Abstracts
Single superphosphate is currently one of the mostly used fertilizers as an alternative source for phosphorus and sulphur. Sulphur presents four stable isotopes (32S, 33S, 34S, and 36S) with natural abundances of 95.00; 0.76; 4.22; and 0.014% in atoms, respectively. Single superphosphate labeled with the 34S isotope was obtained from a chemical reaction in stoichiometric amounts between Ca(H2PO4)2 and Ca34SO4.2H2O. Calcium sulphate (Ca34SO4.2H2O) was enriched with 5.85 ± 0.01 atoms % of 34S. The Ca(H2PO4)2 reagent was obtained from a reaction between CaCl2.2H2O and H3PO4. The reaction between the Ca(H2PO4)2 thus produced and the labeled Ca34SO4.2H2O compound was then performed to obtain the 34S-labeled single surperphosphate. The thermal decomposition of the labeled superphosphate for the production of gaseous 34SO2 was carried out under a vacuum line at 900ºC in the presence of NaPO3. The isotopic determination of S (atoms % of 34S) was carried out on an ATLAS-MAT model CH-4 mass spectrometer. The production yield of Ca(H2PO4)2 and labeled single superphosphate were approximately 97 and 99% respectively, and the purity level of the labeled single superphosphate was estimated as 96%. No isotopic fractionation was observed in the production process of 34S-labeled single superphosphate.
isotopic determinations; sulphur; stable isotopes; mass spectrometry; labeled compounds
O superfosfato simples é um dos fertilizantes mais utilizados atualmente como fonte de fósforo e uma alternativa para enxofre. O enxofre apresenta quatro isótopos estáveis, 32S, 33S, 34S e 36S, com abundância natural de 95,00; 0,76; 4,22 e 0,014% em átomos, respectivamente. O superfosfato simples marcado com 34S foi obtido a partir da reação química em proporção estequiométrica entre o Ca(H2PO4)2 e o Ca34SO4.2H2O. O Ca34SO4.2H2O foi enriquecido com 5,85 ± 0,01% em átomos de 34S. O Ca(H2PO4)2 foi obtido a partir da reação entre CaCl2.2H2O com o H3PO4. A decomposição térmica do superfosfato marcado para produção do 34SO2 gasoso foi realizada em linha de vácuo a 900ºC na presença de NaPO3. A determinação isotópica do S (% em átomos de 34S) foi realizada no espectrômetro de massas. O rendimento da produção do Ca(H2PO4)2 e do superfosfato simples marcado foi em média 97 e 99%, respectivamente, e a pureza do superfosfato marcado foi estimada como 96%. Não foi observado fracionamento isotópico no processo de produção do superfosfato simples marcado com 34S.
determinação isotópica; enxofre; isótopos estáveis; espectrometria de massas; compostos marcados
NOTE
Production of single superphosphate labeled with 34S
Produção de superfosfato simples marcado com 34S
Alexssandra Luiza Rodrigues Molina RosseteI; Josiane Meire Toloti CarneiroI; José Albertino BendassolliII, * * Corresponding author < jab@cena.usp.br> ; Claudineia Raquel Oliveira TavaresII; Carlos Roberto Sant'Ana FilhoII
IUSP/CENA - Programa de Pós-Graduação em Ciências
IIUSP/CENA - Lab. de Isótopos Estáveis - C.P. 96 - 13400-970 - Piracicaba, SP - Brasil
ABSTRACT
Single superphosphate is currently one of the mostly used fertilizers as an alternative source for phosphorus and sulphur. Sulphur presents four stable isotopes (32S, 33S, 34S, and 36S) with natural abundances of 95.00; 0.76; 4.22; and 0.014% in atoms, respectively. Single superphosphate labeled with the 34S isotope was obtained from a chemical reaction in stoichiometric amounts between Ca(H2PO4)2 and Ca34SO4.2H2O. Calcium sulphate (Ca34SO4.2H2O) was enriched with 5.85 ± 0.01 atoms % of 34S. The Ca(H2PO4)2 reagent was obtained from a reaction between CaCl2.2H2O and H3PO4. The reaction between the Ca(H2PO4)2 thus produced and the labeled Ca34SO4.2H2O compound was then performed to obtain the 34S-labeled single surperphosphate. The thermal decomposition of the labeled superphosphate for the production of gaseous 34SO2 was carried out under a vacuum line at 900ºC in the presence of NaPO3. The isotopic determination of S (atoms % of 34S) was carried out on an ATLAS-MAT model CH-4 mass spectrometer. The production yield of Ca(H2PO4)2 and labeled single superphosphate were approximately 97 and 99% respectively, and the purity level of the labeled single superphosphate was estimated as 96%. No isotopic fractionation was observed in the production process of 34S-labeled single superphosphate.
Key words: isotopic determinations, sulphur, stable isotopes, mass spectrometry, labeled compounds
RESUMO
O superfosfato simples é um dos fertilizantes mais utilizados atualmente como fonte de fósforo e uma alternativa para enxofre. O enxofre apresenta quatro isótopos estáveis, 32S, 33S, 34S e 36S, com abundância natural de 95,00; 0,76; 4,22 e 0,014% em átomos, respectivamente. O superfosfato simples marcado com 34S foi obtido a partir da reação química em proporção estequiométrica entre o Ca(H2PO4)2 e o Ca34SO4.2H2O. O Ca34SO4.2H2O foi enriquecido com 5,85 ± 0,01% em átomos de 34S. O Ca(H2PO4)2 foi obtido a partir da reação entre CaCl2.2H2O com o H3PO4. A decomposição térmica do superfosfato marcado para produção do 34SO2 gasoso foi realizada em linha de vácuo a 900ºC na presença de NaPO3. A determinação isotópica do S (% em átomos de 34S) foi realizada no espectrômetro de massas. O rendimento da produção do Ca(H2PO4)2 e do superfosfato simples marcado foi em média 97 e 99%, respectivamente, e a pureza do superfosfato marcado foi estimada como 96%. Não foi observado fracionamento isotópico no processo de produção do superfosfato simples marcado com 34S.
Palavras-chave: determinação isotópica, enxofre, isótopos estáveis, espectrometria de massas, compostos marcados
INTRODUCTION
Phosphorus is essential for plant development, helping in cell division and stimulating root development. In the industry, the decomposition of rock phosphate with sulphuric acid yields many different kinds of phosphate compounds, including the Single Superphosphate (SSP), which is one of the most important fertilizers applied as a phosphorus source for plants. These compounds also contain sulphur (S) (Havlin et al., 2005), an element presenting deficiency in different soils and plants (Bissani & Tedesco, 1988; Tisdale et al., 1986). Isotope dilution techniques and the use of compounds labeled with stable isotopes have been widely employed in order to obtain information about the cycle of many elements especially nitrogen and sulphur (Awonaike et al., 1993).
Sulphur presents four stable isotopes (32S, 33S, 34S, and 36S) with natural abundances of 95.02; 0.75; 4.21; and 0.02% in atoms, respectively (Krouse et al., 1996). Most studies based on the application of labeled S have used the 35S radioisotope (Sharma & Kamath, 1991), however, the application of compounds labeled with a stable isotope like 34S presents advantages especially due to the fact of not being radioactive. Today it is important to stress a world tendency to use stable isotopes as tracers to replace radioactive techniques, especially in studies involving field experiments (Zhao et al., 2001). The first studies based on the application of 34S-labeled stable isotopes were developed by Hamilton et al. (1991), Awonaike et al. (1993), and Trivelin et al. (2002). Studies aiming at the separation of the 34S isotope developed by Bendassolli et al. (1997) were able to produce many different labeled compounds such as (15NH4)234 SO4, K234SO4, H234SO4 and Ca34SO4.2H2O (Maximo et al., 2005; Rossete et al., 2006).
The aim of the present work was to produce a single superphosphate labeled with the 34S isotope in the form of 3Ca(H2PO4)2 + 7Ca34SO4.2H2O and the subsequent S isotopic determination (atoms % of 34S) by mass spectrometry.
MATERIAL AND METHODS
The ATLAS-MAT model CH4 mass spectrometer was equipped with an admission system by molecular flow and a single ion collector with a Faraday cup. The vacuum line was built of Pyrex® and quartz, and the system comprised: an Edwards model 2M8 mechanical vacuum pump; an Edwards model E050 high vacuum diffusion pump; an Edwards active vacuum gauge display (AGD), and an Edwards model APG-M Pirani vacuum filament display. The following reagents, all of analytical grade, were used: CaSO4.2H2O; CaCl2.2H2O; H3PO4 and NaH2PO4.H2O.
Production of 34S-labeled single superphosphate (SSP)
The SSP labeled with the 34S isotope was obtained from a chemical reaction in stoichiometric amounts between Ca(H2PO4)2 and Ca34SO4.2H2O. Calcium sulfate (Ca34SO4.2H2O) enriched with 5.85 ± 0.01 atoms % of 34S was initially produced as shown by Rossete et al. (2006). The Ca(H2PO4)2 reagent was obtained from the chemical reaction between CaCl2.2H2O and H3PO4 in stoichiometric amounts, according to the reaction shown in Equation 1.
After running for approximately 72 h, the produced Ca(H2PO4)2 was dried at 60ºC to eliminate water and the excess acid possibly formed during the reaction, and its yield production was evaluated by gravimetry. Next, 10 mg Ca(H2PO4)2 were dissolved into 100 mL water and phosphate/calcium concentrations were determined by ICP-AES (Gine et al., 2004). To verify the yield production of contaminant in the production process, the chloride concentration was determined by spectrometry (Zagatto et al., 1981). Next, the reaction between the produced Ca(H2PO4)2 and the 34S-labeled Ca34SO4.2H2O (5.81 ± 0.01 atoms % of 34S) was developed. After running for 7 days, the labeled SSP was dried at 60ºC and its yield production was evaluated by gravimetry.
Sample preparation and S isotopic determination
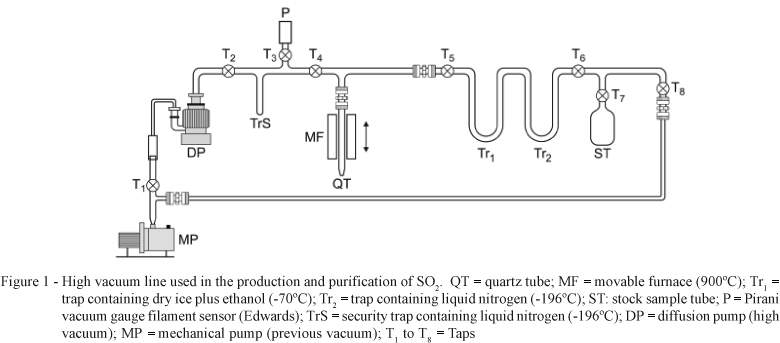
The high vacuum line used in the production and purification of SO2 with subsequent 34S isotopic determination (atoms % of 34S) by mass spectrometry is shown in Figure 1. According to the proposed procedure, 10.00 mg of labeled SSP (approximately 1.35 mg S) and 60 mg sodium metaphosphate were placed inside a quartz tube of 30 cm length. The sodium metaphosphate was obtained by submitting approximately 10 g NaH2PO4.H2O at 200ºC for 2 h to remove structural water and possible organic compounds formed. The mass ratio between superphosphate and NaPO3 was 1:6 (w/w) (Halas & Wolacewicz, 1981). The quarts tube (QT) was then connected into the high vacuum line (Figure 1) and the vacuum system was activated by mechanic (MP) and diffusion (DP) pumps. A bottle containing dry ice plus ethanol solution (-70ºC) and another containing liquid nitrogen (-196ºC) were introduced around the Tr1 and Tr2 traps, respectively. The objective of the traps was to retain water vapor in Tr1 and SO2 (formed during the combustion process) in Tr2. The high vacuum line configuration was based on the method developed by Bailey & Smith (1972).
In the next step, the MF furnace adjusted to 900ºC is moved to the QT tube and is heated for approximately 10 min to produce SO2 after the combustion reaction between SSP and NaPO3. Depending on the O2 partial pressure, an undesirable production of SO3 may occur, with consequent isotopic fractionation. In order to retain oxide compounds formed during the combustion process and to convert SO3 to SO2 thus avoiding isotopic fractionation (Yanagisawa & Sakal, 1983; Rafter, 1957) a metallic copper ring supported by a piece of quartz wool was placed inside the QT tube approximately 2 cm above the volume occupied by the sample and the NaPO3 reagent. The SO2 gas retained inside the Tr2 trap was then transferred into the stock tube (ST) by replacing the bottle containing liquid N2 from the trap Tr2 to the stock sample tube (ST). Next, the ST tube containing SO2 gas was removed from the high vacuum line and connected to the mass spectrometer admission system and the isotopic determination of S (atoms % of 34S) was accomplished (Bendassolli et al., 1997).
The mass spectrometer equipment worked with an admission system heated to 60ºC due to the polarity of the SO2 molecules, thus avoiding interference on the analysis. A cryogenic trap (Tr1) containing dry ice and ethanol (-70ºC) was adapted on the spectrometer admission system to retain water from coming the samples.
RESULTS AND DISCUSSION
With regard to the stoichiometric reaction between H3PO4 and CaCl2.2H2O, using 14.7 g of CaCl2.2H2O for each test and slowly adding 13.5 mL of concentrated H3PO4, it should be theoretically possible to obtain 27.0 g Ca(H2PO4)2, considering the HCl mass formed in the process. Based on the theoretical value, the yield conversion (%) and total Ca(H2PO4)2 mass lost in the process were calculated. Results are in Table 1, where the Ca(H2PO4)2 mass produced and the yield conversion values for each test can be seen.
The concentrations of Ca2+ and PO42- samples produced in Ca(H2PO4)2 as determined by ICP-AES (Gine et al., 2004) were estimated to be 17 and 80% (w/w), respectively. Results were considered satisfactory and in agreement with the gravimetric method.
In relation to the chlorine determination exploring the flow injection analysis system (FIA), the contamination by chlorine in the Ca(H2PO4)2 production process was approximately 5%. This contamination is not significant, thus allowing the application of the 34S-labeled compound in agronomic studies without interferences. After the analysis related to the yield production of 34S-labeled SSP by the gravimetric method, results showed yield conversion values of approximately 99%, with a purity level in the labeled compound estimated approximately at 96%, thus confirming the efficiency of the proposed method. In relation to the results obtained in the isotopic determination of S (atoms % of 34S) in the labeled compound (Ca34SO4.2H2O) by mass spectrometry, no isotopic fractionation was observed. The results obtained by S isotopic determination (atoms % of 34S) in the mass spectrometry using CaSO4.2H2O p.a. with natural abundance and two different S compounds for the production of 34S-labeled SSP can be observed in Table 2.
Results obtained with the CaSO4.2H2O analysis were satisfactory (Table 2), since the natural abundance of the compound is approximately 4.15 to 4.38 atoms % of 34S. Results for 34S determination (atoms % of 34S) using different compounds prove the efficiency of the proposed method, especially in relation to the production of SO2 in the presence of NaPO3 and the isotopic determination of 34S by mass spectrometry.
CONCLUSIONS
The proposed method is feasible and suitable for the production of 34S-labeled single superphosphate by the reaction between Ca(H2PO4)2 and Ca34SO4.2H2O, and for the production of SO2 in a high vacuum line with S isotopic determination (atoms % of 34S) by mass spectrometry. The application of the 34S stable isotope can help identify sulphur sources. Therefore, the production of 34S-labeled single superphosphate represents an important alternative to the application of 34S aiming at studies related to sulphur dynamics in the soil-plant system.
ACKNOWLEDGEMENTS
To FAPESP for partial support and infra-structure.
Received July 28, 2006
Accepted September 18, 2007
- AWONAIKE, K.O.; DANSO, S.K.A.; ZAPATA, F. The use of a double isotope (N-15 and S-34) labeling technique to assess the suitability of various reference crops for estimating nitrogen fixation in gliricidia-sepium and leucaena-leucocephala. Plant and Soil, v.155-156, p.325-328, 1993.
- BAILEY, S.A.; SMITY, I.W. Improved method for the preparation of sulphur dioxide from Barium sulphate for isotope ratio studies. Analytical Chemistry, v.44, p.1542-1543, 1972.
- BENDASSOLLI, J.A.; TRIVELIN, P.C.O.; CARNEIRO JR., F.C. Stable sulphur isotope fractionation by anion exchange chromatography production of compounds enriched in 34S. Journal of Brazilian Chemical Society, v.8, p.13-17, 1997.
- BISSANI, C.A.; TEDESCO, M.J. O enxofre no solo. In: REUNIÃO BRASILEIRA DE FERTILIDADE DO SOLO, 17., Londrina, 1988. Enxofre e micronutrientes na agricultura. Londrina: EMBRAPA, IAPAR, 1988. p.11.
- GINE, M.F.; BELLATO, A.C.S.; MENEGARIO, A.A. Determination of trace elements in serum samples by isotope dilution inductively coupled plasma mass spectrometry using on-line dialysis. Journal of Analytical Atomic Spectrometry, v.19, p.1252-1256, 2004.
- HALAS, S.; WOLACEWICZ, W. Direct extraction of sulphur dioxide from sulfates for isotopic analysis. Analytical Chemistry, v.53, p.686-689, 1981.
- HAMILTON, S.D.; CHALK, P.M.; UNDOVICH, M.J.; SMITH, C.J. The measurement of fertilizer- S uptake by plants using radioactive and stable isotopes. Applied Radiation and Isotopes, v.42, p.1099-1101, 1991.
- HAVLIN, J.L.; BEATON, J.D.; TISDALE, S.L.; NELSON, W.L. Phosphorus. In: Soil fertility and fertilizers Englewood Cliffs: Pearson Prentice Hall, 2005. p.160-197.
- KROUSE, H.R.; BERNHARD, M.; SCHOENAU, J.J. Applications of stable isotopes techniques to soil sulphur cycling. In: BOUTTON, T.W.; YAMASAKI, S. (Ed.) Mass spectrometry of soil New York: Marcel Dekker, 1996. p.246-285.
- MAXIMO, E.; BENDASSOLLI, J.A.; TRIVELIN, P.C.O.; ROSSETE, A.L.R.M.; OLIVEIRA, C.R.; PRESTE, C.V. Produção de sulfato de amônio duplamente marcado com os isótopos estáveis 15N e 34S. Química Nova, v. 28, p. 211-216, 2005.
- RAFTER, T.A. The preparation of sulphur dioxide for mass spectrometer examination. Journal of Science and Technology, v.38, p.849-857, 1957.
- ROSSETE, A.L.R.M.; BENDASSOLLI, J.A.; MAXIMO, E.; SANT ANA FILHO, C.R.; IGNOTO, R.F. Production of 34S labeled gypsum (Ca34SO42H2O). Scientia Agricola, v.63, p.399-404, 2006.
- SHARMA, V.K.; KAMATH, M.B. Effect of sulphur, phosphorus and calcium on sulphur utilization by mustard (Brassica juncea L.) and Pea (Pissum sativum L.). Journal of Nuclear Agriculture and Biology, v.20, p.123-127, l991.
- TISDALE, S.L.; RENEAU, R.B.; PLATOU, J.S. Atlos of sulphur deficiencies. In: TABATABAI, M.A. (Ed.) Sulphur in agriculture Madison: ASA, CSSA, SSSA, 1986. p.295-322.
- TRIVELIN, P.C.O.; BENDASSOLLI, J.A.; CARNEIRO JR., F.; MUROAKA, T. Sulphur utilization by rice and crotalaria juncea from sulfate-34S applied to the soil. Scientia Agricola, v.59, p.205-207, 2002.
- YANAGISAWA, F.; SAKAL, H. Thermal decomposition of barium sulfate-vanadium pentaoxide-silica glass mixtures for preparation of sulphur dioxide in sulphur isotope ratio measurements. Analytical Chemistry, v.55, p.985-987, 1983.
- ZAGATTO, E.A.G.; JACINTO, A.O.; REIS, B.F.; KRUG, F.J.; PESSENDA, L.C.R.; MORTATTI, J.; GINÉ, M.F. Manual de análise de plantas e águas empregando sistemas de injeção em fluxo Piracicaba: USP/CENA, 1981. 45p.
- ZHAO, F.J.; VERKAMPEN, K.C.J.; BIRDSEY, M.; BLAKE-KALFF, M.M.A.; McGRATH, S.P. Use of the enriched stable isotope 34S to study sulphur uptake and distribution in wheat. Journal of Plant Nutrition, v.24, p.1551-1560, 2001.
Publication Dates
-
Publication in this collection
17 Jan 2008 -
Date of issue
Feb 2008
History
-
Accepted
18 Sept 2007 -
Received
28 July 2006