Abstracts
Glyphosate is the herbicide with the greatest participation in the world market, and it has been used intensively in agriculture for over 30 years, especially because of its relatively low cost and high effectiveness on different species. This study describes a synthesis method of glyphosate in the form of the isopropylamine salt enriched in the stable nitrogen isotope (15N). The 15N-herbicide synthesis was performed using a phosphomethylation reaction with dialkyl phosphite and 15N-glycine. The tests were conducted at a microscale and equimolar quantities. A 20% yield was obtained at the established conditions. To test the effectiveness of the synthesized glyphosate, an experiment was performed in a growth chamber with the weed Lolium multiflorum (ryegrass), which is mentioned to be effectively controlled as indicated in package inserts ofisopropylamine salt-based products (glyphosate). The experimental design was completely randomized, with four replicates, and treatments consisted of isopropylamine salt sources at the commercial dose recommended for the product (720 g a.i. ha-1). The production of green and dry phytomass of the above-ground part and roots was evaluated 21 days after application (DAA), and the treatment toxicity was evaluated 7, 14, and 21 DAA. The isopropylamine salt sources did not differ, recognizing them as useful isotopic tracers.
Lolium multiflorum; isopropylamine salt; stable isotope
O glifosato é o herbicida de maior participação no mercado mundial, sendo utilizado intensivamente na agricultura há mais de 30 anos, principalmente devido ao custo relativamente baixo e à alta eficiência sobre diferentes espécies. Descreve-se um método para a síntese do glifosato na forma do sal isopropilamina enriquecido no isótopo estável do nitrogênio (15N). A síntese do herbicida-15N foi realizada utilizando-se da reação de fosfometilação com diaquil fosfito e glicina-15N. Os testes foram realizados em microescala e em quantidades equimolares. Foi possível obter um rendimento de 20%. Para testar a eficiência do glifosato sintetizado, realizou-se experimento em câmara de crescimento com a planta daninha Lolium multiflorum (azevém), a qual é eficazmente controlada segundo à bula dos produtos a base do sal de isopropilamina (glifosato). O delineamento experimental foi inteiramente casualizado, com quatro repetições, sendo os tratamentos provenientes de fontes de sal de isopropilamina na dose comercial recomendada do produto (720 g i.a. ha-1). Avaliou-se a produção de fitomassa fresca e seca, da parte aérea e radicular, aos 21 dias após a aplicação (DAA), e a toxicidade dos tratamentos aos 7, 14 e 21 DAA. As fontes de sal de isopropilamina empregados não diferiram, o que deverá permitir sua utilização como traçador isotópico.
Lolium multiflorum; sal de isopropilamina; isótopo estável
NOTE
15N-labeled glyphosate synthesis and its practical effectiveness
Síntese do glifosato marcado com nitrogênio-15 e sua eficiência prática
Claudinéia Raquel de Oliveira TavaresI; José Albertino BendassolliI, * * Corresponding author < jab@cena.usp.br> ; Daniela Neves RibeiroII; Alexssandra Luiza Rodrigues Molina RosseteI; Clélber Vieira PrestesI; Glauco Arnold TavaresI
IUSP/CENA - Lab. de Isótopos Estáveis, C.P. 96 - 13400-970 - Piracicaba, SP - Brasil
IIUSP/ESALQ - Programa de Pós-Graduação em Fitotecnia, Av. Pádua Dias, 11 - 13418-900 - Piracicaba, SP - Brasil
ABSTRACT
Glyphosate is the herbicide with the greatest participation in the world market, and it has been used intensively in agriculture for over 30 years, especially because of its relatively low cost and high effectiveness on different species. This study describes a synthesis method of glyphosate in the form of the isopropylamine salt enriched in the stable nitrogen isotope (15N). The 15N-herbicide synthesis was performed using a phosphomethylation reaction with dialkyl phosphite and 15N-glycine. The tests were conducted at a microscale and equimolar quantities. A 20% yield was obtained at the established conditions. To test the effectiveness of the synthesized glyphosate, an experiment was performed in a growth chamber with the weed Lolium multiflorum (ryegrass), which is mentioned to be effectively controlled as indicated in package inserts ofisopropylamine salt-based products (glyphosate). The experimental design was completely randomized, with four replicates, and treatments consisted of isopropylamine salt sources at the commercial dose recommended for the product (720 g a.i. ha-1). The production of green and dry phytomass of the above-ground part and roots was evaluated 21 days after application (DAA), and the treatment toxicity was evaluated 7, 14, and 21 DAA. The isopropylamine salt sources did not differ, recognizing them as useful isotopic tracers.
Key words:Lolium multiflorum, isopropylamine salt, stable isotope.
RESUMO
O glifosato é o herbicida de maior participação no mercado mundial, sendo utilizado intensivamente na agricultura há mais de 30 anos, principalmente devido ao custo relativamente baixo e à alta eficiência sobre diferentes espécies. Descreve-se um método para a síntese do glifosato na forma do sal isopropilamina enriquecido no isótopo estável do nitrogênio (15N). A síntese do herbicida-15N foi realizada utilizando-se da reação de fosfometilação com diaquil fosfito e glicina-15N. Os testes foram realizados em microescala e em quantidades equimolares. Foi possível obter um rendimento de 20%. Para testar a eficiência do glifosato sintetizado, realizou-se experimento em câmara de crescimento com a planta daninha Lolium multiflorum (azevém), a qual é eficazmente controlada segundo à bula dos produtos a base do sal de isopropilamina (glifosato). O delineamento experimental foi inteiramente casualizado, com quatro repetições, sendo os tratamentos provenientes de fontes de sal de isopropilamina na dose comercial recomendada do produto (720 g i.a. ha-1). Avaliou-se a produção de fitomassa fresca e seca, da parte aérea e radicular, aos 21 dias após a aplicação (DAA), e a toxicidade dos tratamentos aos 7, 14 e 21 DAA. As fontes de sal de isopropilamina empregados não diferiram, o que deverá permitir sua utilização como traçador isotópico.
Palavras-chave:Lolium multiflorum, sal de isopropilamina, isótopo estável.
Introduction
Glyphosate (C3H8NO5P), also known as N-phosphonomethyl glycine is the herbicide with the greatest participation in the world market. It is a non-selective, systemic, and post-emergent herbicide, included in the Brazilian toxicological classification (class IV - little toxic), as intensively used in weed control (Kertesz et al., 1995; Vencill, 2002). It is an easy-to-apply product, with relatively low cost and high effectiveness over different species (Giolo et al., 2005). Its herbicidal action was first reported in 1971. It is sold in the form of aqueous concentrates of water-soluble salts, facilitating its application (Franz, 1985). Although there are more than 90 commercial brands of glyphosate worldwide (Heap, 1997), all of them present the same action mechanism, regardless of the used salts (Hartzler, 2006; Giolo et al., 2005).
When this molecule is used indiscriminately, the precautions associated with its potential environmental contamination must be carefully evaluated (Prata et al., 2000), since there has been evidence of the appearance of resistant varieties, which will result in increased number of applications. Therefore, it becomes important to conduct studies dealing with the physicochemical properties of glyphosate, its interaction with water and soil, as well as the development of analytical tools that will allow its detection and quantification in natural samples.
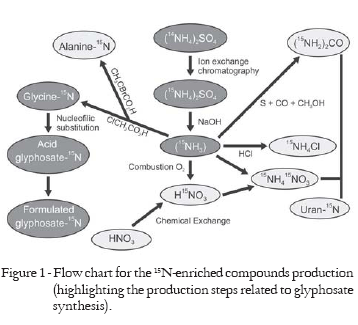
Considering that the technology for the separation and production of glyphosate compounds enriched with the 15N isotope is restrict, efforts were directed toward allowing the synthesis of the herbicide glyphosate, making an important tool available for evaluations of the fate of nitrogen in the soil-plant system, particularly in experiments conducted in the field, since no precautions with radioprotection would be needed, including the advantage of no generation of radiolabeled wastes.
In addition to synthesizing the compound in the laboratory, there is also the interested in evaluating whether the produced molecule has the same herbicidal potential as the commercial products. As a result, the effectiveness of sources of the isopropylamine salt was evaluated through the control of ryegrass (Lolium multiflorum), an annual, winter grass weed species that reproduces via seeds, characterized as typically sensitive to glyphosate. This species is frequently considered an undesired plant in wheat crops in Southern Brazil. (Roman et al., 2004).
Material and Methods
Production Methods
All steps required to produce 15N-enriche glyphosate are represented schematically in Figure 1. A series of nitrogen compounds have already been obtained in recent years (Tavares et al., 2006; Bendassolli et al., 2002; Máximo et al., 2005). Early studies were conducted using reagents with natural isotopic abundance (0.366% 15N atoms), considering that physical and chemical parameters would remain unchanged. Potential isotopic fractionation was evaluated from the production of glycine enriched with 0.9 or 6.7% 15N atoms.
Glyphosate synthesis from dialkyl phosphite and glycine (Patent No. 4,237,065 - 1980)
The mentioned patent reports to a process to obtain glyphosate in several steps, proposed by Ehrat and Dinhard (1980), aimed to use low-cost reagents that can be easily obtained in the market, as well as being exempt of purification methods in the final product. The reaction can be seen in the flow chart presented in Figure 2. According to the proposed synthesis, paraformaldehyde is dissolved in an alcohol under simultaneous depolarization, in the presence of a tertiary base (catalyst agent). Glycine is added to the obtained solution, forming hydroxymethylglycine as an intermediate product. This intermediate compound reacts immediately, under reflux, with added dialkyl phosphite. The resulting ester is saponified by the addition of a 30% NaOH solution (w/v); after this step, the tertiary base and the alcohol are removed. Finally, the obtaind alkaline solution is acidified with the precipitation of N-phosphonomethyl glycine.
Under particular conditions, the amino acid glycine can be considered a simple amine soluble in alcohol or water. Glycine reacts extraordinarily as an amine in the Mannich reaction, involving formaldehyde, amine, and diethyl phosphite, resulting in phosphonomethyl amine. Nevertheless, with the conditions established in the patented invention the Mannich reaction does not produce an amine, but n-phosphonomethyl glycine. In this reaction, 1.5 mol paraformaldehyde are initially dissolved in 500 mL methanol, under simultaneous depolarization, in the presence of 1 mol triethylamine (tertiary base). One mol of glycine is added to the obtained solution, reacting with the formaldehyde from depolarization process, in a condition known as Mannich reaction (Holy et al., 1979). The solution is maintained at 70°C for 60 min, under reflux. Next, the solution is completed with 1 mol dialkyl phosphite by slowly dripping for 30 minutes. After agitation for 90 min, the initial solution generates an ester, which is then saponified with 3.5 mol of a 30% sodium hydroxide solution (w/v). In this step, triethylamine and methanol are recovered by fractionated distillation at 70°C. Immediately after recovering the solvents, the system goes back into reflux at 105°C for a period of 2 h. Next, the obtained alkaline solution is acidified with concentrated hydrochloric acid until pH 1.5. The mixture is left to rest at 15°C for 3 to 4 h, during which glyphosate becomes crystallized and is then separated by filtration. A reaction yield of about 48 to 52% is achieved, producing a weight ranging from 81 to 88 g.
The preliminary tests were conducted with three replicates, at a reduced scale of 1:100, using glycine with natural isotopic abundance (0.366% 15N atoms). After establishing the best experimental conditions, two tests were made, starting from glycine with isotopic abundances of 6.70 and 0.90% in 15N atoms, in order to evaluate potential isotopic fractionation during the process. The system used in the synthesis process was identical to the one illustrated in Figure 2. The isotopic determination in the synthesized glyphosate was made using an ANCA-SL 20/20 mass spectrometer.
After synthesis, the determinations of interest were made in order to confirm the presence of the herbicide, based on fusion tests, infrared spectroscopy, and total nitrogen content determination by mass spectrometry. The synthesized samples were compared against the Sigma standard (p.a. grade). Another possibility to confirm the presence of the herbicide would be to start by nuclear magnetic resonance in the solid phase (Li and Evans, 1995).
Production of formulated Glyphosate
One of the steps in obtaining the formulated herbicide consists in the synthesis of the glyphosate salt, which can be achieved by neutralizing the acid glyphosate with a base, such as monoisopropylamine. This step of the production process is referred to as pre-mixture or first formulation step, containing a 62% acid concentration.
Acid (or solid) glyphosate is relatively insoluble in water, making its application in the field not viable. Because of this, glyphosate is typically formulated in the form of salts, making the molecule soluble. This particular trait allowed several salts to be obtained with a commercial objective in mind. Studies comparing the efficiency of various salts have demonstrated that isopropylamine has an increased phytotoxicity in plant. However, the salt, in association with a surfactant, increases absorption by the plant (Duke, 1988).
In this investigation, it was chosen to obtain the herbicide under the same conditions of the commercial product. To achieve that, the acid glyphosate obtained in the previous step was submitted to amination with 13.5% oleamide monoisopropylamine (MIPA), in association with 13% tensioactive agent (surfactant ultramine 200).
Ultramine 200 is an ethoxylated fatty amine with moisturizing characteristics. It is recommended for agrochemical formulations in its pure or combined form as a moisturizing or solubilizing agent in soluble concentrates of water-based herbicidal formulations, with a spreading effect and as an aid to improve penetration.
Samples obtained from this chemical reaction were analyzed by HPLC with a fluorescence detector, using middle-infrared spectroscopy for solid and liquid samples. Two assays were conducted to determine the 15N isotopic dilution in the formulated glyphosate, using glycine enriched at 6.7 or 0.9% in 15N atoms as precursors.
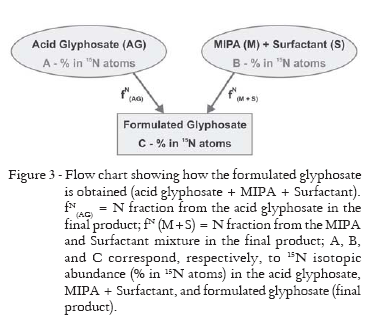
The contribution of the nitrogen source from the acid glyphosate and from amination (MIPA), as well as from the tensioactive agent (surfactant) can be determined in the final product based on the isotopic mass balance. Figure 3 illustrates schematically how the glyphosate formulation is obtained from the MIPA acid added of Surfactant (S). The isotopic mass balance equation can be represented by Equation 1.
where: A, B, and C: isotopic abundances (% in 15N atoms) in the acid glyphosate, MIPA + Surfactant mixture, and formulated glyphosate, respectively; FN(AG) and FN(M+S) = nitrogen fraction in the final product, from the acid glyphosate and the MIPA and Surfactant mixture, respectively.
Considering that the MIPA (oleamide monoisopropylamide) and Surfactant (ultramine) mixture have natural isotopic abundance (0.366% in 15N atoms), and also the nitrogen mass balance given by equation 2, equation 3 can be obtained.
Effectiveness test for the synthesized product
The experiment was performed in a growth chamber at 24/19°C day/night with 250 µmolm-2 s photosynthetically active radiation, and a 16-h photoperiod. The relative humidity oscillated between 60 and 80%. These conditions approximately correspond to the normal ones found in the field when glyphosate is applied. The weed Lolium multiflorum, which is susceptible to the herbicide glyphosate was used in this study. The seeds were initially placed to germinate in trays. When 50% + 1 plants reached the two-leaf phenological stage, they were transplanted to the experimental plots, at a density of threeplants per pot. The plots consisted of 0.5 L-pots filled with commercial substrate and fertilized with a dose proportional to the application of 500 kg ha1 of the commercial formulation 10-10-10 (N-P2O5-K2O), daily irrigated. The experimental design was completely randomized with four replicates.
The treatments consisted of isopropylamine salt sources at the commercial dose recommended for the control of Lolium multiflorum, corresponding to 720 g a.i. ha-1 plus a control treatment. The treatments were applied when the plants had four completely-expanded leaves, on average. The sprays were made in a closed application chamber, using a flat-fan nozzle, with the jet calibrated at a 50 cm height from the target surface, with a relative mix volume in the order of 200 L ha-1. After the herbicide had been applied, the pots were returned to the growth chamber and they were irrigated on the following day, ensuring the leaf absorption of the herbicide.
Treatment toxicity evaluations were made at 7, 14, and 21 days after application (DAA), using a percentage scale, in which a zero rating meant no damage effect to the plant and a 100 rating represented complete plant suppression or death. After the last evaluation, the above-ground part and root of the plants were collected to determine green and dry mass yield. All sampled materials were dried in a forced air circulation oven at 60°C for 72 h, allowing dry phytomass determination.
The F test on analysis of variance was applied to the obtained data. The means for green and dry phytomass data and weed control treatment toxicity values were compared by the Tukey test (p < 0.05).
Results and Discussion
Glyphosate synthesis from dialkyl phosphite and glycine (Patent No. 4,237,065 - 1980)
The evaluation of the parameters involved in the production of glyphosate from synthesized glycine and dialkyl phosphite, a process established by Erhat & Dinhard, (1980), showed that this is the most promising alternative for glyphosate synthesis under the operatinonal conditions available at the Stable Isotopes Laboratory of CENA.
To evaluate the N percentage in the herbicide, synthesis assays were performed using glycine p.a. (Sigma) and the laboratory-synthesized glycine as the starting points. The results for these tests are presented in Table 1. The glyphosate synthesized from dialkyl phosphite had N content (%) and isotopic abundance (% in 15N atoms) similar to the Sigma and the standards (commercial standard). The natural isotopic abundance of the heavy nitrogen isotope is 0.366 ± 0.004. According to the infrared analyses, the results were similar between synthesized samples and the Sigma standard.
Under the tested conditions, a yield about 26% was obtained as compared to the 50% yield predicted in the literature (Table 2). The use of synthesized or natural glycine did not influence the final result.
Production of formulated Glyphosate
In relation to the samples analyzed by HPLC via a FMOC derivative, a similar retention time between the standard (27.60 min), the synthesized sample with natural abundance (27.56 min), and the synthesized 15N-labeled sample (27.55) was observed. When compared against the standard, the results apparently indicate the same substance. The results can be seen in Figures 4, 5, and 6, respectively, for the standard, the synthesized sample with natural abundance, and the synthesized 15N-labeled sample.
At the same time, analyses were made using middle infrared with raw samples. When the glyphosate sample with natural abundance, the 15N-labeled sample, and the formulated standard provided (commercial product) were compared, the emitted peaks practically overlapped. These tests were conducted using a Thermo Nicolet IR200 spectrometer with Fourier transform (FTIR).
Regarding the 15N-enriched glyphosate samples, in which glycine previously enriched in the isotope was used (6.70 or 0.90% in 15N atoms), it was possible to obtain acid and formulated glyphosate with the isotopic characteristics listed in Table 3. The same Table presents the isotopic values and contributions of both nitrogen sources (N-acid glyphosate and N-MIPA + Surfactant) in the formulated product (acid glyphosate + MIPA + Surfactant). The contribution from each nitrogen source was obtained from isotopic balance equations.
There was no isotopic fractionation in the conversion from 15N-glycine into acid glyphosate, and the isotopic dilution while obtaining the formulated glyphosate was practically the same of the assays that used acid glyphosate containing 6.70 or 0.90% in 15N atoms (Table 3). The 15N isotopic dilution in the final (formulated) product due to the MIPA and Surfactant introduction does not represent a problem in the use of the herbicide as tracer, considering that what must be taken into account is the active principle enrichment.
Effectiveness Test
Equal toxicity response injuries were obtained among the tested treatments, only differing from the control, in the evaluated periods (Figure 7). Consequently, in relation to the control effectiveness parameter against the weed Lolium multiflorum by different isopropylamine salt sources, all of them were not statistically different. The variation coefficient found in the variable above-ground for fresh and dry mass were 22.47% and 24.28%, respectively; in the root for fresh and dry mass were 46.74% and 25.68%, respectively, and in the control percentage was 33.86%.
For each treatment, a general decrease in fresh of the root system was observed relative to its corresponding control. These results agree with those obtained in the toxicity evaluation (Figures 7 and 8). This pattern was also maintained for dry mass of the above-ground part of Lolium multiflorum (Figure 8). The fresh and dry phytomass for the root system had the same pattern observed for the above-ground part of this plant. However, smaller decreases than those for the above-ground part of Lolium multiflorum were observed relative to the control. (Figure 8).
Conclusion
The method that best suited the interests of the proposed study was the one that employed dialkyl phosphite, allowing acid glyphosate to be obtained with a yeld of about 25%, corresponding to 0.44 g glyphosate in the acid form, starting from 0.75 g 15N-glycine. Regarding the glyphosate formulated in the isopropylamine salt form, an isotopic dilution of about 60% was obtained. However, in the production of highly enriched glyphosate (80 90% in 15N atoms) this problem can be overcome. As a result, active principle-labeling becomes the factor that should be taken into consideration. The herbicide can be obtained under the same conditions as the commercial product, including a lack of formation of undesired impurities in the synthesis pathway.
Acknowledgements
The authors thank FAPESP for the financial support.
Received September 10, 2007
Accepted August 21, 2009
- Bendassolli, J.A.; Trivelin, P.C.O.; Ignoto, R.F. 2002. Produção de amônia anidra e aquamônia enriquecida em 15N a partir de (15NH4)2SO4 . Scientia Agricola 59: 595-603.
- Duke, S. 1988. Glyphosate. p.1-57. In: Kearney, P.C.; Kaufman, D.D., eds. Herbicides Chemistry, Degradation, and Mode of Action. Marcel Dekker, New York, NY, USA.
- Ehrat, R.; Dinhard, S. 1980. Process for the preparation of N-Phosphonomethyl Glycine. Biological Chemical Activities Patents (US Patent 4,237,065). Luxemburgo - LU.
- Franz, J.E. 1985. Discovery, development and chemistry of glyphosate. p.1-17. In: Grossbard, E.; Atkinson, D., eds. 1985. The Herbicide Glyphosate. Butterworths, CIDADE, UK.
- Giolo, F.P.; 2005. Grützmacher, A.D.; Procópio, S.O.; Manzoni, C.G; Lima, C.A.B.; Nörnberg, S.D. Seletividade de formulações de glyphosate a Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Planta Daninha 23: 457-462.
- Hartzler, B. 2006. Which glyphosate product is best? Available at: http/www.weeds.iastate.edu/mgmt/qtr01-1/glyphosateformulations.htm [Accessed Jan. 25, 2006]
- Heap, I.M. 1997. The occurrence of herbicide resistant weeds world wide. Pesticide Science 51: 235-243.
- Holy, N.; Fowler, R.; Burnett, E.; Lorenz, R.1979. Tetrahedron 35: 613.
- Kertesz, M.A.; Cook, A.M.; Leisinger, T. 1995. Microbial metabolism of sulfur and phosphorus containing xenobiotics. FEMS Microbiology Review 15: 195-215.
- Li, Y.; Evans, J.N.S. 1995. The importance of XY-8 Phase Cycling in the Rotational-Echo Double-Resonance Experiment with Total Sideband Suppression. Journal of Magnetic Resonance Series A 116: 150-155.
- Máximo, E.; Bendassolli, J.A.; Trivelin, P.C.O.; Rossette, A.L.M.; Oliveira, C.R.O.; Prestes, C.V. 2005. Produção de sulfato de amônio duplamente marcado com os isótopos estáveis 15N e 34S. Química Nova28: 211-216.
- Prata, F.; Lavorenti, A.; Regitano, J.B.; Tornisielo, V. 2000. Influência da matéria orgânica na sorção e dessorção do glifosato em solos com diferentes atributos mineralógicos. Revista Brasileira de Ciência do Solo 24: 945-951.
- Roman, E.S.; Vargas, L.; Rizzardi, M.A.; Mattei, R.W. 2004. Resistência de azevém (Lolium multiflorum) ao herbicida glyphosate. Planta Daninha 22: 301-306.
- Tavares, C.R.O.; Bendassolli, J.A.; Coelho, F.; Sant´ana Filho, C.R.; Prestes, C.V. 2006. 15N-Labeled glycine synthesis. Anais da Academia Brasileira de Ciências 78: 441-449.
- Vencill, W.K., ed. 2002. Herbicide handbook. 8ed. Weed Science Society of America, Lawrence, KS, USA.
Publication Dates
-
Publication in this collection
16 Mar 2010 -
Date of issue
Feb 2010
History
-
Received
10 Sept 2007 -
Accepted
21 Aug 2009