Abstracts
The effect of cadmium (Cd) on mycorrhizal association and on shoot and root Cd concentration was investigated in jackbean plants under hydroponic conditions. The treatments consisted of the inoculation of three different species of arbuscular mycorrhizal fungi (AMF), Glomus etunicatum, G. intraradices and G. macrocarpum, and a non-inoculated control, two Cd (0 and 5 µmol L-1) and two P (1 and 10 mg L-1) levels in the nutrient solution. Mycorrhizal colonization, length of AMF extraradical mycelium, guaiacol peroxidase activity in roots, plant growth and root and shoot Cd and P concentrations were determined. Mycorrhizal status did not promote jackbean growth but in most of the cases mycorrhization increased root and shoot Cd concentrations. Cd ions were accumulated mainly in roots and only small amounts were translocated to the shoot. Cd addition did not affect root colonization by AMF but the AM extraradical mycelium (ERM) was sensitive to the added Cd. ERM length was reduced by 25% in the presence of Cd. This reduction was more pronounced under conditions of low P concentration. Also at this P concentration, Cd addition decreased guaiacol peroxidase activity in non-mycorrhizal roots and in roots colonized by G. macrocarpum. However, mycorrhizal roots maintained lower values of peroxidase activity. G. etunicatum showed the best performance when associated to jackbean plants and it could be a promising association for phytoremediation of Cd- contaminated soil.
peroxidase; Cd accumulation; extraradical mycelium; phytoremediation; hydroponics
O efeito do cádmio na associação micorrízica e no teor e acúmulo de Cd na raiz e parte aérea de feijão de porco foi avaliado em condição de hidroponia. Os tratamentos consistiram da inoculação ou não de três espécies de fungos micorrízicos arbusculares (FMAs), Glomus etunicatum, G. intraradices e G. macrocarpum, e uma testemunha (ausência de FMA), duas concentrações de Cd ( 0 e 5 µmol L-1) e de P (1 e 10 mg L-1) na solução nutritiva. Foram determinados a colonização micorrízica, o comprimento do micélio extraradical, atividade da peroxidase nas raízes, crescimento das plantas e teor e acúmulo de Cd e P na raiz e na parte aérea das plantas. A associação micorrízica não promoveu crescimento das plantas mas aumentou a concentração foliar e radicular de Cd. O metal pesado foi acumulado principalmente nas raízes e somente uma pequena quantidade foi translocada para a parte aérea. A colonização micorrízica não foi influenciada pelo Cd adicionado, mas o micélio extrarradicular mostrou-se sensível ao metal, tendo sido reduzido em 25%, principalmente na menor concentração de P adicionado. Nesta mesma concentração de P, a adição de Cd reduziu a atividade da peroxidase nas raízes das plantas não colonizadas e nas colonizadas por G. macrocarpum. Entretanto, as raízes micorrizadas mostraram valores menores de atividade da enzima. Melhor desempenho da associação micorrízica foi constatado nas plantas colonizadas por G. etunicatum, o qual mostrou-se promissor na fitorremediação de solos contaminados por Cd quando em associação ao feijão de porco.
peroxidase; acúmulo de Cádmio; micélio extrarradicular; fitorremediação; hidroponia
SOILS AND PLANT NUTRITON
Cadmium effect on the association of jackbean (Canavalia ensiformis) and arbuscular mycorrhizal fungi
Efeito do cadmio na associação de feijão de porco (Canavalia ensiformis) e fungos micorrízicos arbusculares
Sara Adrián López de AndradeI; Renato Atílio JorgeII; Adriana Parada Dias da SilveiraI, * * Corresponding author < apdsil@iac.sp.gov.br>
IIAC - Centro de Pesquisa e Desenvolvimento de Solos e Recursos Ambientais, C.P. 28 - 13001-970 - Campinas, SP - Brasil
IIUNICAMP/Instituto de Quimica - Depto. de Físico-Química, C.P. 6154 - 13083-970 - Campinas, SP - Brasil
ABSTRACT
The effect of cadmium (Cd) on mycorrhizal association and on shoot and root Cd concentration was investigated in jackbean plants under hydroponic conditions. The treatments consisted of the inoculation of three different species of arbuscular mycorrhizal fungi (AMF), Glomus etunicatum, G. intraradices and G. macrocarpum, and a non-inoculated control, two Cd (0 and 5 µmol L-1) and two P (1 and 10 mg L-1) levels in the nutrient solution. Mycorrhizal colonization, length of AMF extraradical mycelium, guaiacol peroxidase activity in roots, plant growth and root and shoot Cd and P concentrations were determined. Mycorrhizal status did not promote jackbean growth but in most of the cases mycorrhization increased root and shoot Cd concentrations. Cd ions were accumulated mainly in roots and only small amounts were translocated to the shoot. Cd addition did not affect root colonization by AMF but the AM extraradical mycelium (ERM) was sensitive to the added Cd. ERM length was reduced by 25% in the presence of Cd. This reduction was more pronounced under conditions of low P concentration. Also at this P concentration, Cd addition decreased guaiacol peroxidase activity in non-mycorrhizal roots and in roots colonized by G. macrocarpum. However, mycorrhizal roots maintained lower values of peroxidase activity. G. etunicatum showed the best performance when associated to jackbean plants and it could be a promising association for phytoremediation of Cd- contaminated soil.
Key words: peroxidase, Cd accumulation, extraradical mycelium, phytoremediation, hydroponics
RESUMO
O efeito do cádmio na associação micorrízica e no teor e acúmulo de Cd na raiz e parte aérea de feijão de porco foi avaliado em condição de hidroponia. Os tratamentos consistiram da inoculação ou não de três espécies de fungos micorrízicos arbusculares (FMAs), Glomus etunicatum, G. intraradices e G. macrocarpum, e uma testemunha (ausência de FMA), duas concentrações de Cd ( 0 e 5 µmol L-1) e de P (1 e 10 mg L-1) na solução nutritiva. Foram determinados a colonização micorrízica, o comprimento do micélio extraradical, atividade da peroxidase nas raízes, crescimento das plantas e teor e acúmulo de Cd e P na raiz e na parte aérea das plantas. A associação micorrízica não promoveu crescimento das plantas mas aumentou a concentração foliar e radicular de Cd. O metal pesado foi acumulado principalmente nas raízes e somente uma pequena quantidade foi translocada para a parte aérea. A colonização micorrízica não foi influenciada pelo Cd adicionado, mas o micélio extrarradicular mostrou-se sensível ao metal, tendo sido reduzido em 25%, principalmente na menor concentração de P adicionado. Nesta mesma concentração de P, a adição de Cd reduziu a atividade da peroxidase nas raízes das plantas não colonizadas e nas colonizadas por G. macrocarpum. Entretanto, as raízes micorrizadas mostraram valores menores de atividade da enzima. Melhor desempenho da associação micorrízica foi constatado nas plantas colonizadas por G. etunicatum, o qual mostrou-se promissor na fitorremediação de solos contaminados por Cd quando em associação ao feijão de porco.
Palavras-chave: peroxidase, acúmulo de Cádmio, micélio extrarradicular, fitorremediação, hidroponia
INTRODUCTION
Cadmium accumulation in biotic systems due to anthropogenic activities is becoming a growing environmental problem. Atmospheric deposition of industrial emissions, sludge, phosphate fertilizers and mining are some of the sources of Cd in soils (Jackson & Alloway, 1992). The main symptoms of Cd toxicity in plants are leaf chlorosis, leaf and root necrosis and general decrease in growth (Hernandez & Cooke, 1997).
Little is known about the interactions between Cd and arbuscular mycorrhiza. Some reports showed that arbuscular mycorrhizal fungi (AMF) decreased or did not affect foliar Cd concentrations in soils with high amounts of this metal (Gildon & Tinker, 1983; Heggo et al., 1990; Tonin et al., 2001). However, AMF attenuated the negative effects of Cd in Pisum sativum, in spite of higher, or similar, concentrations of Cd in mycorrhizal than in non-mycorrhizal plants (Rivera-Becerrril et al., 2002). Some authors suggest that negatively charged surfaces on AMF mycelium adsorbed Cd decreasing its transfer to root cells (Joner et al., 2000), or that AMF hyphae do in fact transfer Cd from the soil to the roots but with restricted translocation to the shoot (Joner & Leyval, 1997). Thus, the interaction between plants, AMF and Cd involves a variety of arranged factors, which influence plant and fungal development and Cd availability. The enhanced nutrient supply, mainly phosphorus, to the host plant by the AMF may attenuate the effect of physiological stress caused by Cd (Meharg & Cairney, 2000). Some scarce data suggest that the antioxidative system is affected by mycorrhizal fungi in conditions of high metal concentrations (Schutzendubel & Polle, 2002).
The aim of this study was to investigate the effects of Cd on the association of jackbean and different species of AMF, the participation of AMF in Cd uptake and the relation between the activity of root guaiacol peroxidase and mycorrhization.
MATERIAL AND METHODS
Experimental design
A pot experiment was carried out under greenhouse conditions in Campinas, SP, Brazil, from April to May 2002, using a hydroponic sand culture. Greenhouse conditions were 28/16ºC day/night temperature, about 1200 µmol m-2 s-1 light intensity and 12h photoperiod. The experiment consisted of a 2 ´ 2 ´ 4 factorial scheme and completely randomised design, with five replications. Treatments were two Cd concentrations, 0 and 5 µmol L-1, two P concentrations, 1 and 10 mg L-1 in the nutrient solution and the inoculation or not of three AMFs.
AMF inoculum
The mycorrhizal fungi were Glomus etunicatum (Becker & Gerdemann) (IAC-42), G. intraradices (Schenck & Smith) (IAC-43) and G. macrocarpum (Tul. & Tul.) (IAC-50). Spores used as inoculum arose from a non-contaminated soil. AMF inoculum was obtained from stock -cultures with Brachiaria brizantha Stapf and belong to the collection maintained by IAC, in Campinas, Brazil. The inoculum consisted of sand-soil mixture containing spores, mycelium and colonized root fragments, with approximately 1,500 spores cm-3 of soil-inoculum. Non-mycorrhizal treatments received washings (20 mL) of the soil-inoculum mixture filtered through Whatman nº42 filter paper.
Pot culture experiment
Two litters of sterilised quartz sand (0.045 to 1 mm of size) were used to fill in 2.79 L plastic pots. Jackbean (Canavalia ensiformis L. D.C) seeds were disinfected with 1:3 (v/v) of 2.5% sodium hypochlorite solution for 10 minutes and then rinsed several times with sterilized water. Three seeds were sown per pot and after emergence thinned to one plant per pot. Jackbeans were cultivated in hydroponics system with sand, and irrigated with complete nutrient solution (N-NO3 154.6; N-NH4 19.5; S-SO4 18.7; Ca 151.2; K 70.9; Mg 18.8; B 0.53; Fe 1.99; Mn 0.97; Cu 0.076; Zn 0.3; Mo 0.15 mg L-1) (Furlani & Furlani, 1988), with P and Cd concentrations adjusted for each treatment. Cd was supplied as Cd(NO3)2. Cd-nutrient solutions had a pH of 5.58. Speciation calculations using Visual MINTEQ ver. 2.23 (Gustafsson, 2003) indicated that 92 and 90% of the Cd in solution was free Cd2+ ion, for solutions with 1 and 10 mg L-1 of phosphorus, respectively. The total volume added to each pot during the experiment was of 5 L corresponding to 0.554 mg of Cd added cumulatively. The plants were harvested after 45 days at the flowering stage.
Analytical methods
Shoots and roots were separated at harvest. The shoots were washed in distilled water, dried at 60°C, weighed and the leaves ground. Root subsamples were: 1) washed, dried and ground, 2) stored in ethanol 50% in order to evaluate mycorrhizal colonization and 3) stored in liquid nitrogen until enzyme analysis. Plant Cd and P concentrations were determined after dry-digesting with HNO3 and HClO4 by ICP-AES (Inductively-Coupled Plasma Atomic Emission Spectrometry). Mycorrhizal colonization was evaluated by the grid-line intersected method (Giovannetti & Mosse, 1980) by first clearing the roots with 25 g L-1 KOH, followed by root acidification in 1% HCl and staining with 0.05% trypan blue. The length of the extraradical mycelium (ERM) of AMF in the substrate was estimated according to Melloni & Cardoso (1999). The ERM was extracted by wet-sieving from 10 g of substrate, which was mixed in a blender with 1.5 L of tap water. A subsample of 11 mL was vacuum-filtered onto nitro-cellulose membrane (0.40 mm pore size). The extracted ERM was stained by 0.05% trypan blue in lactoglycerol and the total length of ERM assessed under compound microscope (125x magnification). Sixty four fields were counted and the results expressed as cm of mycelium in 1g of dry soil.
Guaiacol peroxidase (EC 1.11.1.7) (GPX) was assayed spectrophotometrically using a diode array spectophotometer (8452.A, Hewlett Packard, USA), according to Boscolo et al. (2003), based on the method of Calmak & Horst (1991). Liquid nitrogen frozen roots were washed three times in deionized water and about 0.05 g were homogenized in a phosphate buffer (KH2PO4/K2HPO4, pH 6.8). The homogenates were centrifuged at 10,000 x g for 8 minutes and in the supernatant was immediately determined the peroxidase activity. The reaction mixture contained 500 mmol L-1 phosphate buffer, 8 mmol L-1 guaiacol, 8 mmol L-1 H2O2 and protein extract. The increase in absorbance due to tetraguaicol formation was recorded at 470 nm (e = extinction coefficient of 26.6 mmol L-1 cm-1). Proteins in each extract were assayed according to Bradford (1976). Determinations of enzyme activity were performed with three parallels in three replicates per treatment.
All data were processed by analysis of variance and Tukey test at 5% for mean comparisons. Data expressed as percentage were arcsin-square root transformed prior to statistical analysis.
RESULTS AND DISCUSSION
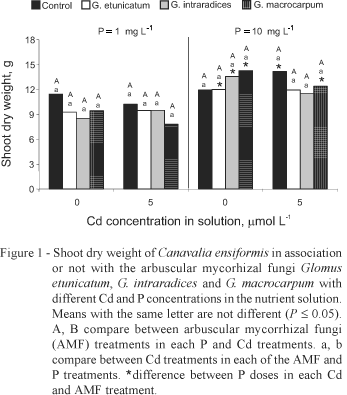
Cadmium addition to the nutrient solution did not affect jackbean growth and no chlorosis symptoms were observed in plants. As expected, high P concentration in the nutrient solution (10 mg L-1) promoted plant growth (Figure 1). AMF did not influence plant growth (Figure 1), because in sand culture with salts as nutrient supply, the mycorrhizal growth effect, which is usually related to an enhanced nutrient absorption, is less relevant than in the soil (Weissenhorn & Leyval, 1995). The similar growth between mycorrhizal an non-mycorrhizal plants allowed to confront plants with comparable biomass production, avoiding dilution or concentration effects on nutrients and Cd contents (Jarrell & Beverly, 1981).
Although the amount of Cd added cumulatively during the experiment (0.554 mg pot-1) was not very high, it can be considered of environmental relevance. The added Cd concentration may simulate a chronic Cd stress condition (Sanita di Toppi & Gabbrielli, 1999) since soil solutions with moderate level of Cd pollution have concentrations varying from 0.32 to 1 µmol Cd L-1 (Wagner, 1993). Most of the publications regarding Cd toxicity in plants are related to acute stress with non-realistic concentrations of Cd (Sanita di Toppi & Gabbrielli, 1999).
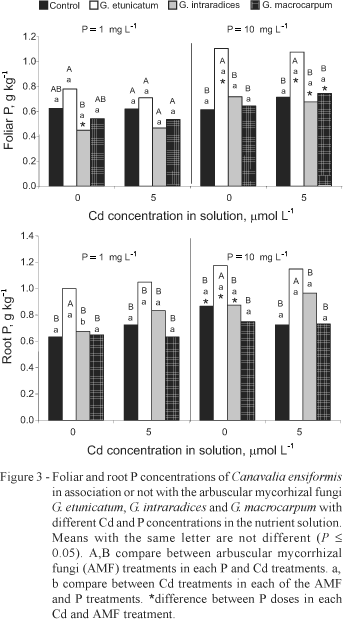
Cd was taken up by jackbean roots and translocated to leaves, however its concentration was more than 20 times higher in roots than in leaves (Figure 2). On the average, 96% of the absorbed Cd was retained in the roots, which was already observed for bean, pea and maize plants (Weigel & Jäger, 1980; Rivera-Becerril et al., 2002; Rauser, 2000). Mycorrhizal roots accumulated higher amounts of Cd (16% more) than non-mycorrhizal roots. The association of C. ensiformis and G. etunicatum lead to higher Cd accumulation in both, roots and leaves, and in addition, only this AMF promoted a higher foliar P concentration (Figure 3). Therefore, the association C. ensiformis-G. etunicatum might be a promising symbiosis for Cd polluted soils.
Root AMF colonization did not change by Cd addition, but P concentration in the nutrient solution had an inhibitory effect, being 24% lower in plants cultivated under 10 mg P L-1 (Figure 4). Several authors showed that high heavy metal concentrations in soils can reduce mycorrhizal colonization (Gildon & Tinker, 1983; Liao et al., 2003; Andrade et al., 2004) and some reported even an elimination of root colonization due to the presence of Cd in the nutrient solution (Weissenhorn & Leyval, 1995), in spite of these concentrations being higher than that added in the present study. The colonization rates were different among the inoculated AMF. The highest rate of root colonization was observed for G. etunicatum, followed by G. intraradices and G. macrocarpum (Figure 4). However, the amount of ERM was reduced by Cd and was also influenced by P concentration in the nutrient solution. In general, the ERM length was 25% lower in the presence of Cd and the reduction was more pronounced at low P concentration (Figure 4). However, the length of ERM was about 1.5 time higher at low P than at high P concentration (Figure 2). G. intraradices presented the highest amounts of external mycelia followed by G. etunicatum and G. macrocarpum which had low amounts of ERM (Figure 2). Root colonization and amount of ERM were highly correlated (R2=0.908, P < 0.001) and both were positively correlated with root P concentration, possibly due to phosphorus transfer to the roots by AMF hyphae and P accumulation inside intraradical hyphae. Despite the importance of the ERM on mycorrhizal symbiosis functioning, studies on this subject have been usually neglected. Extraradical hyphae of mycorrhizal fungi are able to transport Cd from the soil solution to the plant and even restrict metal transfer to the shoot due to fungal immobilization in the roots (Joner & Leyval, 1997; Joner et al., 2000).
Among the antioxidant enzymes, which play an important role in the cellular defence against oxidative stress, peroxidases can transform peroxides into non-reactive species (Chaoui et al., 1997). An induction in the activity of antioxidant enzymes is expected as a strategy to overcome oxidative stress due to excess of Cd. However, the addition of 5 mmol Cd L-1 reduced guaiacol peroxidase (GPX) activity only in non-mycorrhizal roots and in roots colonized by G. macrocarpum (Table 1), which had a very low infection rates (Figure 4), at low P condition. Since increase and decrease of antioxidant activity in response to Cd have been reported in different plant species and Cd concentrations, it is possible that, besides the detoxification function, enzyme molecules may also be sensitive targets of Cd toxicity in plant roots (Gallego et al., 1996). P concentration in the nutrient solution modified GPX activity in roots, which was 25% lower in plants at high P concentration (Table 1). This suggests that higher P concentration in the solution could be buffering the effect of added Cd.
The response of AM symbiosis under heavy metal stress conditions depends on plant and fungi species. In the present experiment, G. etunicatum associated to jackbean plants had the best performance and, so, this could be a promising association for phytoremediation of Cd-contaminated soils.
ACKNOWLEDGEMENTS
To CNPq and to the IB-UNICAMP for the fellowship to the first author. To Dr. Mônica Ferreira de Abreu for ICP-AES analysis and to Miss Rosana Gierts Gonçalves for technical support.
GUSTAFSSON, J.P. Visual Minteq ver. 2.15. Disponível em: <http://www.lwr.kth.se/english/OurSoftware/vminteq>. Accesso em: ago. 2003.
Received June 30, 2004
Accepted May 25, 2005
- ANDRADE, S.A.L.; ABREU, C.A.; de ABREU, M.F.; SILVEIRA, A.P.D. Influence of lead addition on arbuscular mycorrhiza and Rhizobium symbioses under soybean plants. Applied Soil Ecology, v.26, p.123-131, 2004.
- BOSCOLO, P.R.S.; MENOSSI, M.; JORGE, R.A. Aluminum-induced oxidative stress in maize. Phytochemistry, v.62, p.181-189, 2003.
- BRADFORD, N.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, v.72, p.248-254, 1976.
- CALMAK, I.; HORST, W.J. Effect of aluminum on lipid peroxidation, superoxide dismutase, catalase and peroxidase activities in root tips of soybean (Glycine max). Physiologia Plantarum, v.83, p.463-468, 1991.
- CHAOUI, A.; MAZHOUDI, S.; GHORBAL, M.H.; EL-FERJANI, E. Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.) Plant Science, v.127, p.139-147, 1997.
- FURLANI, A.M.C.; FURLANI, P.R. Composição e pH de soluções nutritivas para estudos fisiológicos e seleção de plantas em condições nutricionais adversas Campinas: Instituto Agronômico, 1988. 34p. (Boletim Técnico).
- GALLEGO, S.M.; BENAVIDES, M.P.; TOMARO, M. Effect of heavy metal ion excess on sunflowers leaves: evidence for involvement of oxidative stress. Plant Science, v.121, p.151-159, 1996.
- GILDON, A.; TINKER, P.B. Interactions of vesicular arbuscular mycorrhizal infection and heavy metals in plants: I. The effect of heavy metals on the development of vesicular-arbuscular mycorrhizas. New Phytologist, v.95. p.247-261, 1983.
- GIOVANNETTI, M.E.; MOSSE, B. An evaluation of techniques for measuring vesicular-arbuscular mycorrrhizal infection in roots. New Phytologist, v.84, p.482-500, 1980.
- HEGGO, A.; ANGLE, J.S.; CHANEY R.L. Effects of vesicular-arbuscular mycorrhizal fungi on heavy metal uptake by soybeans. Soil Biology and Biochemistry, v.22, p.865-869, 1990.
- HERNANDEZ, L.E.; COOKE, D.T. Modifications of root plasma membrane lipid composition of cadmium treated Pisum sativum Journal of Experimental Botany, v.48, p.1375-1381, 1997
- JACKSON, A.P.; ALLOWAY, B.J. The transfer of cadmium from agricultural soils to the human food chain. In: ADRIANO D. C. (Ed.) Biogeochemistry of trace metals. Boca Raton: Lewis Publishers , 1992. p.109-158.
- JARRELL, W.M.; BEVERLY, R.B. The dilution effect in plant nutrition studies. Advances in Agronomy, v.34, p.197-224, 1981.
- JONER, E.J.; LEYVAL, C. Uptake of 109Cd by roots and hyphae of a Glomus mosseae/Trifolium subterraneum mycorrhiza from soil amended with high and low concentrations of cadmium. New Phytologist, v.135, p.353-360, 1997.
- JONER, E.J.; BRIONES, R.; LEYVAL, C. Metal-binding capacity of arbuscular mycorrhizal mycelium. Plant and Soil, v.226, p.227-234, 2000.
- LIAO, J.P.; LIN, X.G.; CAO, Z.H.; SHI, Y.Q.; WONG, M.H. Interactions between arbuscular mycorrhizae and heavy metals under sand culture experiment. Chemosphere, v.50, p.847-853, 2003.
- MEHARG, A.A.; CAIRNEY, J.W.G. Co-evolution of mycorrhiza symbionts and their host too metal contaminated environments. Advances in Ecological Research, v.30, p.69-112, 2000.
- MELLONI, R.; CARDOSO, E.J.B.N. Quantificação de micélio extrarradicular de fungos micorrízicos arbusculares em plantas cítricas e endófitos. I. Método empregado. Revista Brasileira de Ciência do Solo, v.23, p.53-58, 1999.
- RAUSER, W.E. Roots of maize seedlings retain most of their cadmium through two complexes. Journal of Plant Physiology, v.156, p.545-551, 2000.
- RIVERA-BECERRIL, F.; CALANTZIS, C.; TURNAU, K.; CAUSSANEL, J.P.; BELISMOV, A.A.; GIANINAZZI, S.; STRASSER, J.R.; GIANINAZZI-PEARSON V. Cadmium accumulation and buffering of cadmium-induced stress by arbuscular mycorrhiza in three Pisum Sativum L. genotypes. Journal of Experimental Botany, v.371, p.1177-1185, 2002.
- SANITÀ DI TOPPI, L.; GABBRIELLI, R. Response to cadmium in higher plants. Environmental and Experimental Botany, v.41, p.105-130, 1999.
- SCHÜTZENDUBEL, A.; POLLE, A. Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. Journal of Experimental Botany, v.372, p.1351-1365, 2002.
- TONIN, C.; VANDENKOORNHUYSE, P.; JONER, E.J.; STRACZEK, J.; LEYVAL, C. Assessment of arbuscular mycorrhizal fungi diversity in the rhizosphere of Viola calaminaria and effect of these fungi on heavy metal uptake by clover. Mycorrhiza, v.10, p.161-168, 2001.
- WAGNER, G.J. Accumulation of cadmium in crop plants and its consequences to human health. Advances in Agronomy, v.51, p.173-212, 1993.
- WEIGEL H.J.; JÄGER, H.J. Subcelular distribution and chemical form of cadmium in bean plants. Plant Physiology, v.65, p.480-482, 1980.
- WEISSENHORN, I.; LEYVAL, C. Root colonization of maize by a Cd-sensitive and a Cd-tolerant Glomus mosseae and cadmium uptake in sad culture. Plant and Soil, v.175, p.233-238, 1995.
Publication Dates
-
Publication in this collection
23 Aug 2005 -
Date of issue
Aug 2005
History
-
Accepted
25 May 2005 -
Received
30 June 2004