Abstracts
Sugar cane silages are characterized by extensive yeast activity, alcohol production and great dry matter - DM - losses. Better knowledge of the fermentation process is fundamental to the development of efficient ensilage techniques for this forage. This study evaluates temporal changes in chemical composition, DM losses and epiphytic microflora in sugar cane silage. Mature sugar cane, variety RB835486 (12 months of vegetative growth), was hand harvested, processed in a stationary chopper and ensiled in 20-L plastic buckets provided with valves for gas release and a device for effluent collection. Laboratory silos were kept at ambient temperature and sampled after ½, 1, 2, 3, 7, 15, 45, 90, 120 and 180 days. Ethanol concentration reached 6.4% in DM after 15 days of ensilage, followed by 71% water soluble carbohydrates - WSCs - disappearance. Gas and total DM losses reached a plateau on day 45 (16% and 29% of DM, respectively). Yeast count was higher on the second day (5.05 log cfu g-1). Silage pH declined to below 4.0 on the third day. Effluent yield was negligible (20 kg t-1). DM content in the forage decreased (35% to 26%) from day 0 to day 45. The increase in ethanol concentration showed an opposite trend to WSCs and true in vitro dry matter digestibility reductions in the silage. Developing methods to control yeasts, most probably through the use of additives, will enable more efficient production of sugar cane silage by farmers.
ethanol; water soluble carbohydrates; losses; yeasts; bacteria
Silagens de cana-de-açúcar caracterizam-se pela extensa atividade de leveduras, alto teor de álcool e grandes perdas de matéria seca - MS. Conhecer melhor o processo fermentativo é fundamental para o desenvolvimento de técnicas eficientes de ensilagem da cana. Este trabalho avalia a mudança temporal na composição química, nas perdas de MS e na microflora epífita nestas silagens. Cana-de-açúcar (RB835486) foi colhida manualmente (12 meses de crescimento), picada em picadora estacionária e ensilada em baldes de plástico de 20 L com válvulas para gases e aparato para colheita de efluentes. Os silos laboratoriais foram mantidos sob temperatura ambiente e amostrados após ½, 1, 2, 3, 7, 15, 45, 90, 120 e 180 dias. Etanol atingiu 6,4% na MS no 15º dia após ensilagem, seguido pelo desaparecimento de 71% dos carboidratos solúveis - CHOs. As perdas gasosas e a perda total de MS estabilizaram-se após 45 dias (16% e 29% da MS). A contagem de leveduras foi máxima no segundo dia (5,05 log ufc g-1). O pH atingiu nível abaixo de 4,0 no terceiro dia. A produção de efluentes foi insignificante (20,1 kg t-1). O teor de MS da forragem decresceu (35% para 26%) do dia 0 ao 45º dia. O padrão de variação na concentração de etanol foi inverso à concentração de CHOs e à redução da digestibilidade da silagem. O desenvolvimento de métodos de controle das leveduras, provavelmente com o uso de aditivos, melhorará a eficiência no uso de silagens de cana-de-açúcar pelos pecuaristas.
etanol; carboidratos solúveis em água; efluentes; gases; leveduras
ANIMAL SCIENCE AND PASTURES
Fermentation and epiphytic microflora dynamics in sugar cane silage
Dinâmica da fermentação e da microflora epífita em silagem de cana-de-açúcar
André de Faria PedrosoI, * * Corresponding author < andref@cppse.embrapa.br> ; Luiz Gustavo NussioII; Solidete de Fátima PazianiIII; Daniele Rebouças Santana LouresIV; Mauricio Scoton IgarasiV; Rodrigo Michelini CoelhoVI; Irineu Humberto PackerII; Jorge HoriiVII; Luiz Humberto GomesVIII
IEmbrapa Pecuária Sudeste, C.P. 339 - 13560-970 - São Carlos, SP - Brasil
IIUSP/ESALQ - Depto. de Zootecnia, C.P. 9 - 13418-900 - Piracicaba, SP - Brasil
IIISítio Jataí - Bairro Jataí - 15840-000 - Itajobi, SP - Brasil
IVVila Gianetti, 21- Campus UFV - 36570-000 - Viçosa, MG - Brasil
VUNESP/FMVZ - Programa de Pós-Graduação - Depto de Nutrição e Melhoramento Animal - Botucatu, SP - Brasil
VIRua Pedra Bonita, 1111 - Alto Barroca - 30430-390 - Belo Horizonte, MG - Brasil
VIIUSP/ESALQ - Depto. de Agroindústria, Alimentos e Nutrição
VIIIUSP/ESALQ - Depto. de Genética, C.P.83 - 13418-900 - Piracicaba, SP - Brasil
ABSTRACT
Sugar cane silages are characterized by extensive yeast activity, alcohol production and great dry matter - DM - losses. Better knowledge of the fermentation process is fundamental to the development of efficient ensilage techniques for this forage. This study evaluates temporal changes in chemical composition, DM losses and epiphytic microflora in sugar cane silage. Mature sugar cane, variety RB835486 (12 months of vegetative growth), was hand harvested, processed in a stationary chopper and ensiled in 20-L plastic buckets provided with valves for gas release and a device for effluent collection. Laboratory silos were kept at ambient temperature and sampled after ½, 1, 2, 3, 7, 15, 45, 90, 120 and 180 days. Ethanol concentration reached 6.4% in DM after 15 days of ensilage, followed by 71% water soluble carbohydrates - WSCs - disappearance. Gas and total DM losses reached a plateau on day 45 (16% and 29% of DM, respectively). Yeast count was higher on the second day (5.05 log cfu g-1). Silage pH declined to below 4.0 on the third day. Effluent yield was negligible (20 kg t-1). DM content in the forage decreased (35% to 26%) from day 0 to day 45. The increase in ethanol concentration showed an opposite trend to WSCs and true in vitro dry matter digestibility reductions in the silage. Developing methods to control yeasts, most probably through the use of additives, will enable more efficient production of sugar cane silage by farmers.
Key words: ethanol, water soluble carbohydrates, losses, yeasts, bacteria
RESUMO
Silagens de cana-de-açúcar caracterizam-se pela extensa atividade de leveduras, alto teor de álcool e grandes perdas de matéria seca - MS. Conhecer melhor o processo fermentativo é fundamental para o desenvolvimento de técnicas eficientes de ensilagem da cana. Este trabalho avalia a mudança temporal na composição química, nas perdas de MS e na microflora epífita nestas silagens. Cana-de-açúcar (RB835486) foi colhida manualmente (12 meses de crescimento), picada em picadora estacionária e ensilada em baldes de plástico de 20 L com válvulas para gases e aparato para colheita de efluentes. Os silos laboratoriais foram mantidos sob temperatura ambiente e amostrados após ½, 1, 2, 3, 7, 15, 45, 90, 120 e 180 dias. Etanol atingiu 6,4% na MS no 15º dia após ensilagem, seguido pelo desaparecimento de 71% dos carboidratos solúveis - CHOs. As perdas gasosas e a perda total de MS estabilizaram-se após 45 dias (16% e 29% da MS). A contagem de leveduras foi máxima no segundo dia (5,05 log ufc g-1). O pH atingiu nível abaixo de 4,0 no terceiro dia. A produção de efluentes foi insignificante (20,1 kg t-1). O teor de MS da forragem decresceu (35% para 26%) do dia 0 ao 45º dia. O padrão de variação na concentração de etanol foi inverso à concentração de CHOs e à redução da digestibilidade da silagem. O desenvolvimento de métodos de controle das leveduras, provavelmente com o uso de aditivos, melhorará a eficiência no uso de silagens de cana-de-açúcar pelos pecuaristas.
Palavras-chave: etanol, carboidratos solúveis em água, efluentes, gases, leveduras
INTRODUCTION
The use of sugar cane has at least two major advantages in comparison to feeding green forage to animals: avoids daily operations of harvesting, chopping and hauling of the crop and prevents crop loss by fire and frosts. In addition, field's production life span increases due to uniform harvesting and better post-harvesting management.
The major limitation of ensiling sugar cane is extensive ethanol production, which results in high dry matter - DM - loss and low quality forage. Ethanol is produced mainly through fermentation of sugars by yeasts in a metabolic process which leads to proximately 49% loss of substratum (McDonald et al., 1991). Yeasts are also undesirable because they contribute to aerobic deterioration of silages (Rotz & Muck, 1994).
Sugar cane forage can loose up to 90% of water soluble carbohydrates - WSCs,, present 44% elevation in acid detergent fiber - ADF - (Alli et al., 1982) and reach 15% ethanol in DM during ensiling (Kung Jr. & Stanley (1982). Only a few research works done in Brazil contributed for the characterization of the fermentative process in sugar cane silages. High ethanol production, decrease in DM and increase in fiber content and pH rapid decline to below 4,0 have been reported (Andrade et al., 2001; Bernardes et al., 2002; Coan et al., 2002; Molina et al., 2002). The objective of this study was to supply broader information on the fermentation dynamics of sugar cane silage, establishing the temporal variation for: chemical components; true in vitro dry matter digestibility - IVDMD; DM losses, and epiphytic microflora population.
MATERIAL AND METHODS
Silage was produced with sugar cane, variety RB83-5486 approximately 12 months old (1st cut), picked manually and chopped, in a stationary chopper adjusted for theoretical cut length of 10 mm without cleaning dead parts. Approximately 9 kg of chopped material was conditioned into 20-L plastic buckets (minisilos), sealed with tight lids containing Bunsen valves. Each minisilo was provided with an apparatus for effluent quantification - 2 kg of dry sand separated from the silage by a thin plastic screen and two layers of cheesecloth. Four replicates were prepared for each date of sampling (10 dates), in a total of 40 minisilos.
The minisilos were weighted and the silages were sampled at ½, 1, 2, 3, 7, 15, 45, 90, 120 and 180 days after ensilage. Gaseous DM loss was estimated by gross weight loss. Effluent was estimated by weight gain of the minisilos with sand, plastic screen and cheesecloth. Total DM loss was calculated by DM weight loss in the silage.
Samples for WSCs, pH and ethanol determinations were kept frozen (-10°C) before analysis. Samples for microorganisms counts (approximately 50 g) were placed in plastic bags and kept in ice, before analysis on the same day. Other samples were dried in air forced oven (60°C, 48 h) and grounded in a Wiley mill through a 1 mm screen. Dry chemistry analysis for DM, ash, crude protein - CP, neutral detergent fiber - NDF, ADF and lignin was performed by Near Infrared Reflectance Spectroscopy - NIRS - (Berzaghi et al., 1997; Cozzolino et al., 2001) in a spectrophotometer model NIRS 5000® (NIRSystems, Silver Spring, MD, USA). The equipment's software identified samples distant more than 3 H (standardized distance of Mahalanobis) from the average, which were excluded as outliers. Considering a minimum distance of 0.6 H between samples (Shenk & Westerhaus, 1991), the software indicated the samples which had to be analyzed by wet chemistry. Wet chemical analysis followed AOAC (1990) recommendations. IVDMD was performed in a ANKON® Fiber Analyzer (ANKON Technology Corporation, Fairport, NY). WSCs and pH were determined in aqueous extracts produced according to method described by Kung Jr. (1996). WSCs were determined by the phenol sulfuric acid method (Dubois et al., 1956). Ethanol was analyzed by gas chromatography (Gregório and Cioli®; model CG-37D with column PAAD 2499-CG and electronic integrator CG 200).
Yeasts and lactic acid bacteria (LAB) were enumerated using the method described by Lin et al. (1992). The group containing lactobacilli, pediococci and leuconostocs was enumerated in Rogosa SL medium; streptococci were enumerated in Slanetz and Bartley medium (oxoid Ltd., Hampshire, England). The plates were incubated at 35°C for two days. Yeasts were counted in Malt agar (Difco), with addition of ampicilin (200 µg mL-1) and tetraciclin (200 mg mL-1) to inhibit bacterial growth, after incubation at 25°C for two days.
Data were analyzed through a completely randomized design. The experiment was set in 11 treatments (Pj) with four replicates (minisilos). Treatments were characterized by the opening periods: 0, ½, 1, 2, 3, 7, 15, 45, 90, 120, 180 days. Though there was a possibility for a time trend analysis, silages were spoiled and damaged at each sampling period which did not allow a repeated measure procedure at the same experimental unit. The proposed model (Yij = µ + Pij + eij) for each variable (ethanol, WSCs, gas losses, effluent, total DM loss, IVDMD, DM, NDF, ADF, lignin, CP, ash, pH) was analyzed by the PROC GLM of the SAS system (SAS, 1988). In the multiple comparisons of averages among treatments the LSMEANS was used at a = 0.05. To study the association among some variables (ethanol, WSCs, gas losses, total DM loss, IVDMD, NDF), the coefficient of simple correlation was determined by the PROC CORR of the SAS system (SAS, 1988).
RESULTS AND DISCUSSION
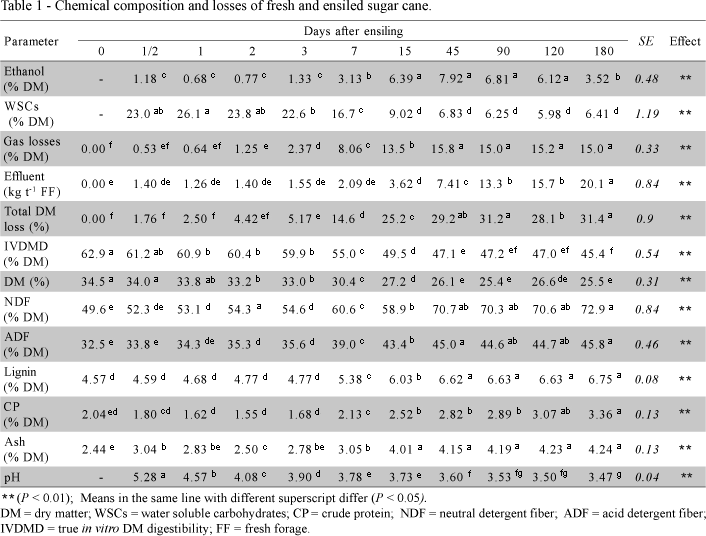
The fresh sugar cane presented high DM and WSCs concentrations (Table 1) compared to 12 months old sugar canes used in previous research in Brazil which presented values around 27% DM and 18% WSCs (Bernardes et al., 2002; Molina et al., 2002). Values as high as 34% WSCs and 37% DM were reported by Alli et al. (1983) for sugar cane harvested at 16.5 months.
Ethanol concentration in the silage (Table 1) lied within levels ordinarily found in silages produced with mature sugar cane (Bernardes et al., 2002; Alli et al., 1983). Alcohol production was low during the first three days of ensilage, increasing linearly from that point until the 15th day, when the highest level was reached. From the 15th to the 120th day, alcohol concentration remained constant, suffering 50% reduction from this point to the 180th day, probably because of volatilization.
The temporal variation of WSCs showed an opposite trend in relation to the variation of ethanol content in the silage with disappearance of approximately 71% of sugars during the firs 15 days of ensilage (Table 1). This phase of intense ethanol production and WSCs consumption was coincident with the period when occurred 85% of gaseous and 86% of total DM losses and was characterized by 21% reduction in the forage's IVDMD (Table 1).
Using values from Table 1, and assuming 51% recovery of DM in the fermentation of sugars to ethanol by yeasts (McDonald et al., 1991), its possible to calculate that 56% of the WSCs disappeared during the first 15 days of ensilage would be consumed by yeasts to produce the amount of ethanol detected at the end of that period, evidencing that other microorganisms were also very active in the silage.
Total DM loss though was excessive in relation to the amount of ethanol detected in the silage. Losses expected from the metabolism of sugars by yeasts, to produce the amount of ethanol detected on day 15, could respond to just 30% of the total DM lost in the period, even considering that intense heterolactic fermentation could result in up to 10% DM loss in the silage (McDonald et al., 1991). Since very little DM was lost through effluent, it is possible that significant amounts of ethanol were not recovered due to volatilization during sample processing. In addition, the loss of volatile components during determination of DM by oven drying, which can reach up to 8.8% of correct DM (McDonald & Dewar, 1960 in Porter & Barton, 1997), may have lead to underestimation of DM contents of the silage and, consequently, to overestimation of DM losses.
Gaseous losses responded for 54% of the total DM losses, which occurred until the 45th day of ensilage (Table 1). The remaining losses can have resulted mainly from conversion of substrate to water during ethanol synthesis by yeasts, although water may be produced also during bacterial fermentation of sugars. This water generated by microbial metabolism caused 8.4 percent units decrease in the DM content of the silage (Table 1), contributing to effluent production.
Effluent production was small (Table 1) and compatible with the DM concentration in the silage (26% in average), according to data by McDonald et al. (1991). Although effluents were produced during the whole period of ensilage, the rate of accumulation of effluents varied, remaining practically constant in 0.14 kg t-1 day-1 during the first 90 days, and reducing to 0.07 kg t-1 day-1 between the 90th and 180th days. Considering that there was not an increase in total DM loss after the 45th day, despite the continuous production of effluent, it can be concluded that losses through effluents were very small during the last 135 days of ensilage. Once average DM concentration in effluents is 5% (McDonald et al., 1991), it can be estimated that effluents in this experiment probably carried 0.37 kg of DM t-1 of silage, until day 45, corresponding to just 0.12% of the DM originally present in the forage.
Yeast population reached its peak on the second day after ensiling (5.05 log10 of colony forming units per gram of fresh forage - log cfu g-1) (Figure 1). After 15 days, yeast number decreased to 4.50 log cfu g-1, coinciding with the point when ethanol concentration ceased to increase (Table 1), that is, from that forth, although yeasts were present, their metabolism was inhibited. From the 45th to 120th day yeast population remained stable in approximately 2 log cfu g-1. Number of yeast cell was below detection in the fresh sugar cane and on the 180th day after ensilage.
Data on epiphytic microflora in sugar cane silage is scarce. Yeast count for the 45th and 90th days of ensilage in this experiment, corresponding to 159.5 cfu g-1, was higher than the 38.7 cfu g-1 reported by Bernardes et al. (2002) in sugar cane ensiled during 55 days. The pattern of yeast development was similar to the variation of fungi (yeasts and molds) population reported by Alli et al. (1983). Those researchers detected fungi count of 6.15 log cfu g-1 in the fresh sugar cane, increasing to approximately 7 log cfu g-1 after the first day, decreasing constantly from this point to 3.3 log cfu g-1 on the 21st day of ensilage. In this case, ethanol accumulation in the silage ceased after the 7th day, when fungi population reached approximately 5.1 log cfu g-1.
LAB population developed in a similar way (Figure 1). Initial LAB count of 4.58 log cfu g-1 increased to 7.47 log cfu g-1 on the third day, coinciding with the period of intense acidification of the silage (Table 1). On the third day, pH was low enough (3.9) to inhibit the development of most bacteria ordinarily present in silages (Rotz & Muck, 1994) and, therefore, LAB number began to decrease accentuated and constantly until day 45, reaching 3.60 log cfu g-1. From that on, LAB population remained practically stable, resulting in small reductions in the pH until the end of the evaluation period. A similar curve was obtained for lactobacilli by Alli et al. (1983), who detected an initial population of 6.2 log cfu g-1 in the fresh sugar cane, increasing to 8.3 log cfu g-1 after the first day of ensilage and decreasing constantly afterwards.
Intense alcoholic fermentation will cause slow decline in pH and final pH above levels considered adequate to silage conservation. In an extensive study, Driehuis & Wikselaar (2000) observed that grass silages containing 4.8% to 6.3% ethanol in DM, with the alcohol molar proportion higher than 0.5 of the total of fermentation products, presented final pH higher than 5.3. Sugar cane silages behave differently though, and despite their high levels of ethanol, these silages normally present rapid decline in pH and final pH around 3.5 (Kung Jr. & Stanley, 1982; Bernardes et al., 2002). It is considered that the low buffering capacity of sugar cane results in a rapid drop in pH even with relatively small amounts of acids in the silage (Alli et al., 1983).
Probably, stabilization in sugar cane silages results from their high ethanol content and not from inhibition of fermentation by low pH or lack of substratum, since yeasts develop well in pH as low as 3,5 (McDonald et al., 1991) and fermentable WSCs are ordinarily not exhausted in these silages. However, high ethanol concentrations can inhibit yeast growth (Gutierrez et al, 1991). Driehuis & Wikselaar (2000) reported that ethanol concentrations between 1.4 and 5.2% in the liquid phase of grass silages were high enough to affect microbial development, contributing to preservation of the silages. In this experiment, ethanol reached 2.3% in the liquid phase of the silage (45th day) coincidentally with the cessation of DM losses (Table 1), indicating that yeast activity was inhibited by the alcohol, and the silage, stabilized.
During the period of intense alcohol production, first 15 days, there was approximately 10 percent units increase in NDF and ADF, leading to 13 percent units reduction in the digestibility of the silage during the same period (Table 1). NDF and ADF, as well as lignin, ash and CP became more concentrated in the silage's DM due to the extensive loss of soluble nutrients in the form of gases, conversion to water and, in a much smaller scale, through effluents, as discussed. However, there was a numeric reduction in the concentration of CP during the first two days of ensilage, probably because of the loss of nitrogen via ammonia in the period in which the pH was not sufficiently low to inhibit proteolysis by enzymes of the forage, enterobacteria and clostridia.
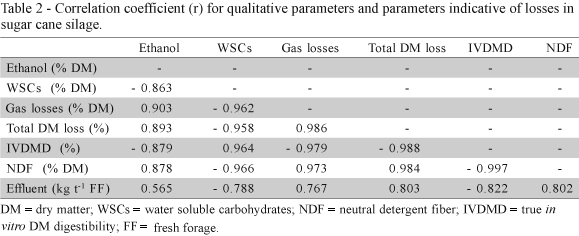
Ethanol concentration was inversely related to WSCs and IVDMD and directly related to DM losses and NDF content in the silage. Gaseous and total DM losses presented direct relationship with NDF content and were inversely related to WSCs concentration and IVDMD. Effluent production was direct related to other parameters indicative of losses and inversely related to WSCs concentration and IVDMD (Table 2).
ACKNOWLEDGMENTS
To FAPESP, for financial support, and to Embrapa, for a doctoral fellowship granted to the first author.
Received January 17, 2005
Accepted July 21, 2005
- ALLI, I.; BAKER, B.E.; GARCIA, G. Studies on the fermentation of chopped sugarcane. Animal Feed Science and Technology, v.7, p.411-417, 1982.
- ALLI, I.; FAIRBAIRN, R.; BAKER, B.E.; GARCIA, G. The effects of ammonia on the fermentation of chopped sugarcane. Animal Feed Science and Technology, v.9, p.291-299, 1983.
- ANDRADE, J.B.; JÚNIOR, E.F.; POSSENTI, R.A.; LEINZ, F.F.; BIANCHINI, D.; RODRIGUES, C.F.C. Valor nutritivo de silagem de cana-de-açúcar, cortada aos 7 meses de idade, tratada com uréia e adicionada de rolão de milho. In: REUNIÃO DA SOCIEDADE BRASILEIRA DE ZOOTECNIA, 38., Piracicaba, 2001. Anais. Piracicaba: SBZ, 2001. 1 CD-ROM.
- AOAC. Association of Official Analytical Chemistis. Official methods of analysis. 15. ed. Arlington: AOAC International, 1990. v.1, 1117p.
- BERNARDES, T.F.; SILVEIRA, R.N.; COAN, R.M.; REIS, R.A.; MOREIRA, A.L.; ITURRINO, R.P.S. Características fermentativas e presença de levedura na cana-de-açúcar crua ou queimada ensilada com aditivo. In: REUNIÃO DA SOCIEDADE BRASILEIRA DE ZOOTECNIA, 39., Recife, 2002. Anais. Recife: SBZ, 2002. 1 CD-ROM.
- BERZAGHI, P.; COZZI, G.; ANDRIGHETTO, I. The use of near infrared analysis for in situ studies. Journal of Dairy Science, v.80, p.3263-3270, 1997.
- COAN, R.M.; SILVEIRA, R.N.; BERNARDES, T.F.; REIS, R.A.; MORENO, T.T.B.; MOREIRA, A.L. Composição química da cana-de-açúcar crua ou queimada ensilada com aditivo. In: REUNIÃO DA SOCIEDADE BRASILEIRA DE ZOOTECNIA, 39., Recife, 2002. Anais. Recife: SBZ, 2002. 1 CD-ROM.
- COZZOLINO, D.; ACOSTA, Y.; GARCIA, J. Application of near infrared reflectance spectroscopy (NIRS) to forage evaluation in Uruguay. In: INTERNATIONAL GRASSLANDS CONGRESS, 19., São Pedro, 2001. Proceedings Piracicaba: FEALQ, 2001. p.370.
- DRIEHUIS, F.; WIKSELAAR, P.G. The occurrence and prevention of ethanol fermentation in high-dry-matter grass silage. Journal of Science of Food and Agriculture, v.80, p.711-718, 2000.
- DUBOIS, M.; GILLES, K.A.; HAMILTON, J.K.; REBERS, P.A.; SMITH, F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry, v.28, p.350, 1956.
- GUTIERREZ, L.E.; AMORIM, H.V.; BASSO, L.C. Inibidores da fermentação alcoólica. STAB - Açúcar, Álcool e Subprodutos, v.10, p.24-30, 1991.
- KUNG Jr., L. Preparation of silage water extracts for chemical analyses. Standard operating procedure - 001 6.03.9. 6.ed. Newark: University of Delaware, Ruminant Nutrition Laboratory, 1996.
- KUNG JUNIOR, L.; STANLEY, R.W., 1982. Effect of stage of maturity on the nutritive value of whole-plant sugarcane preserved as silage. Journal of Animal Science, v.54, p.689-696.
- LIN, C.; BOLSEN, K.K.; HART, R.A.; DICKERSON, J.T.; FEYERHERM, A.M.; AIMUTIS, W.R. Epiphytic microflora on alfalfa and whole-plant corn. Journal of Dairy Science, v.75, p.2484-2493, 1992.
- McDONALD, P.; DEWAR, W.A. Determination of dry matter and volatiles in silage. Journal of Science of Food and Agriculture, v.11, p.566-570, 1960.
- McDONALD, P.; HENDERSON, A.R.; HERON, S.J.E. The biochemistry of silage. 2.ed. Marlow: Chalcomb Publ., 1991. 340 p.
- MOLINA, L.R.; FERREIRA, D.A.; GONÇALVES, L.C.; CASTRO NETO, A.G.; RODRIGUES, N.M. Padrão de fermentação da silagem de cana-de-açúcar (Saccharum officinarum L.) submetida a diferentes tratamentos (compact disc). In: REUNIÃO DA SOCIEDADE BRASILEIRA DE ZOOTECNIA, 39., Recife, 2002. Anais. Recife: SBZ, 2002.
- PORTER, M.G.; BARTON, D. A comparison of methods for the determination of dry matter concentration in grass silage including an extraction method for water. Animal Feed Science and Technology, v.68, p.67-76, 1997.
- ROTZ, C.A.; MUCK, R.E. Changes in forage quality during harvest and storage. In: FAHEY JR., G.C.; COLLINS, M.; MERTENS, D.R.; MOSER, L.E. (ED) Forage quality, evaluation and utilization. Madison: ASA; CSSA; SSSA, 1994. p.828-868.
- SAS. Statistical Analysis System. User's Guide: Statistics, Version 6. SAS Institute. Cary, NC, USA, 1988.
- SHENK, J.S.; WESTERHAUS, M.O. Population definition, sample selection, and calibration procedures for near infrared reflectance spectroscopy. Crop Science, v.31, p.469-474, 1991.
Publication Dates
-
Publication in this collection
26 Sept 2005 -
Date of issue
Oct 2005
History
-
Received
17 Jan 2005 -
Accepted
21 July 2005