % in all digestates. The digestates increased P uptake of the tested crops and concentrations of bioavailable P in the soil to the same extent as highly soluble TSP. Activities of soil enzymes were lower after application of the digestates in comparison to unfermented slurry. The fertilizer management of digestates can be improved by a solid-liquid separation since the solid fraction showed a relatively high concentration of P resulting in a reduction in application doses required to meet the P demands of crops.
Keywords:
phosphorus fractions; anaerobic digestion; amaranth; mixed cropping; soil enzymes
Introduction
The anaerobic digestion of organic wastes is becoming more important as a source of energy and a treatment of wastes. Most biogas installations use animal slurries which are co-fermented with energy crops. Other substrates are by-products from slaughterhouses, the food sector, and bio-wastes from private households (Nkoa, 2014Nkoa, R. 2014. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: a review. Agronomy for Sustainable Development 34: 473-492.). The digestates which are left after the biogas process contain important plant nutrients like phosphorus (P), nitrogen (N), potassium (K) (Arthurson, 2009Arthurson, V. 2009. Closing the global energy and nutrient cycles through application of biogas residues to agricultural land. Energies 2: 226-242.). During anaerobic digestion the total amount of nutrients generally remains stable but other characteristics of the input substrate may change. It has been shown that the C/N ratio decreases, while the mineral N (NH4-N) content and substrate pH may increase (Möller and Müller, 2012Möller, K.; Müller, T. 2012. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Engineering in Life Science 12: 242-257.). P solubility may decrease after anaerobic digestion due to the formation of struvite (MgNH4PO4 * 6 H2O) and poorly soluble hydroxylapatite (Ca5(PO4)2OH) compounds (Field et al., 1984Field, J.A.; Caldwell, J.S.; Jeyanayagam, S.; Reneau, R.B.; Kroontje, W.; Collins, E.R. 1984. Fertilizer recovery from anaerobic digesters. Transactions of the ASAE 27: 1871-1876., Güngör et al., 2007Güngör, K.; Jürgensen, A.; Karthikeyan, K.G. 2007. Determination of phosphorus speciation in dairy manure using XRD and XANES Spectroscopy. Journal of Environmental Quality 36: 1856-1863.). However, previous studies have shown that an application of digestates increases such crop P uptake and readily available P pools in soil to an extent comparable with high soluble P fertilizers like TripleSuper-P (TSP) (Bachmann et al., 2011Bachmann, S.; Wentzel, S.; Eichler-Löbermann, B. 2011. Co-digested dairy slurry as a phosphorus and nitrogen source for Zea maysL. and Amaranthus cruentus L. Journal of Plant Nutrition and Soil Science 174: 908-915.; Loria and Sawyer, 2005Loria, E.; Sawyer, J. 2005. Extrable soil phosphorus and inorganic nitrogen following application of raw and anaerobically digested swine manure. Agronomy Journal 97: 879-885.). The high water content of digestates, makes a separation of solid and liquid parts an important tool for achieving better handling of these residues. Mechanical separation with screw presses or decanter centrifuges are efficient and simple techniques and are widely used, whereby the results of the separation process also depend on the type and the dry matter content of the slurries (Møller et al., 2002Møller, H.B.; Sommer, S.G.; Ahring, B.K. 2002. Separation efficiency and particle size distribution in relation to manure type and storage conditions. Bioresource Technology 85: 189-196.). Furthermore, mechanical solid-liquid-separation of residues efficiently leads to an enrichment of P in the solid phase, whereas N is often found in higher concentrations in the liquid phase. According to the state of the art research, it can be assumed that the solid fraction of biogas residues is an effective P fertilizer, but to our knowledge, very little data regarding P solubility in separated digestates and their impact on P nutrition of crops is available. The aim of our study was to analyse the P fractions in biogas digestates before and after solid-liquid separation. Furthermore, we wanted to evaluate the P fertilizer effects they may have in combination with different crop species, as crops develop various strategies to influence either the spatial or chemical availability of P in soil (Eichler et al., 2004Eichler, B.; Zachow, B.; Bartsch, S.; Köppen, D.; Schnug, E. 2004. Influence of catch cropping on nitrate contents in soil and soil solution. Landbauforschung Volkenrode 54: 7-12.; Richardson et al., 2011Richardson, A.E.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A.; Smith, S.E.; Harvey, P.R.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; Oberson, A.; Culvenor, R.A.; Simpson, J.R. 2011. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant and Soil 349: 121-156.) and thus influence the use-efficiency of fertilizer applied.
Materials and Methods
Digestate sampling and analysis
The digestates were sampled from commercially operating biogas plants close to the city of Rostock in Northern Germany. The digestate from biogas Plant A was a mixture of dairy slurry (57 %, w/w) and maize silage (43 %, w/w). The solid-liquid separation in Plant A was performed by a screw press, where the digestates were compressed into a gradually decreasing screw channel between a screw shaft and screen mantle, squeezing out the liquid. Approximately 92 % of the fresh digestate was separated into liquid and 8 % into solid. After separation the solid fraction was further treated and dried with the waste heat from the biogas plant. An unfermented dairy slurry from this farm was also included in the study. The digestate from biogas Plant B was based on energy crops (87 %, maize silage, 9 % cereal whole plant silage, and 4 % grass silage, w/w) only. Here the solid-liquid separation was done via a decanter centrifuge, which works on the principle of different specific gravities of the material. The digestates are pumped into a cylinder, which rotates at about 2000 and 4500 rpm. The centrifuge separates approximately 89 % of the fresh digestate into liquid and 11 % solid. Samples from both plants were taken directly before and after the separation device. The digestates from both biogas plants were sampled two times; i.e. in September 2012 for characterization of the nutrient composition and P availability, and in April 2013 for analysis of the nutrient composition and the following application in a pot experiment.
The dry matter (DM) content of the dairy slurry, the digestates and the solid and liquid fractions were determined by freeze drying and subsequent oven drying at 105 °C. Organic matter content (OM) in the dry material of the products was determined after ashing in an oven at 550 °C and weighing (Bauer et al., 2009Bauer, A.; Mayr, H.; Hopfner-Sixt, K.; Amon, T. 2009. Detailed monitoring of two biogas plants and mechanical solid-liquid separation of fermentation residues. Journal of Biotechnology 142: 56-63.). Total P, K and Mg concentrations in the slurry and digestate samples were determined after digesting the ash with 25 % HCl. Phosphorus contents were determined spectrometrically via the Vanadate-molybdate method (Page et al., 1982Page, A.L.; Miller, R.H.; Keeney, D.R. 1982. Methods of Soil Analysis. Part 2. Chemical and Microbial Properties. ASA/CSSA/SSSA, Madison, WI, USA.), K via flame spectroscopy, and Mg spectrophotometrically. Total N contents were determined in the fresh slurry using a Kjeldahl analyzer. NH4-N contents were determined after shaking 20 g of fresh sample in 500 mL ultrapure water and analyzing the N contents in the supernatant using a Kjeldahl apparatus (Schmitt, 1954Schmitt, L. 1954. Book of the agricultural methods and analyses. The Analyses of Fertilizers = Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik. Die Untersuchung von Düngemitteln. Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten, 2ed. Neumann, Radebeul, Germany (in German).). Sulfur was measured with the CNS elemental analyzer. The pH value (H2O) was determined in the fresh slurry with a pH electrode according to DIN EN 12176.
The separation index (Et(x)) for a respective compound was calculated according to Hjorth et al., (2010)Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. 2010. Soli-liquid separation of animal slurry in theory and practice: a review. Agronomy for Sustainable Development 30: 153-180. and expresses the distribution of the respective compound between the solid and liquid fractions. For the solid fraction this value was calculated as follows:
where mx,solid is the mass (g) of the respective compound in the solid fraction and mx,slurry is the mass of the respective compound in the slurry/digestate treated. For the liquid fraction the value was calculated in a similar way:
The solubility of P in the dairy slurry, the digestates, and their solid and liquid fractions were determined according to Dou et al., (2000)Dou, Z.; Toth, J.D.; Galligan, D.T.; Ramberg, C.F.; Ferguson, J.D. 2000. Laboratory procedures for characterizing manure phosphorus. Journal of Environmental Quality 29: 508-514.. In brief, 0.3 g of the freeze-dried slurry sample was shaken in 30 mL of deionized water for 16 hours on a horizontal shaker at 150 rpm. Samples were centrifuged for 20 minutes at 1700 g and the supernatant was decanted. Subsequently, the residue was extracted with 0.5 mol L−1 NaHCO3 (pH 8.5), 0.1 mol L−1 NaOH and 1.0 mol L−1 H2SO4 as described above. Total P content in the supernatant of each phase was measured using inductively coupled plasma spectroscopy (ICP-OES) on a wavelength of 214.91 nm. Inorganic P content was determined using the molybdate-blue method as described by Schinner et al., (1996)Schinner, F.; Öhlinger, R.; Kandeler, E. 1996. Methods in Soil Biology. Springer, Berlin, Germany.. Residual P content was calculated as follows: total P – (H2O-P+NaHCO3-P+NaOH-P+H2SO4-P). All samples were analyzed in parallel. P solubility in the digestates was analysed only for samples taken in September 2012.
Experimental design of the pot experiment
An outdoor pot experiment covered by a wire cage was carried out at an experimental station in Rostock (Mecklenburg-Vorpommern, Germany: 54°3′41.47″N and 12°5′5.59″ E) in April 2013. The soil which was sampled from a field experiment of the experimental station was a moderately acidic loamy sand and according to the World Reference Base for Soil Resources is classified as Stagnic Cambisol. To achieve a very low bioavail-able P content (double lactate-soluble phosphorus Pdl: 37 mg kg−1), a 1:1 mixture of soil from the A horizon and the B horizon was used. The pots (height 21 cm, diameter 19.5 cm) were filled with approximately 6 kg of soil. Four different crops were cultivated, amaranth (Amaranthus cruentus), sorghum (Sorghum bicolor), maize (Zea mays) and beans (Phaseolus coccineus; four seeds) intercropped with maize (four seeds). Crops were cultivated for 8 weeks with distilled water used for irrigation. The pots could freely drain to field capacity, and percolated water was collected in a bowl below the pots and was replenished.
Nine different treatments were established: I) mineral treatment without P (NK), II) mineral treatment with TripleSuperPhosphate (TSP) (NPK), III) unfermented dairy slurry, IV) digestate A (from biogas Plant A – see above), V) liquid phase A, VI) solid phase A, VII) digestate B (from biogas Plant B – see above) VIII) liquid phase B, IX) solid phase B. The P containing products were applied in an amount equivalent to 200 mg P per pot (except the control). As other nutrients besides P were applied with the digestates (see Table 1), the addition of N, K, and Mg was adapted to ensure a uniform supply of these nutrients to all treatments according to the following amounts per pot: NH4-N: 1 g, K: 1.3 g, and Mg: 0.25 g with nutrient salts (NH4-N as calcium ammonium nitrate, K as potash (KCl) and Mg as magnesium sulfate (MgSO4·7 H2O)). Each treatment consisted of 4 replications. For this experiment the digestates sampled in April 2013 were used.
At the end of the experiment, plant and soil samples were taken from each pot for laboratory analysis. For the analysis of soil microbial activity a sub-sample was stored frozen.
Plant and Soil analyses
The dry matter yield of the crops was determined by drying the harvested biomass in an oven at 60 °C for seven days and then weighing it. To determine the P content of the plant tissue, the dried plant material was ground and the P content was measured after dry ashing using the vanadate-molybdate method (Page et al., 1982Page, A.L.; Miller, R.H.; Keeney, D.R. 1982. Methods of Soil Analysis. Part 2. Chemical and Microbial Properties. ASA/CSSA/SSSA, Madison, WI, USA.). The P uptake was calculated by multiplying dry matter yield by P or N concentration.
For the analysis of the chemical soil parameters, the soil was air-dried and sieved (< 2 mm). The double lactate soluble P (Pdl) concentration, which is a standard procedure for estimating plant available P in soil in Germany, the water soluble P (Pw) and the pH values were measured using the method described by Blume et al., (2000)Blume, H.P.; Deller, B.; Leschber, R.; Paetz, A.; Schmidt, S.; Wilke, B.M. 2000. Book of Soil Analyses. Physical, Chemical and Biological Methods = Handbuch der Bodenuntersuchung. Physikalische, Chemische und Biologische Untersuchungsverfahren. Wiley-VCH, Weinheim, Germany (in German).. To determine the effect of the digestates on the activity of soil microorganisms the frozen samples were thawed and kept at room temperature for 24 hours before analysis. Thawed samples were sieved and roots were removed as quickly as possible using tweezers in order to avoid drying. Dehydrogenase activity (DHA) in the soil was measured after incubating 1 g of soil for 24 hours with triphenyltetrazolium chloride solution and the DHA was estimated after measuring the triphenylformazan (TPF) concentration photometrically (Schinner et al., 1996Schinner, F.; Öhlinger, R.; Kandeler, E. 1996. Methods in Soil Biology. Springer, Berlin, Germany.). The alkaline phosphatase (alkPase) activities were determined after incubating 1 g soil with p-nitrophenolphosphate for 1 hour at pH 11.0 and after, the subsequent photometrical measurement of the p-nitrophenol (pNP) according to Tabatabai and Bremner (1969)Tabatabai, M.A.; Bremner, J.M. 1969. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biology and Biochemistry 1: 301-307..
Statistics
For statistical analysis, the software package PASW Statistics 18 was used. Soil and plant data corresponding to four replications were subjected to a two factorial analysis of variance (ANOVA, general linear model). The means of soil and plant parameters were compared using the Duncan multiple range test. Significance was determined at p ≤ 0.05 and significantly different means were indicated by different letters. Data from digestate analysis were subjected to a one factorial ANOVA.
Results and Discussions
Effect of mechanical solid-liquid separation on the nutrient content and nutrient distribution in the digestates
Results of both sampling dates (Sep 2012 and Apr 2013) consistently showed, that after mechanical separation the solid fraction is characterized by a relative high dry matter and organic matter content (Table 2 and Figure 1). After mechanical separation the dry matter (DM) content was about 5 % in the liquid fraction and about 30 % in the solid fraction. This makes the solid fraction better for storing and easier to transport, as less water must be moved. Our results are consistent with studies from Bauer et al., (2009)Bauer, A.; Mayr, H.; Hopfner-Sixt, K.; Amon, T. 2009. Detailed monitoring of two biogas plants and mechanical solid-liquid separation of fermentation residues. Journal of Biotechnology 142: 56-63. and Möller et al., (2010)Möller, K.; Rudolf, S.; Müller, T. 2010. Substrate inputs, nutrient flows and nitrogen loss of two centralized biogas plants in southern Germany. Nutrient Cycling in Agroecosystems 87: 307-325. who found a DM content between 4 - 8 % in the liquid fraction and 19 -23 % in the solid fraction of digestates from on-farm biogas plants.
Composition of the dairy slurry and digestates from two biogas plants and their liquid and solid phases after mechanical separation at two sampling dates (sampling date Sept 2012 – first number, Apr 2013 – second number, n = 3).
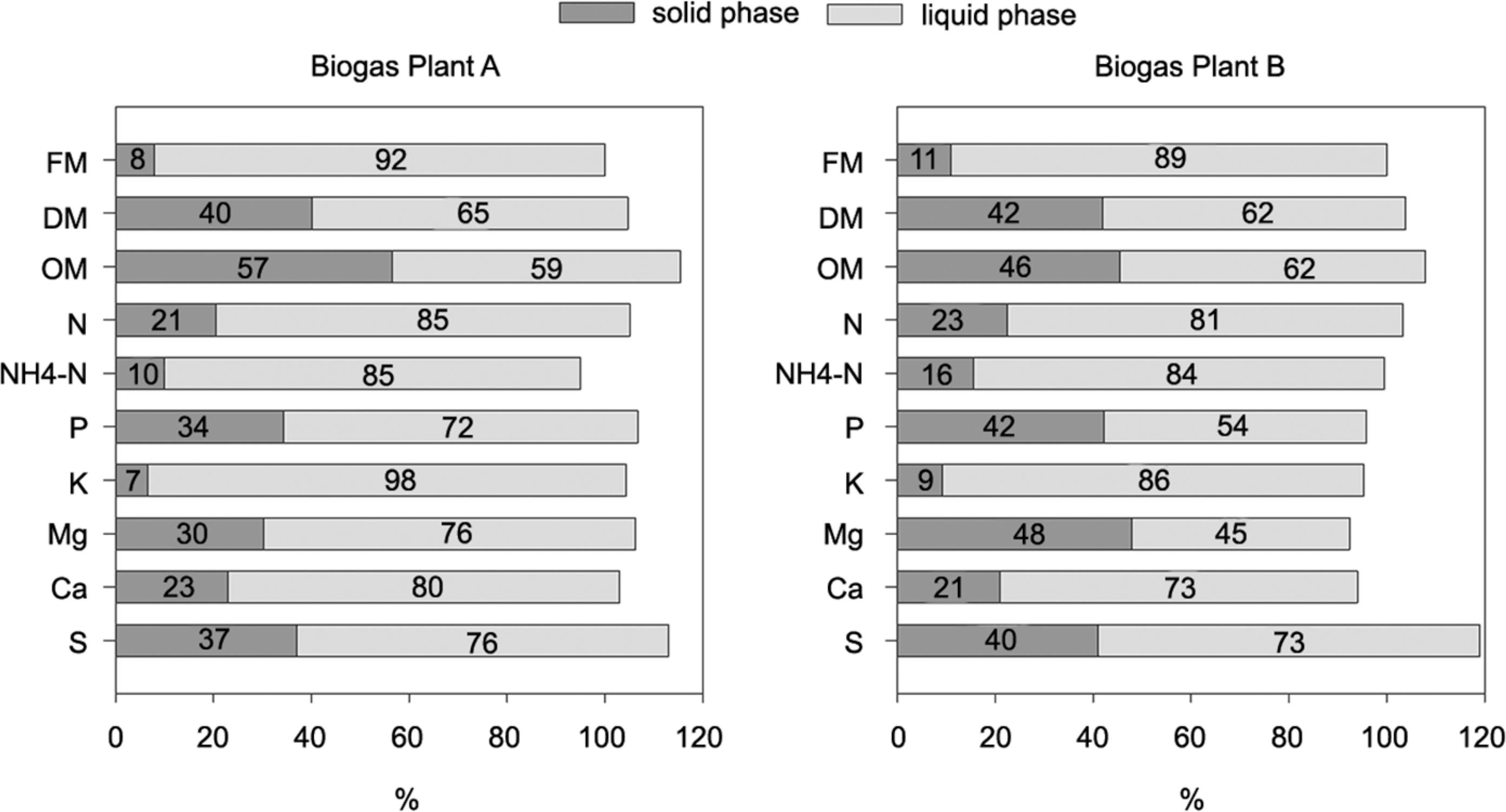
Distribution of nutrients for the solid and liquid phases after mechanical separation of digestates from two different biogas plants as expressed by the separation index (sampling date Sept 2012). DM = dry matter; OM = organic matter; FM = fresh matter.
Additionally, except for K, the N, P, Mg, Ca and S concentrations were high in the solid fraction of the digestates, which makes them, in combination with the relative low water content, a potentially viable organic fertilizer. P concentration in the solid fraction (2.8 to 3.2 g kg−1 FW) was about four times as high as in the untreated digestate (0.6-0.8 g kg−1 FW), and five to six times higher than in the liquid fraction (0.4-0.6 g kg−1 FW). Comparable P contents of the solid and liquid fractions of digestates and slurry were also reported by other authors (Fangueiro et al., 2012Fangueiro, D.; Lopes, C.; Surgy, S.; Vasconcelos, E. 2012. Effect of the pig slurry separation techniques on the characteristics and potential availability of N to plants in the resulting liquid and solid fractions. Biosystems and Engineering 113: 187-194.; Jørgensen and Stoumann-Jensen, 2009Jørgensen, K.; Stoumann-Jensen, L. 2009. Chemical and biochemical variation in animal manure solids separated using different commercial separation technologies. Bioresource Technology 100: 3088-3096.). The N:P ratio was considerably lower in the solid fraction (about 3.0 – Plant A, 2.5 – Plant B) than in the liquid fraction (about 7.0 – Plant A and 8.0 – Plant B) or untreated digestate (5.5 – Plant A, 6.0 – Plant B) (the average of both sampling dates). A low N:P ratio of 3.4:1 in the solid fraction of separated digestates was also reported by Möller et al. (2010)Möller, K.; Rudolf, S.; Müller, T. 2010. Substrate inputs, nutrient flows and nitrogen loss of two centralized biogas plants in southern Germany. Nutrient Cycling in Agroecosystems 87: 307-325., and demonstrates the preferential accumulation of P in the solid fraction. The low N:P ratio is also positive in terms of plant nutrition because the P demand of field crops could be met without excessive N supply.
Although total N concentration in the solid phase was high, only less than 30 % of the total N was analyzed as NH4-N, which is regarded as being easily available to crops. In the liquid fraction and in the non-separated digestates the proportion of NH4-N to total N was about 50 % for biogas from Plant A and about 40 % from Plant B. The solid fraction is also a potential source of S, as the S concentrations were about four times higher compared to that of the untreated digestate. In addition, the pH value of the solid fraction was high (8.7-9.0), probably due to the high Ca content. Additional drying of the solid fraction with waste heat from biogas plant A did not increase DM content significantly, but led to a reduction in total N and NH4-N content. The composition of the liquid fraction was very similar to that of the untreated digestate.
To evaluate the efficiency of the separation process, the simple separation index (Et) was calculated as described by Hjorth et al. (2010)Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. 2010. Soli-liquid separation of animal slurry in theory and practice: a review. Agronomy for Sustainable Development 30: 153-180.. Separation efficiency depends on the separator, as well as on the type and composition of the input slurry, amongst other factors such as the organic matter content, the particle size distribution and the pH value (Bauer et al., 2009Bauer, A.; Mayr, H.; Hopfner-Sixt, K.; Amon, T. 2009. Detailed monitoring of two biogas plants and mechanical solid-liquid separation of fermentation residues. Journal of Biotechnology 142: 56-63.;Hjorth et al., 2010Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. 2010. Soli-liquid separation of animal slurry in theory and practice: a review. Agronomy for Sustainable Development 30: 153-180.; Møller et al., 2002Møller, H.B.; Sommer, S.G.; Ahring, B.K. 2002. Separation efficiency and particle size distribution in relation to manure type and storage conditions. Bioresource Technology 85: 189-196.). In both biogas plants studied, about 40 % of the DM previously present in the untreated digestate was separated to the solid fraction, while 60 % of the DM remained in the liquid fraction. These results are comparable to the findings of Popovic et al., (2012)Popovic, O.; Hjorth, M.; Stoumann-Jensen, L. 2012. Phosphorus, copper and zinc in solid and liquid fractions from full-scale and laboratory-separated pig slurry. Environmental Technology 33: 2119-2131. who reported that 46 % of the DM slurry was transferred to the solid separated fraction when using a commercial screw press.
Only 7-20 % of the N, NH4-N and K previously present in the digestate was separated into the solid fraction, whereas over 80 % remained in the liquid phase. On the other hand, between 30 and 40 % of the total P previously present in the digestate was found in the solid phase after mechanical separation. Bauer et al., (2009)Bauer, A.; Mayr, H.; Hopfner-Sixt, K.; Amon, T. 2009. Detailed monitoring of two biogas plants and mechanical solid-liquid separation of fermentation residues. Journal of Biotechnology 142: 56-63. reported that as much as 52 % of P found in the inflow was found in the solid fraction after digestate separation. NH4 and K salts are very soluble and most of the NH4 and K in slurries is present in dissolved form, and thus remains in the liquid fraction after separation. On the other hand, more than 80 % of the P in untreated slurry is present in crystalline form (mainly Ca-P or Mg-P) or adsorbed onto particles, and therefore a higher proportion is separated into the solid fraction (Massé et al., 2005Massé, L.; Massé, D.I.; Beaudette, V.; Muir, M. 2005. Size distribution and composition of particles in raw and anaerobically digested swine manure. Transactions of the ASAE 48: 1943-1949.).
More P was separated into the solid fraction in Plant B than Plant A. Apart from the different original material this could also be attributed to the separation technology. A screw press, which was applied at Plant A, has a lower efficiency when separating P into solids than other separation techniques (Hjorth et al., 2010Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. 2010. Soli-liquid separation of animal slurry in theory and practice: a review. Agronomy for Sustainable Development 30: 153-180.; Møller et al., 2002Møller, H.B.; Sommer, S.G.; Ahring, B.K. 2002. Separation efficiency and particle size distribution in relation to manure type and storage conditions. Bioresource Technology 85: 189-196.). In the screw press the DM-rich solid fraction is pressed against a filter plate to press out more liquid, and the small particles are forced to pass through the filter and end up in the liquid fraction (Popovic et al., 2012Popovic, O.; Hjorth, M.; Stoumann-Jensen, L. 2012. Phosphorus, copper and zinc in solid and liquid fractions from full-scale and laboratory-separated pig slurry. Environmental Technology 33: 2119-2131.). Interestingly, Mg showed the same partition tendencies between the solid and liquid fractions as P, demonstrating that P is associated with Mg in slurries and digestates, e.g. as struvite (Dou et al., 2000Dou, Z.; Toth, J.D.; Galligan, D.T.; Ramberg, C.F.; Ferguson, J.D. 2000. Laboratory procedures for characterizing manure phosphorus. Journal of Environmental Quality 29: 508-514.).
Effect of mechanical solid-liquid separation on P availability in the digestates
The sequential P fractionation has often been used to evaluate P solubility and potential P availability in farmyard manure or agroindustrial by-products (Ajiboye et al., 2004Ajiboye, B.; Akrinremi, O.O.; Racz, G.J. 2004. Laboratory characterization of phosphorus in fresh and oven-dried organic amendments. Journal of Environmental Quality 33: 1062-1069.; Dou et al., 2000Dou, Z.; Toth, J.D.; Galligan, D.T.; Ramberg, C.F.; Ferguson, J.D. 2000. Laboratory procedures for characterizing manure phosphorus. Journal of Environmental Quality 29: 508-514.; Kruse et al., 2010Kruse, J.; Negassa, W.; Appathurai, N.; Zuin, L.; Leinweber, P. 2010. Phosphorus speciation in sequentially extracted agro-industrial by-products: Evidence from X-ray absorption near edge structure spectroscopy. Journal of Environmental Quality 39: 2179-2184.; Sharpley and Moyer, 2000Sharpley, A.N.; Moyer, B.G. 2000. Forms of phosphorus in manure and their release during rainfall. Journal of Environmental Quality 29: 1462-1469.). In both biogas plants, 70 to 90 % of the total P in the digestates and its solid and liquid fractions were extracted with mild agents such as H2O and NaHCO3 (Figure 2). Neither the mechanical separation technique nor the subsequent drying process (in plant A) had a significant effect on the readily soluble P (H2O+NaHCO3-P; p ≤ 0.178). The H2O-P and the NaHCO3-P fractions generally represent labile and highly soluble P forms, such as dicalcium phosphate dihydrate (CaHPO4*2H2O), struvite (MgNH4PO4*6H2O), hydrated aluminum phosphate (AlPO4*2H2O), P sorbed to CaCO3 or phospholipids, DNA and simple phosphate monoesters (Ajiboye and Akrin-remi, 2007Ajiboye, B.; Akrinremi, O.O. 2007. Phosphorus speciation of sequential extracts of organic amendments using nuclear magnetic resonance and x-ray absorption near-edge structure spectroscopies. Journal of Environmental Quality 36: 1563-1576.; Turner and Leytem, 2004Turner, B.L.; Leytem, A.B. 2004. Phosphorus compounds in sequential extracts of animal manures: chemical speciation and a novel fractionation procedure. Environmental Science & Technology 38: 6101-6108.). Thus, our results indicate, that the bioavailablity of P in digestates is high, even in the P-rich solid phase after digestate separation. The fact that most of the P contained in slurries and digestates is present in easily bioavailable form has also been demonstrated in previous studies (Bachmann et al., 2011Bachmann, S.; Wentzel, S.; Eichler-Löbermann, B. 2011. Co-digested dairy slurry as a phosphorus and nitrogen source for Zea maysL. and Amaranthus cruentus L. Journal of Plant Nutrition and Soil Science 174: 908-915.; Güngör and Karthikeyan, 2005Güngör, K.; Karthikeyan, K.G. 2005. Probable phosphorus solid phases and their stability in anaerobically digested dairy manure. Transactions of the ASAE 48: 1509-1520.).
P-fractions of the total P in digestates from two different biogas plants as influenced by mechanical solid-liquid separation (sampling date Sept 2012). Drying of the solid digestate was applied only in biogas plant A.
Between 5 - 15 % of the total P in the digestates and its solid and liquid fractions were extracted with NaOH and H2SO4. This proportion is also comparable with our previous results (Bachmann et al., 2011Bachmann, S.; Wentzel, S.; Eichler-Löbermann, B. 2011. Co-digested dairy slurry as a phosphorus and nitrogen source for Zea maysL. and Amaranthus cruentus L. Journal of Plant Nutrition and Soil Science 174: 908-915.) where 12 % of the total P in the digestate was extracted with NaOH and H2SO4. The NaOH and H2SO4 fractions are considered to represent P forms of moderate to low solubility. In the NaOH-fraction of farmyard manure organic P forms dominate, whereas complex Ca-P compounds i.e. low soluble hydroxylapatite (Ca5(PO4)2OH) or phytic acid and Ca-phytate, dominate in the H2SO4/HCl-P fraction (Ajiboye and Akrinremi, 2007Ajiboye, B.; Akrinremi, O.O. 2007. Phosphorus speciation of sequential extracts of organic amendments using nuclear magnetic resonance and x-ray absorption near-edge structure spectroscopies. Journal of Environmental Quality 36: 1563-1576.; Kruse et al., 2010Kruse, J.; Negassa, W.; Appathurai, N.; Zuin, L.; Leinweber, P. 2010. Phosphorus speciation in sequentially extracted agro-industrial by-products: Evidence from X-ray absorption near edge structure spectroscopy. Journal of Environmental Quality 39: 2179-2184.; Turner and Leytem, 2004Turner, B.L.; Leytem, A.B. 2004. Phosphorus compounds in sequential extracts of animal manures: chemical speciation and a novel fractionation procedure. Environmental Science & Technology 38: 6101-6108.). Between 5 - 23 % of total P was not extracted by the different agents and was considered Residual-P.
The mineral P predominated in all digestate samples. The organic P represented only 5 - 18 % of the total P (Figure 3). This range is quite similar to what was reported by Dou et al., (2000)Dou, Z.; Toth, J.D.; Galligan, D.T.; Ramberg, C.F.; Ferguson, J.D. 2000. Laboratory procedures for characterizing manure phosphorus. Journal of Environmental Quality 29: 508-514. in dairy slurry samples. In contrast, a higher proportion of organic P, between 29 - 45 % of the total P, was found for solid manures (Aijboye et al., 2004). The proportion of organic P to total P in the di-gestates varied between the two biogas plants and was influenced by the use of mechanical separation (p = 0.020). The digestate from Plant B had a higher proportion of organic P (18 % of the total P) than that from plant A (12 % of the total P). This might be attributed to the different input substrates used. Animal slurries are composed of organic and inorganic P forms, but inorganic P is usually the principal P form. In a wide variety of organic wastes of different origins and treatments animal slurry was found to have a low concentration of organic P (about 1 %) in relation to total P (García-Albacete et al., 2012García-Albacete, M.; Martín, A.; Cartagena, M.C. 2012. Fractionation of phosphorus biowastes: characterisation and environmental risk. Waste Management 32: 1061-1068.). Biogas Plant A mainly used dairy slurry (57 %, w/w) which may explain the relatively low proportion of organic P in the digestates. Surprisingly, in both biogas plants, we found a considerably lower proportion of organic P to total P in the solid fraction (5 - 7 % of the total P for plant B and plant A) than in the liquid fraction (13 - 14 % of the total P for plant B and plant A). This indicates that organic P compounds in digestates probably have a very low particle size and thus remain in the liquid fraction.
Proportion of organic P in each P fraction in digestates from two different biogas plants as influenced by mechanical solid-liquid separation (sampling date Sep 2012). One-Way ANOVA with post hoc comparison of means (Tukey-Test p ≤ 0.05). Different letters indicate significant differences of the organic P concentration between the digestates.
Effect of the digestates on plant and soil parameters
The dry matter yield and P uptake differed between crops and were affected by the amendments applied. Significant interactions between crops and fertilizer treatments indicate that the efficacy of the organic amendments also depended on the crop cultivated.
All types of digestates resulted in higher P uptake than the control and were in the range of that of TSP and unfermented dairy slurry (Table 3). The digestates can, therefore, be considered a suitable P source for plants. These results confirmed previous outcomes of an experiment with maize and amaranth (Bachmann et al., 2011Bachmann, S.; Wentzel, S.; Eichler-Löbermann, B. 2011. Co-digested dairy slurry as a phosphorus and nitrogen source for Zea maysL. and Amaranthus cruentus L. Journal of Plant Nutrition and Soil Science 174: 908-915.). As regards yield, the positive effect of the amendments was not so clear. All amendments resulted in higher yield of maize and sorghum in comparison to the control whereas for amaranth only the unseparated digestates increased yield and none of the amendments resulted in higher yields of the mixed cropped maize and beans (see also below). On average, we could not detect an effect of separation on the crops and the unseparated digestates had the same effect as the solid or liquid fraction of the separated digestates. Differences occurred, however, between the digestates and the digestate B and its liquid fraction resulted in higher P uptake than di-gestate A and its liquid fraction (average of all crops). As the same amount of P was provided to all pots we assume that other components in the digestates affected the soil characteristics and the P delivery to plant. Due to the varying P concentration, the digestates were applied in different amounts to warrant a P supply of 200 mg per pot. Other ingredients except P, NH4-N, K and Mg (which were balanced for all pots) were thus also applied in different amounts.
Yield and P uptake of different crop species as influenced by different amendments at the end of an eight week pot experiment on a P-poor loamy sand.
Soil P concentration (Pw and Pdl) also increased when P was applied (Table 4). The only exception was the Pdl concentration in combination with amaranth cultivation, where only two treatments resulted in higher values than the control without P. The Pw concentration in soil of all pots almost doubled when P was applied in comparison to the control. As mentioned above, we could not detect stringently a different effect of the digestates or differences between the solid or liquid fraction.
Bioavailable P concentration and pH of the soil after application of different amendments and cultivation of different crop species in an eight week pot experiment on a P-poor loamy sand.
The pH values in soil rose when the organic amendments were applied, which can be explained by the high pH values of the substrates (see Table 4).
The microbial metabolitic activity (DHA) was highest when undigested dairy slurry was applied (Table 5). This was the case in combination with all crops tested. It is well documented that the application of organic materials enhances the microbial biomass and microbial activity of soils (Ehlers et al., 2010Ehlers, K.; Bakken, L.R.; Frostegard, A.; Frossard, E.; Bünemann, E.K. 2010. Phosphorus limitation in a Ferralsol: impact on microbial activity and cell internal P pools. Soil Biology and Biochemistry 42: 558-566.; Krey et al., 2013Krey, T.; Vassilev, N.; Baum, C.; Eichler-Löbermann, B. 2013. Effects of long-term phosphorus application and plant growth promoting rhizobacteria on maize phosphorus nutrition under field conditions. European Journal of Soil Biology 55: 124-130.; Requejo and Eichler-Löbermann, 2014Requejo, M.; Eichler-Löbermann, B. 2014. Organic and inorganic phosphorus forms in soil as affected by long-term application of organic amendments. Nutrient Cycles in Agroecosystems 100: 245-255.). Nonetheless, this depends on the material applied. After digestate application (separated or unseparated) the DHA was generally lower than after application of dairy slurry. This was also found in previous experiments with digested and undigested animal slurries (Bachmann et al., 2011Bachmann, S.; Wentzel, S.; Eichler-Löbermann, B. 2011. Co-digested dairy slurry as a phosphorus and nitrogen source for Zea maysL. and Amaranthus cruentus L. Journal of Plant Nutrition and Soil Science 174: 908-915., Bachmann et al., 2014Bachmann, S.; Gropp, M.; Eichler-Löbermann, B. 2014. Phosphorus availability and soil microbial activity in a 3 year field experiment amended with digested dairy slurry. Biomass and Bioenergy 70: 429-439.; Elfstrand et al., 2007Elfstrand, S.; Bath, B.; Martensson, A. 2007. Influence of various forms of green manure amendment on soil microbial community composition, enzyme activity and nutrient levels in leek. Applied Soil Ecology 36: 70-82.). Elevated values in comparison to the control after digestate application were only found in combination with maize and maize + bean. The activity of the dehydrogenase in soil is closely related to microbial activity and can be regarded as a sensitive indicator of changes in the nutrient and carbon turnover in soil because this enzyme is an integral part of their cellular metabolism (Watts et al., 2010Watts, D.B.; Torbert, H.A.; Feng, Y.; Prior, S.A. 2010. Soil microbial community dynamics as influenced by composted dairy manure, soil properties, and landscape position. Soil Science 175: 474-486.; Brookes, 2001Brookes, P. 2001. The soil microbial biomass. Microbes Environment 16: 131-140.).
Microbial metabolic activity and activity of alkaline phosphatase in soil after application of different amendments and cultivation of different crop species in an eight week pot experiment on a P-poor loamy sand.
As the activity of soil microorganisms strongly depends on the presence of available organic C, the lower microbial activity in soil after application of the digestates can be attributed, in part, to the lower amount of applied organic matter. Furthermore, the quality of the organic matter in the digestates could also have affected the microbial activity. Obviously, the organic matter applied with the digestate did not stimulate the growth of the soil microorganisms. After anaerobic digestion, mainly stable lignin-like organic C compounds remain, which can hardly be used as carbon and energy sources by most soil microorganisms (Marcato et al., 2009Marcato, C.E.; Mohtar, R.; Revel, J.C.; Pouech, P.; Hafidi, M.; Guiresse, M. 2009. Impact of anaerobic digestion on organic matter quality in pig slurry. International Biodeterioration and Biodegradation 63: 260-266.; Thomsen et al., 2013Thomsen, I.K.; Olesen, J.E.; Møller, H.B.; Sørensen, P.; Christensen, B.T. 2013. Carbon dynamics and retention in soil after anaerobic digestion of dairy cattle feed and faeces. Soil Biology and Biochemistry 58: 82-827.). Our results, therefore, show that changes in substrate composition due to anaerobic digestion influence the activity of enzymes, which in turn can affect the P turnover in the soil. In our experiment we found a significant correlation between Pw contents in soil and DHA (0.349, p ≤ 0.001).
The activities of the alkaline phosphatase (alkPase) were also influenced by the fertilizer application. Although averaged for all crops the addition of dairy slurry and digestate A resulted in higher activities in comparison to the NK and the NPK treatment, the effect of organic matter supply was not as clear as was found for DHA. Obviously it was not decisive if the applied organic material was digested or not. The activity of alkPase is also affected by pH value and alkPase develops higher activities when soil pH rises (Tabatabai and Bremner, 1969Tabatabai, M.A.; Bremner, J.M. 1969. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biology and Biochemistry 1: 301-307.). The correlation between pH values and alkPase was highly significant (0.262, p > 0.001) when all treatments and crops were included, and even higher (until 0.47p ≤ 0.001) when only amaranth or sorghum were considered. A higher activity of Pase indicates a greater potential for mineralizing P from organic compounds. The alkPase is mainly produced by soil microorganisms, and the extent of its synthesis and excretion should be coupled to the microbial activity and/or population (Albiach et al., 2000Albiach, R.; Canet, R.; Pomares, F.; Ingelmo, F. 2000. Microbial biomass content and enzymatic activities after the application of organic amendments to a horticultural soil. Bioresource Technology 75: 43-48.). Thus, correlations between DHA and alkPase were also found (0.291 p ≤ 0.001, including all crops and fertilizer treatments). Higher activity of alk-Pase after addition of organic fertilizers (compost and manure) in a field experiment was also shown in a study of Krey et al., (2013)Krey, T.; Vassilev, N.; Baum, C.; Eichler-Löbermann, B. 2013. Effects of long-term phosphorus application and plant growth promoting rhizobacteria on maize phosphorus nutrition under field conditions. European Journal of Soil Biology 55: 124-130., where this was also related to an increase in pH and soil organic matter.
On average for all fertilizer treatments, the shoot yield ranged as follows: maize > maize + bean > sorghum > amaranth (Table 3). The relatively small effect of P supply on yields (see above) may indicate that the amount of P in the soil (despite the low Pdl contents in the treatment without fertilization) was still sufficient to cover plant demand. Even P compounds with relatively low solubility can be mobilized in soil by crops and microorganisms, and such processes might not be reflected adequately by standard soil-P extraction like the double-lactate method (Requejo and Eichler-Löbermann, 2014Requejo, M.; Eichler-Löbermann, B. 2014. Organic and inorganic phosphorus forms in soil as affected by long-term application of organic amendments. Nutrient Cycles in Agroecosystems 100: 245-255.; Dakora and Phillips, 2002Dakora, F.D.; Phillips, D.A. 2002. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245: 35-47.). To improve the spatial or chemical availability of P in soil, crops can change their root morphology (mainly the formation of fine roots) to explore a larger soil volume; others excrete P-solubilizing compounds like organic acids, organic and inorganic ions, sugars, vitamins, nucleosides, and enzymes (Richardson et al., 2011Richardson, A.E.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A.; Smith, S.E.; Harvey, P.R.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; Oberson, A.; Culvenor, R.A.; Simpson, J.R. 2011. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant and Soil 349: 121-156.). This can be especially advantageous in mixed cropping where two major processes occur: complementarity and facilitation. Resource complementarity minimizes the niche overlap in space and time and facilitation enhances the resource availability and growth of a crop by another crop (Hinsinger et al., 2011Hinsinger, P.; Betencourt, E.; Bernard, L.; Brauman, A.; Plassard, C.; Shen, J.; Tang, X.; Zhang, F. 2011. P for two, sharing a scarce resource: soil phosphorus acquisition in the rhizosphere of intercropped species. Plant Physiology 156: 1078-1086.). This can be the reason, that in the present study for the mixed cropping with maize and beans no yield effect of the treatments was observed.
The internal P utilization efficiency also varied between crops. Despite the comparable low yield, shoot uptake of P by amaranth was, with 171 mg per pot, considerably higher than the P uptake of the other crops and unproportionally high in relation to the shoot bio-mass, because of the high P concentration in the plant tissue (data not shown). This implies a low internal P utilization (biomass production in relation to P uptake). However, the high P uptake of amaranth did not result in lower bioavailable P concentrations in the soil (Pdl) in comparison to the other crops. This suggests on the other hand a relatively high efficiency for P acquisition. The lowest pH values in soil were also found for amaranth which can probably be explained by the release of organic acids which was shown by Li et al., (2006) who cultivated amaranth under K stress. We assume that in our experiment the amaranth roots released organic acids to mobilize P resources in soil to cover the high P demand of this crop. Outstandingly high P uptakes by amaranth were also found in other studies (Ojo et al., 2010Ojo, O.D.; Akinrinde, E.A.; Akoroda, M.O. 2010. Residual effects of phosphorus sources in grain amaranth production. Journal of Plant Nutrition 33: 770-783.; Brandt et al., 2011Brandt, C.; Balko, C.; Eichler-Löbermann, B. 2011. Interactive effects of soil water content and phytin supply on phosphorus nutrition of different crops species. Landbauforschung Volkenrode 4: 317-326.; Bachmann et al., 2011Bachmann, S.; Wentzel, S.; Eichler-Löbermann, B. 2011. Co-digested dairy slurry as a phosphorus and nitrogen source for Zea maysL. and Amaranthus cruentus L. Journal of Plant Nutrition and Soil Science 174: 908-915.). Lower pH values after amaranth cultivation were probably also the reason for the decrease in enzyme activities.
Conclusion
Digestates showed the same P fertilizer effects as the high soluble mineral P fertilizer (TSP) and undigested dairy slurry and thus represent a valuable P source. Nutrient management and the nutrition of crops can be improved by a solid-liquid separation of digestates as the solid phase showed relatively high concentration of P and a low N:P ratio. Because of the low water content of the solid phase, it is more easily transported, and allows for targeted application on soils with low P status and low risk of P losses. The reduced activity of enzymes in soil after application of the digestates (mainly activity of dehydrogenase) indicated a lower C and P turnover in the digestate-amended soils which needs to be investigated for a longer time span under field conditions.
References
- Ajiboye, B.; Akrinremi, O.O.; Racz, G.J. 2004. Laboratory characterization of phosphorus in fresh and oven-dried organic amendments. Journal of Environmental Quality 33: 1062-1069.
- Ajiboye, B.; Akrinremi, O.O. 2007. Phosphorus speciation of sequential extracts of organic amendments using nuclear magnetic resonance and x-ray absorption near-edge structure spectroscopies. Journal of Environmental Quality 36: 1563-1576.
- Albiach, R.; Canet, R.; Pomares, F.; Ingelmo, F. 2000. Microbial biomass content and enzymatic activities after the application of organic amendments to a horticultural soil. Bioresource Technology 75: 43-48.
- Arthurson, V. 2009. Closing the global energy and nutrient cycles through application of biogas residues to agricultural land. Energies 2: 226-242.
- Bachmann, S.; Wentzel, S.; Eichler-Löbermann, B. 2011. Co-digested dairy slurry as a phosphorus and nitrogen source for Zea maysL. and Amaranthus cruentus L. Journal of Plant Nutrition and Soil Science 174: 908-915.
- Bachmann, S.; Gropp, M.; Eichler-Löbermann, B. 2014. Phosphorus availability and soil microbial activity in a 3 year field experiment amended with digested dairy slurry. Biomass and Bioenergy 70: 429-439.
- Bauer, A.; Mayr, H.; Hopfner-Sixt, K.; Amon, T. 2009. Detailed monitoring of two biogas plants and mechanical solid-liquid separation of fermentation residues. Journal of Biotechnology 142: 56-63.
- Blume, H.P.; Deller, B.; Leschber, R.; Paetz, A.; Schmidt, S.; Wilke, B.M. 2000. Book of Soil Analyses. Physical, Chemical and Biological Methods = Handbuch der Bodenuntersuchung. Physikalische, Chemische und Biologische Untersuchungsverfahren. Wiley-VCH, Weinheim, Germany (in German).
- Brandt, C.; Balko, C.; Eichler-Löbermann, B. 2011. Interactive effects of soil water content and phytin supply on phosphorus nutrition of different crops species. Landbauforschung Volkenrode 4: 317-326.
- Brookes, P. 2001. The soil microbial biomass. Microbes Environment 16: 131-140.
- Dakora, F.D.; Phillips, D.A. 2002. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245: 35-47.
- Dou, Z.; Toth, J.D.; Galligan, D.T.; Ramberg, C.F.; Ferguson, J.D. 2000. Laboratory procedures for characterizing manure phosphorus. Journal of Environmental Quality 29: 508-514.
- Ehlers, K.; Bakken, L.R.; Frostegard, A.; Frossard, E.; Bünemann, E.K. 2010. Phosphorus limitation in a Ferralsol: impact on microbial activity and cell internal P pools. Soil Biology and Biochemistry 42: 558-566.
- Eichler, B.; Zachow, B.; Bartsch, S.; Köppen, D.; Schnug, E. 2004. Influence of catch cropping on nitrate contents in soil and soil solution. Landbauforschung Volkenrode 54: 7-12.
- Elfstrand, S.; Bath, B.; Martensson, A. 2007. Influence of various forms of green manure amendment on soil microbial community composition, enzyme activity and nutrient levels in leek. Applied Soil Ecology 36: 70-82.
- Fangueiro, D.; Lopes, C.; Surgy, S.; Vasconcelos, E. 2012. Effect of the pig slurry separation techniques on the characteristics and potential availability of N to plants in the resulting liquid and solid fractions. Biosystems and Engineering 113: 187-194.
- Field, J.A.; Caldwell, J.S.; Jeyanayagam, S.; Reneau, R.B.; Kroontje, W.; Collins, E.R. 1984. Fertilizer recovery from anaerobic digesters. Transactions of the ASAE 27: 1871-1876.
- García-Albacete, M.; Martín, A.; Cartagena, M.C. 2012. Fractionation of phosphorus biowastes: characterisation and environmental risk. Waste Management 32: 1061-1068.
- Güngör, K.; Jürgensen, A.; Karthikeyan, K.G. 2007. Determination of phosphorus speciation in dairy manure using XRD and XANES Spectroscopy. Journal of Environmental Quality 36: 1856-1863.
- Güngör, K.; Karthikeyan, K.G. 2005. Probable phosphorus solid phases and their stability in anaerobically digested dairy manure. Transactions of the ASAE 48: 1509-1520.
- Hinsinger, P.; Betencourt, E.; Bernard, L.; Brauman, A.; Plassard, C.; Shen, J.; Tang, X.; Zhang, F. 2011. P for two, sharing a scarce resource: soil phosphorus acquisition in the rhizosphere of intercropped species. Plant Physiology 156: 1078-1086.
- Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. 2010. Soli-liquid separation of animal slurry in theory and practice: a review. Agronomy for Sustainable Development 30: 153-180.
- Jørgensen, K.; Stoumann-Jensen, L. 2009. Chemical and biochemical variation in animal manure solids separated using different commercial separation technologies. Bioresource Technology 100: 3088-3096.
- Krey, T.; Vassilev, N.; Baum, C.; Eichler-Löbermann, B. 2013. Effects of long-term phosphorus application and plant growth promoting rhizobacteria on maize phosphorus nutrition under field conditions. European Journal of Soil Biology 55: 124-130.
- Kruse, J.; Negassa, W.; Appathurai, N.; Zuin, L.; Leinweber, P. 2010. Phosphorus speciation in sequentially extracted agro-industrial by-products: Evidence from X-ray absorption near edge structure spectroscopy. Journal of Environmental Quality 39: 2179-2184.
- Loria, E.; Sawyer, J. 2005. Extrable soil phosphorus and inorganic nitrogen following application of raw and anaerobically digested swine manure. Agronomy Journal 97: 879-885.
- Marcato, C.E.; Mohtar, R.; Revel, J.C.; Pouech, P.; Hafidi, M.; Guiresse, M. 2009. Impact of anaerobic digestion on organic matter quality in pig slurry. International Biodeterioration and Biodegradation 63: 260-266.
- Massé, L.; Massé, D.I.; Beaudette, V.; Muir, M. 2005. Size distribution and composition of particles in raw and anaerobically digested swine manure. Transactions of the ASAE 48: 1943-1949.
- Møller, H.B.; Sommer, S.G.; Ahring, B.K. 2002. Separation efficiency and particle size distribution in relation to manure type and storage conditions. Bioresource Technology 85: 189-196.
- Möller, K.; Müller, T. 2012. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Engineering in Life Science 12: 242-257.
- Möller, K.; Rudolf, S.; Müller, T. 2010. Substrate inputs, nutrient flows and nitrogen loss of two centralized biogas plants in southern Germany. Nutrient Cycling in Agroecosystems 87: 307-325.
- Nkoa, R. 2014. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: a review. Agronomy for Sustainable Development 34: 473-492.
- Ojo, O.D.; Akinrinde, E.A.; Akoroda, M.O. 2010. Residual effects of phosphorus sources in grain amaranth production. Journal of Plant Nutrition 33: 770-783.
- Page, A.L.; Miller, R.H.; Keeney, D.R. 1982. Methods of Soil Analysis. Part 2. Chemical and Microbial Properties. ASA/CSSA/SSSA, Madison, WI, USA.
- Popovic, O.; Hjorth, M.; Stoumann-Jensen, L. 2012. Phosphorus, copper and zinc in solid and liquid fractions from full-scale and laboratory-separated pig slurry. Environmental Technology 33: 2119-2131.
- Requejo, M.; Eichler-Löbermann, B. 2014. Organic and inorganic phosphorus forms in soil as affected by long-term application of organic amendments. Nutrient Cycles in Agroecosystems 100: 245-255.
- Richardson, A.E.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A.; Smith, S.E.; Harvey, P.R.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; Oberson, A.; Culvenor, R.A.; Simpson, J.R. 2011. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant and Soil 349: 121-156.
- Schinner, F.; Öhlinger, R.; Kandeler, E. 1996. Methods in Soil Biology. Springer, Berlin, Germany.
- Schmitt, L. 1954. Book of the agricultural methods and analyses. The Analyses of Fertilizers = Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik. Die Untersuchung von Düngemitteln. Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten, 2ed. Neumann, Radebeul, Germany (in German).
- Sharpley, A.N.; Moyer, B.G. 2000. Forms of phosphorus in manure and their release during rainfall. Journal of Environmental Quality 29: 1462-1469.
- Tabatabai, M.A.; Bremner, J.M. 1969. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biology and Biochemistry 1: 301-307.
- Thomsen, I.K.; Olesen, J.E.; Møller, H.B.; Sørensen, P.; Christensen, B.T. 2013. Carbon dynamics and retention in soil after anaerobic digestion of dairy cattle feed and faeces. Soil Biology and Biochemistry 58: 82-827.
- Turner, B.L.; Leytem, A.B. 2004. Phosphorus compounds in sequential extracts of animal manures: chemical speciation and a novel fractionation procedure. Environmental Science & Technology 38: 6101-6108.
- Watts, D.B.; Torbert, H.A.; Feng, Y.; Prior, S.A. 2010. Soil microbial community dynamics as influenced by composted dairy manure, soil properties, and landscape position. Soil Science 175: 474-486.
Edited by
Publication Dates
-
Publication in this collection
Jan-Feb 2016
History
-
Received
20 Feb 2015 -
Accepted
30 June 2015