Abstracts
Annona crassiflora Mart known as 'araticum', 'marolo' or 'field araticum' is a typical fruit from the Cerrado biome of Brazil with socio-economic and medicinal importance. Normally, Annona crassiflora is propagated through seeds. However, due to a deep dormancy that the seeds display at dispersion and the difficulty to obtain uniform plants in a short time period, micropropagation may be a feasible alternative. Concentrations of gibberellic acid (GA3) and naphthalene-acetic acid (NAA) and their interactive effects on in vitro seed germination and seedling development of Annona crassiflora were studied. Mature fruits of Annona crassiflora were depulped and the seeds washed in clear water and dried at room temperature. Seed coat was removed and the seeds were placed on Murashige & Skoog (MS) medium supplemented with gibberellic acid (GA3) and naphthalene-acetic acid (NAA), 30 g L-1 sucrose and 6 g L-1 agar-agar. Seeds were kept under these conditions for 30 days. After this period, seedlings were kept for another 90 days on Wood Plant Medium (WPM) with 20 g L-1 sucrose and 5 g L-1 agar-agar supplemented with the same GA3 and NAA concentrations. Cultures were incubated under controlled conditions at 25 ± 2°C temperature, 16: 8 (light: dark) photoperiod of 32 µmol m-2 s-1 irradiance provided by cool white fluorescent tubes (Philips). Use of WPM medium supplemented with 25-32 mg L-1 GA3 or MS with 26-30 mg L-1 GA3 and 2 mg L-1 NAA promoted rooting and plant growth.
tissue culture; culture medium; growth regulators
O araticum ou marolo (Annona crassiflora Mart.) é uma fruta típica de Cerrado com grande importância sócio-econômico e medicinal. Sua propagação pode ser feita através de sementes, porém devido à dormência das sementes e dificuldade de se obterem plantas uniformes e em curto espaço de tempo, a micropropagação poderá ser uma alternativa. Estudaram-se os efeitos do GA3 associado ao ANA sobre a germinação de sementes e desenvolvimento in vitro de marolo. Frutos maduros foram despolpados e suas sementes lavadas e secas. Em seguida retirou-se o tegumento das sementes e estas foram colocadas em tubos contendo 20 mL de meio MS, acrescido de GA3 e ANA. Ao meio foram adicionados 30 g L-1 de sacarose e 6g L-1 de ágar, permanecendo nestas condições por 30 dias. Ao final dos 30 dias, as plântulas foram colocadas em meio WPM, acrescido de 20 g L-1 de sacarose, 5 g L-1 de ágar e suplementado das mesmas concentrações de GA3 e ANA da etapa anterior, onde permaneceram por 90 dias. Melhores resultados em meio WPM para todas as variáveis analisadas foram obtidos na faixa de 25-32 mg L-1 de GA3. Em meio MS a interação significativa foi observada apenas no comprimento da parte aérea e número de raízes, nos quais melhores resultados foram verificados com 26,92 - 30,13 mg L-1 de GA3 associado a 2 mg L-1 de ANA.
cultura de tecidos; meio de cultura; reguladores de crescimento
NOTE
In vitro seed germination and seedling development of Annona crassiflora Mart.
Germinação de sementes e desenvolvimento in vitro de plântulas de Annona crassiflora Mart.
Márcia de Nazaré Oliveira RibeiroI; Moacir PasqualI,* * Corresponding author < mpasqual@ufla.br> ; Fabíola VillaI; Leila Aparecida Salles PioI; Henk William Maria HilhorstII
IUFLA - Depto. de Agricultura, C.P. 3037 - 37200-000 - Lavras, MG - Brasil
IIWUR - Laboratory of Plant Physiology, Arboretumlaan 4, 6703 BD Wageningen - The Netherlands
ABSTRACT
Annona crassiflora Mart known as 'araticum', 'marolo' or 'field araticum' is a typical fruit from the Cerrado biome of Brazil with socio-economic and medicinal importance. Normally, Annona crassiflora is propagated through seeds. However, due to a deep dormancy that the seeds display at dispersion and the difficulty to obtain uniform plants in a short time period, micropropagation may be a feasible alternative. Concentrations of gibberellic acid (GA3) and naphthalene-acetic acid (NAA) and their interactive effects on in vitro seed germination and seedling development of Annona crassiflora were studied. Mature fruits of Annona crassiflora were depulped and the seeds washed in clear water and dried at room temperature. Seed coat was removed and the seeds were placed on Murashige & Skoog (MS) medium supplemented with gibberellic acid (GA3) and naphthalene-acetic acid (NAA), 30 g L-1 sucrose and 6 g L-1 agar-agar. Seeds were kept under these conditions for 30 days. After this period, seedlings were kept for another 90 days on Wood Plant Medium (WPM) with 20 g L-1 sucrose and 5 g L-1 agar-agar supplemented with the same GA3 and NAA concentrations. Cultures were incubated under controlled conditions at 25 ± 2°C temperature, 16: 8 (light: dark) photoperiod of 32 µmol m-2 s-1 irradiance provided by cool white fluorescent tubes (Philips). Use of WPM medium supplemented with 25-32 mg L-1 GA3 or MS with 26-30 mg L-1 GA3 and 2 mg L-1 NAA promoted rooting and plant growth.
Key words: tissue culture, culture medium, growth regulators
RESUMO
O araticum ou marolo (Annona crassiflora Mart.) é uma fruta típica de Cerrado com grande importância sócio-econômico e medicinal. Sua propagação pode ser feita através de sementes, porém devido à dormência das sementes e dificuldade de se obterem plantas uniformes e em curto espaço de tempo, a micropropagação poderá ser uma alternativa. Estudaram-se os efeitos do GA3 associado ao ANA sobre a germinação de sementes e desenvolvimento in vitro de marolo. Frutos maduros foram despolpados e suas sementes lavadas e secas. Em seguida retirou-se o tegumento das sementes e estas foram colocadas em tubos contendo 20 mL de meio MS, acrescido de GA3 e ANA. Ao meio foram adicionados 30 g L-1 de sacarose e 6g L-1 de ágar, permanecendo nestas condições por 30 dias. Ao final dos 30 dias, as plântulas foram colocadas em meio WPM, acrescido de 20 g L-1 de sacarose, 5 g L-1 de ágar e suplementado das mesmas concentrações de GA3 e ANA da etapa anterior, onde permaneceram por 90 dias. Melhores resultados em meio WPM para todas as variáveis analisadas foram obtidos na faixa de 25-32 mg L-1 de GA3. Em meio MS a interação significativa foi observada apenas no comprimento da parte aérea e número de raízes, nos quais melhores resultados foram verificados com 26,92 - 30,13 mg L-1 de GA3 associado a 2 mg L-1 de ANA.
Palavras-chave: cultura de tecidos, meio de cultura, reguladores de crescimento
INTRODUCTION
Annona crassiflora (araticum, marolo or field araticum) is a native tree of Cerrado biome of Brazil with economic, medicinal and social uses. Species of the family Annonaceae show fructiferous and pharmaceutical potential. The seeds are dispersed from the mother plant and display prolonged dormancy. This makes seedling production and maintenance of population a very expensive and time consuming procedure (Da Silva et al., 2004). The A. crassiflora endosperm is a ruminate type mainly composed of galactomannans that are deposited in the thick cell walls and pitted cells. The seeds are dispersed with a small embryo located in the endosperm cap region, which elongates into the lateral endosperm prior to radicle protusion. Upon dispersal the embryo is poorly developed, which could be responsible for the long period required for radicle protrusion (Da Silva et al., 2004).
In the field, germination (as radicle protrusion) starts after 150 days after sowing. At present the type of dormancy that Annona crassiflora seeds display has been classified as non-deep simple morpho-physiological type (Da Silva et al., 2007). Moreover, efforts to cross species of Annona have had no success so far and, hence, tree improvement becomes difficult (Samson, 1986). Propagation of Annona species is usually done by grafting or budding the selected scions onto seedling root stocks. However, seedling root stocks are highly variable in vigor and disease resistance and consequently scion growth and productivity are also variable (George & Nissen, 1987).
The development of a methodology for micropropagation of Annona may help the breeding process (so far nonexistent), the multiplication of elite plants or species under threat, and the exchange of genetic material among research centers. Therefore, the aim of this work was to establish an efficient method for seed germination and in vitro development of Annona crassiflora plantlets.
MATERIAL AND METHODS
Annona crassiflora seeds from mature fruits without seed coat were sterilized with 70% (v/v) ethanol for 5 s and then soaked for 20 min in 2% (v/v) NaOCl solution. The seeds were rinsed three times with sterile distilled water and cultured in glass tubes (150 mm × 25 mm) containing 20 mL of MS medium (Murashige & Skoog, 1962). The treatments consisted of different concentrations of GA3 (0, 5, 10, 20 and 40 mg L-1) and NAA (0, 0.5, 1 and 2 mg L-1) which were added to the MS medium. The medium was also enriched with 30 g L-1 sucrose and solidified with 6 g L-1 agar-agar. The pH was adjusted to 5.8 before autoclaving at 121°C and 1 atm for 20 min. The cultures were incubated under controlled conditions at 25 ± 2°C, 16: 8 (light: dark) photoperiod of 32 μmol m-2 s-1 irradiance. After 30 days the first evaluations were carried out in a laminar flow chamber, assessing length of the aerial part, number of leaves and roots and larger length of roots. Subsequently, the plantlets were transferred to WPM medium (Loyd & McCown, 1980) with 20 g L-1 sucrose, solidified with 5 g L-1 agar-agar containing the same concentrations GA3 and NAA used before. The plantlets were kept in this new medium for 90 days under the same environmental conditions as previously described. Observations were made on length of the aerial part, number of leaves and roots and length of roots. Treatments were arranged in a completely randomized design with four replicates of four tubes each and one seed/tube. Data were submitted to analysis of variance, using SISVAR software (Ferreira, 2000), with polynomial regression for GA3 and NAA concentrations used.
RESULTS AND DISCUSSION
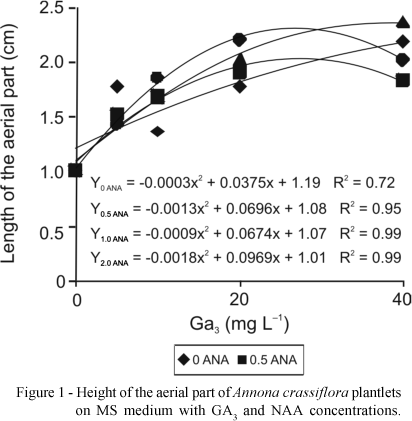
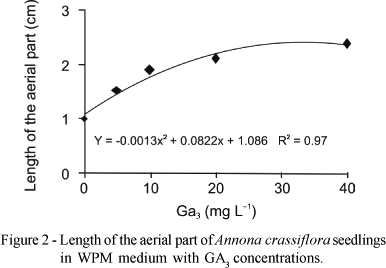
With the increase in GA3 concentrations there was an exponential increase in the length of aerial part of the plantlets, both in the first 30 days in MS medium and 90 days in WPM medium (Figures 1 and 2, respectively). In the MS medium the maximum height of the aerial part (2.3 cm) was obtained at 2 mg L-1 NAA and 26.92 mg L-1 GA3 (Figure 1), whereas in WPM medium with 31.62 mg L-1 GA3 the greatest height of plantlets was observed (2.4 cm) (Figure 2).
The results are in accordance with Diniz (2003), who reported that an increase in GA3 concentration led to greater increase in height of Egletes viscosa (L.) Less plantlets cultivated in vitro. Costa et al. (2002) used 1.0 mg L-1 GA3 for in vitro sprouting of jenipapo (Genipa americana), which promoted growth to 15 mm. However, Deccetti (2000), studying germination in vitro of Annona glabra seeds, found that the use of 2 mg L-1 GA3 and low concentrations of sucrose without GA3 resulted in germination percentages of 80% to 90%.
A similar tendency was observed for number of leaves (Figure 3) in WPM medium, in which 28.56 mg L-1 GA3 exhibited superior results (2.5). Regarding the general aspects of jenipapo (Genipa americana) plants, the treatment with 1 mg L-1 GA3 showed the intense green color, shape and size of a normal leaves (Costa et al., 2002). However, when NAA was supplied to this medium, fewer shoots were produced and callus formed at the cut ends of the explants in Annona squamosa (Nair et al., 1984).
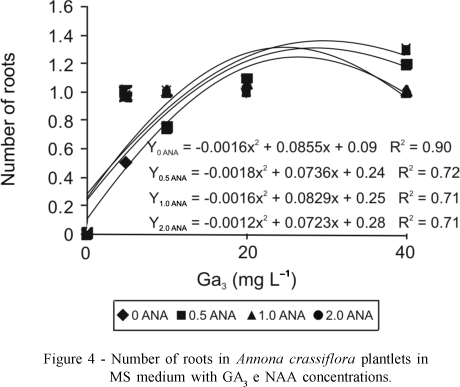
In MS medium, a significant interaction for the number of roots, where the highest value (1.4) was found on 2 mg L-1 NAA associated with 30.13 mg L-1 GA3 (Figure 4), was observed. In WPM medium the increase in GA3 concentration resulted in an exponential increase in the number of roots (Figure 5). There was no significant interaction between the growth regulators used for this parameter. Highest number of roots (2.0) was observed on 26.86 mg L-1 GA3. These results contradict those of Spera (1995), who obtained more roots of Jatropha podagrica in the absence of GA3 in 50% strength MS medium. These results also disagree with Pasqual (2001), who reported that gibberellic acid in culture medium decreased or inhibited root formation, especially when various types of auxins were added. In this study, there was no phytotoxic effect of GA3 on root formation even when higher concentrations were used. NAA at concentrations of 5 mg L-1 and 2 mg L-1 showed higher percentages of rooting and higher number of roots formed in the apical meristem and in sprouting of Eucalyptus globus (Acesita Energética,1987).
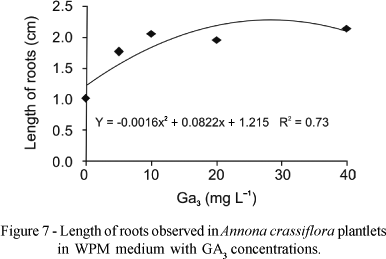
There was no significant interaction among the factors for the length of roots, but there was a tendency of increasing root length with the increase of GA3 concentration in the MS medium (Figure 6) and in the WPM medium (Figure 7). Greater root lengths were observed on MS medium (1.8 cm) with 26.3 mg L-1 GA3. In WPM medium we observed the greatest length of 2.3 cm with 25.7 mg L-1 GA3.
The observation that more roots were formed in medium with GA3 indicated that Annona crassiflora explants do not have sufficient quantities of endogenous gibberellins. The GAs promote synthesis of enzymes involved in the endosperm weakening (endo-ß-mannanase) and/or in the hydrolysis of reserves (amylases), events related mainly with radicle protrusion (Bewley & Black, 1994). These results indicated that GAs act in dormancy breaking, by acting in the gene silencing involved in the maintenance of dormancy (Koornneef et al., 2002) and also in the progression of embryonic prolongation, for promoting the enzyme synthesis involved in reserve mobilization (Bewley, 1997). These observations showed that GAs are the main agents involved in the dormancy breaking (Peng & Harberd, 2002).
CONCLUSIONS
Superior results in Wood Plant Medium for all variables studied were obtained with 25-32 mg L-1 GA3. For maximum height of the aerial parte (2.4 cm), number of roots (2.0), length of roots (2.3 cm) and number of leaves (2.5).
In MS medium a significant interaction was observed only for height of the aerial part (2.3) and number of roots (1.4), where meaningful results were obtained with 26-30 mg L-1 GA3 associated with 2 mg L-1 NAA.
ACKNOWLEDGEMENTS
To CAPES for financial support, and to Drs. Edvaldo Aparecido Amaral da Silva and José Márcio Rocha Faria, from Federal University of Lavras, for valuable suggestions during realization of this study.
Received April 23, 2008
Accepted November 14, 2008
- ACESITA ENERGÉTICA. Sol & solo: enraizamento de estacas e micropropagação de eucaliptos. Belo Hotizonte: Acesita Energética, 1987. 4p. (Acesita Energética, 15).
- BEWLEY, J.D.; BLACK, M. Seeds: physiology of development and germination. 2 ed. New York: Plenum Press, 1994. 445p.
- BEWLEY, J.D. Seed germination and dormancy. The Plant Cell, v.9, p.1055-1066, 1997.
- COSTA, M.A.P.C.; CARMO, D.O.; SOUZA, F.V.D.; MAGALHÃES, G.L.; HANSEN, D.S. Efeito de diferentes concentrações de GA3 no alongamento de brotações in vitro de jenipapo (Genipa americana). In: CONGRESSO BRASILEIRO DE FRUTICULTURA, 17., Belém, 2002. Anais. Belém: SBF, 2002.
- DA SILVA, E.A.A.; DAVIDE, A.C.; MELO, D.L.B.; ABREU, G.B.; NIJSEE, J.; HILHORST, H.W.M. Preliminarys studies on dormancy of Annona crassiflora Mart. seeds. In: INTERNATIONAL SYMPOSIUM ON PLANT DORMANCY, 3., Wageningen, 2004. Proceedings. Wageningen: Wageningen International Conference Centre, 2004. p.4.
- DA SILVA, E.A.A.; MELO, D.L.B.; DAVIDE, A.C.; BODE, N.; ABREU, G.B.; FARIA, J.M.R.; HILHORST, H.W. Germination Ecophysiology of Annona crassiflora Seeds. Annals of Botany, v.99, p.823-830, 2007.
- DECCETTI, S.F.C. Propagação in vitro de Annona glabra L. Lavras: Universidade Federal de Lavras, 2000. 101p. Dissertação (Mestrado).
- DINIZ, J.D.N.; ALMEIDA, J.L.; TEIXEIRA, A. L. A.; GOMES, E.S.; HERNANDEZ, F.F.F. Ácido giberélico (GA3) e 6-Benzilaminopurina (BAP) no crescimento in vitro de macela [Egletes viscosa (L.) Less.]. Ciência e Agrotecnologia, v.27, p.934-938, 2003.
- FERREIRA, D.F. Análises estatísticas por meio do Sisvar para Windows versão 4.0. In: REUNIÃO ANUAL DA REGIÃO BRASILEIRA DA SOCIEDADE INTERNACIONAL DE BIOMETRIA, 45., São Carlos, 2000. Anais São Carlos: UFSCar, 2000. p.255-258.
- GEORGE, A.P.; NISSEN, R.J. Propagation of Annona species: a review. Scientia Horticulturae, v.33, p.75-85, 1987.
- KOORNNEEF, M.; BENTSINK, L.; HILHORST, H.W.M. Seed dormancy and germination. Current Opinion in Plant Biology, v.5, p.33-36, 2002.
- LOYD, G.; McCOWN, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Combined Proceedings International Plant Propagators Society, v.30, p.421-427, 1980.
- MURASHIGE, T.; SKOOG, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiologia Plantarum, v.15, p.473-497, 1962.
- NAIR, S.; GUPTA, P.K.; SHIRGURKAR, M.V.; MASCARENHAS, A.F. In vitro organogenesis from leaf explants of Annona squamosa Linn. Plant Cell Tissue and Organ Culture, v.3, p.29-40, 1984.
- PASQUAL, M. Cultura de tecidos vegetais: tecnologia e aplicações; meios de cultura. Lavras: UFLA/FAEPE, 2001. 74p.
- PENG, J.; HARBERD, N.P. The role of GA-mediated signaling in the control of seed germination. Current Opinion in Plant Biology, v.5, p.376-381, 2002.
- SAMSON, J.A. Tropical fruits 2 ed. New York: New York Scientific & Technical, 1986. 335p.
- SPERA, M.R.N. Propagação in vitro de Jatropha podagrica Hook. Lavras: Universidade Federal de Lavras, 1995, 78p. Dissertação (Mestrado).
Publication Dates
-
Publication in this collection
29 June 2009 -
Date of issue
June 2009
History
-
Received
23 Apr 2008 -
Accepted
14 Nov 2008