Abstracts
Feed regimens alter muscle growth rate, hence they might impact the proteolytic system involved in tenderization during meat conditioning. The aim of this project was to verify the effects of feed restriction regimens on muscular and animal growth and their impact on postmortem myofibrillar fragmentation. The regimens were: 1) Feeding ad libitum for 11 d (Al/2); 2) Feed restriction (60% of Net Energy for maintenance - NEm) for 11 d (Rt/2); 3) Ad libitum for 22 d (Al); 4) Ad libitum for 4 d and feed restriction (60% NEm) for 18 d (Rt); 5) Ad libitum for 19 d and 3 d of fast (Ft); 6) Feed restriction (60% NEm) for 11 d and ad libitum until 22 d (Ral). The regimens Al/2 and Rt/2 had different intestine weights (19.3 ± 1.1 and 15.8 ± 1.9 g, respectively; P < 0.07). At 22 d, Al animals had higher (P < 0.07) intestine weight (21.8 ± 3.8). Moreover, Ral animals had heavier intestine (19.9 ± 1.5) as compared to Rt (16.6 ± 1.6) or Ft (12.8 ± 1.9). The intestine/live weight percentage ratio was lower (P < 0.05) for Ft (6.3%) as compared to Al (8.4%) and to Ral (9.2%), but it was similar to Rt (7.6%). Liver weight (g) in the Ral (9.5 ± 1.1) did not differ from Al (10.7 ± 2.5) or Rt (8.5 ± 1.1), although the two latter were different (P < 0.05). There was an effect of feed restriction over muscle protein degradation verified by Myofibrillar Fragmentation Index (MFI). The animals at Rt, Ft or Ral showed the lowest MFI 0d (42 ± 1.9; 40 ± 2.7; 40 ± 3.6; respectively) and MFI 5d (77 ± 2.7; 74 ± 3.0; 74 ± 2.9; respectively) as compared to Al, whose indexes were 54 ± 3.0 and 82 ± 3.3. Even though the MFI 5d were lower for the restricted animals, the rates of fragmentation postmortem were higher. Feed restriction altered myofibrillar protein degradation, reflected in lower extended fragmentation of the myofibrils.
myofibrillar fragmentation index; muscle; intestine; liver
Regimes alimentares alteram a taxa de crescimento muscular, e podem impactar o sistema proteolítico envolvido no amaciamento da carne durante maturação. O objetivo do trabalho foi verificar a existência de reflexo do regime alimentar sobre crescimento animal e muscular, e o impacto na fragmentação miofibrilar pós-morte. Os regimes foram: 1) Consumo ad libitum por 11 d (Al/2); 2) Restrição alimentar (60% energia de mantença - NEm) por 11 d (Rt/2); 3) Ad libitum por 22 d (Al); 4) Ad libitum por 4 d e restrição alimentar (60% NEm) por 18 d (Rt); 5) Ad libitum por 19 d e 3 d de jejum (Ft); 6) Restrição alimentar (60% NEm) por 11 d e ad libitum até 22 d (Ral). Os regimes Al/2 e Rt/2 apresentaram diferenças no peso de intestino (19,3 ± 1,1 e 15,8 ± 1,9 g, respectivamente; P < 0,07). Nos 22 d, o regime Al resultou em intestinos mais pesados (P < 0,07; 21,8 ± 3,8). Além disso, os animais do regime Ral tiveram maiores pesos de intestino (19,9 ± 1,5) comparados com Rt (16,6 ± 1,6) ou Ft (12,8 ± 1,9). A relação intestino/peso vivo foi menor (P < 0,05) para Ft (6,3%) comparado com Al (8,4%) e com Ral (9,2%), mas foi similar ao Rt (7,6%). O peso do fígado (g) nos animais do regime Ral (9,5 ± 1,1) não diferiu do regime Al (10,7 ± 2,5) ou Rt (8,5 ± 1,1), sendo que os dois últimos foram diferentes (P < 0,05). Houve efeito da restrição alimentar sobre a degradação da proteína muscular verificada pelo Índice de Fragmentação miofibrilar (MFI). Os animais de Rt, Ft ou Ral mostraram os menores valores de MFI 0d (42 ± 1,9; 40 ± 2,7; 40 ± 3,6; respectivamente) e MFI 5d (77 ± 2,7; 74 ± 3,0; 74 ± 2,9; respectivamente) comparados com Al onde os índices foram 54 ± 3,0 e 82 ± 3,3, respectivamente. Embora os animais que experimentaram alguma restrição alimentar tenham apresentado menor MFI 5d, as sua taxas de fragmentação pós-morte foram maiores. Restrição alimentar alterou a degradação da proteína miofibrilar, refletida na menor extensão da fragmentação das miofibrilas.
índice de fragmentação miofibrilar; músculo; intestinos; fígado
ANIMAL SCIENCE AND PASTURES
Differential growth retardation and Myofibrillar fragmentation in rats submitted to feed restriction and realimentation
Taxa de crescimento e fragmentação Miofibrilar diferenciadas em ratos submetidos a restrição alimentar e realimentação
Eric Franchi LeonardoI; Eduardo Francisquine DelgadoII, * * Corresponding author < efdelgad@esalq.usp.br> ; Adriana Regina BagaldoI; Dante Pazzanese Duarte LannaIII; Claudia Cristina Paro de PazIV
IUSP/ESALQ - Programa de Pós-Graduação em Ciência Animal e Pastagens
IIUSP/ESALQ - Depto. Zootecnia - Lab. Anatomia e Fisiologia Animal, C.P. 09 - 13418-900 - Piracicaba, SP - Brasil
IIIUSP/ESALQ - Depto. Zootecnia - Lab. Nutrição e Crescimento Animal
IVAPTA - Regional Centro Leste, C.P. 271 - 14001-970 - Riberão Preto, SP - Brasil
ABSTRACT
Feed regimens alter muscle growth rate, hence they might impact the proteolytic system involved in tenderization during meat conditioning. The aim of this project was to verify the effects of feed restriction regimens on muscular and animal growth and their impact on postmortem myofibrillar fragmentation. The regimens were: 1) Feeding ad libitum for 11 d (Al/2); 2) Feed restriction (60% of Net Energy for maintenance NEm) for 11 d (Rt/2); 3) Ad libitum for 22 d (Al); 4) Ad libitum for 4 d and feed restriction (60% NEm) for 18 d (Rt); 5) Ad libitum for 19 d and 3 d of fast (Ft); 6) Feed restriction (60% NEm) for 11 d and ad libitum until 22 d (Ral). The regimens Al/2 and Rt/2 had different intestine weights (19.3 ± 1.1 and 15.8 ± 1.9 g, respectively; P < 0.07). At 22 d, Al animals had higher (P < 0.07) intestine weight (21.8 ± 3.8). Moreover, Ral animals had heavier intestine (19.9 ± 1.5) as compared to Rt (16.6 ± 1.6) or Ft (12.8 ± 1.9). The intestine/live weight percentage ratio was lower (P < 0.05) for Ft (6.3%) as compared to Al (8.4%) and to Ral (9.2%), but it was similar to Rt (7.6%). Liver weight (g) in the Ral (9.5 ± 1.1) did not differ from Al (10.7 ± 2.5) or Rt (8.5 ± 1.1), although the two latter were different (P < 0.05). There was an effect of feed restriction over muscle protein degradation verified by Myofibrillar Fragmentation Index (MFI). The animals at Rt, Ft or Ral showed the lowest MFI 0d (42 ± 1.9; 40 ± 2.7; 40 ± 3.6; respectively) and MFI 5d (77 ± 2.7; 74 ± 3.0; 74 ± 2.9; respectively) as compared to Al, whose indexes were 54 ± 3.0 and 82 ± 3.3. Even though the MFI 5d were lower for the restricted animals, the rates of fragmentation postmortem were higher. Feed restriction altered myofibrillar protein degradation, reflected in lower extended fragmentation of the myofibrils.
Key words: myofibrillar fragmentation index, muscle, intestine, liver
RESUMO
Regimes alimentares alteram a taxa de crescimento muscular, e podem impactar o sistema proteolítico envolvido no amaciamento da carne durante maturação. O objetivo do trabalho foi verificar a existência de reflexo do regime alimentar sobre crescimento animal e muscular, e o impacto na fragmentação miofibrilar pós-morte. Os regimes foram: 1) Consumo ad libitum por 11 d (Al/2); 2) Restrição alimentar (60% energia de mantença NEm) por 11 d (Rt/2); 3) Ad libitum por 22 d (Al); 4) Ad libitum por 4 d e restrição alimentar (60% NEm) por 18 d (Rt); 5) Ad libitum por 19 d e 3 d de jejum (Ft); 6) Restrição alimentar (60% NEm) por 11 d e ad libitum até 22 d (Ral). Os regimes Al/2 e Rt/2 apresentaram diferenças no peso de intestino (19,3 ± 1,1 e 15,8 ± 1,9 g, respectivamente; P < 0,07). Nos 22 d, o regime Al resultou em intestinos mais pesados (P < 0,07; 21,8 ± 3,8). Além disso, os animais do regime Ral tiveram maiores pesos de intestino (19,9 ± 1,5) comparados com Rt (16,6 ± 1,6) ou Ft (12,8 ± 1,9). A relação intestino/peso vivo foi menor (P < 0,05) para Ft (6,3%) comparado com Al (8,4%) e com Ral (9,2%), mas foi similar ao Rt (7,6%). O peso do fígado (g) nos animais do regime Ral (9,5 ± 1,1) não diferiu do regime Al (10,7 ± 2,5) ou Rt (8,5 ± 1,1), sendo que os dois últimos foram diferentes (P < 0,05). Houve efeito da restrição alimentar sobre a degradação da proteína muscular verificada pelo Índice de Fragmentação miofibrilar (MFI). Os animais de Rt, Ft ou Ral mostraram os menores valores de MFI 0d (42 ± 1,9; 40 ± 2,7; 40 ± 3,6; respectivamente) e MFI 5d (77 ± 2,7; 74 ± 3,0; 74 ± 2,9; respectivamente) comparados com Al onde os índices foram 54 ± 3,0 e 82 ± 3,3, respectivamente. Embora os animais que experimentaram alguma restrição alimentar tenham apresentado menor MFI 5d, as sua taxas de fragmentação pós-morte foram maiores. Restrição alimentar alterou a degradação da proteína miofibrilar, refletida na menor extensão da fragmentação das miofibrilas.
Palavras-chave: índice de fragmentação miofibrilar, músculo, intestinos, fígado
INTRODUCTION
The effect of feed restriction on growth and final weight of different animal organs is well established (Pethes et al., 1985; Hicks et al., 1990; Murphy & Loerch, 1994; Cardoso & Stock, 1996; Wertz et al., 2001; Choat et al., 2002; Farmer et al., 2004). Some of the authors showed that liver, intestine and other non-carcass tissues are the first to respond to feed restriction and realimentation. On the other hand, skeletal muscles give little response, being spared until higher levels of tissue nutrients are imposed to mobilization due to food deprivation (Brandstetter et al., 1998 in bovine; Potokar-Candek et al., 1999 in swine; Maxwell et al., 1992 in rats). The depletion of muscle mass and proteins are linked to changes in the protein degradation systems, which are important contributors to the growth rate of animals (Huang & Forsberg, 1998; Kristensen et al., 2002; Owens et al., 1993).
The growth rate immediately before slaughter is especially interesting due to its possible impact on the postmortem muscle proteolytic process associated to the regulation of endogenous protease systems. The postmortem proteolysis has been known to be a key effector on tenderization during meat conditioning under refrigeration (Koohmaraie, 1992). The enzymatic system involved in the postmortem proteolysis and muscle protein turnover is known as calpain system (Koohmaraie et al., 1990), and its effects on myofibrillar degradation are correlated to Myofibrillar Fragmentation Index (MFI) (Whipple et al., 1990b). There are evidences that feeding regimens may alter the calpain system and myofibrillar fragmentation (McDonagh et al., 2001). However, there are no reports on the effect of different schemes of feed restriction and realimentation in the postmortem myofibrillar fragmentation process.
The aim of this study was to verify whether the changes in animal growth elicited by different feeding restriction regimens during the muscle growth phase (protein accretion) would have an impact on the rate and extension of postmortem proteolysis evaluated by the myofibrillar fragmentation index.
MATERIAL AND METHODS
Animals and feeding regimens
Thirty-six growing Wistar rats (Rattus novergicus), right after weaning (14 days of age), were kept 22 days of experimental period (d.e.p.). Six groups of six rats (three males and three females) were randomly assigned to one of the following feeding regimens: 1) Al/2 - Feeding ad libitum until slaughter on the 11th d.e.p.; 2) Rt/2 Feed restriction (60% of NEm Net Energy for Maintenance) until slaughter on the 11th d.e.p.; 3) Al - Feeding ad libitum until slaughter on the 22th d.e.p.; 4) Rt - Feeding ad libitum for 4 days and feed restriction (60% of NEm) for 18 d; 5) Ft - Feeding ad libitum for 19 d.e.p. and 3 days of fast; and 6) Ral - Feed restriction (60% of NEm) for 11 d.e.p. and feeding ad libitum until slaughter on the 22th d.e.p.. All the animals were fed the same ration (Table 1) and kept in individual cages. The restriction was obtained by reducing the ration offered to the animals in a way that the quantity would attend 80% of the basal energy based on the energy intake level of those at the ad libitum regimen. The correction of feed intake was made weekly.
Growth
The animals were slaughtered at two different time points (11 and 22 d.e.p.). At slaughter the live weight and carcass, intestine, liver, visceral and thigh muscle weights were evaluated using a scale of 0.1 g accuracy. Before slaughtering, the animals were food deprived for six hours. Rats being nocturnal, there was no fasting during this period (exception for the regimen Ft) to avoid deprivation of the animals that were not on restricted regimens by the time of slaughtering. The animals were stunned in a closed glass chamber containing cotton soaked with ethyl ether and exsanguined.
Myofibrillar Fragmentation Index (MFI)
Samples from the muscles located at the posterior part of the thigh (m. semimembranosus, m. semitendinosus, m. gracilis, m. adductor) were kept under refrigerated conditions (8 to 10ºC) for 0, 1 and 5 days postmortem. Afterwards, the samples were weighed (24 h), and 2 g were homogenized mechanically by a Polytron homogenizer (15000 rpm) with MFI buffer and under cooled conditions according to Culler et al. (1978).
Statistical Analysis
The data were analyzed using the procedures of GLM of the SAS package (SAS Inst., Cary, NC, 2001). The basic model used live weight (LW), carcass weight (CW), intestine weight (IW), liver weight (LrW), visceral weight (VW) and total weight of thigh muscles (MW) as dependent variables. The kinetics of postmortem myofibrillar fragmentation resulting from lengthening the time of refrigerated conditioning was evaluated by the following exponential function (Riley et al., 2003):
MFI = k0 + k1 exp{k2 di} + e i
where: k0 represents the maximum MFI after the last day of maturation, k1 represents the difference between the initial MFI and the maximum MFI, k2 represents the increase rate, "di" is the day postmortem and ei is the error associated with the observation of the data (assumed independent and distributed identically). This analysis was made using the procedure NLIN of SAS. The data of each treatment were separated in individual curves. The parameters were compared to evaluate potential differences in the treatment.
RESULTS AND DISCUSSION
Growth and organ weight
LW and CW of the animals that were under Al (i.e., Al/2 and Al) regimens were higher (P < 0.001) than all restricted regimens (i.e., Rt/2 and, Ral, Rt and Ft) at both evaluation times (Table 2). The results were expected and confirmed the undernutrition of the animals that were submitted to different feeding restriction regimens.
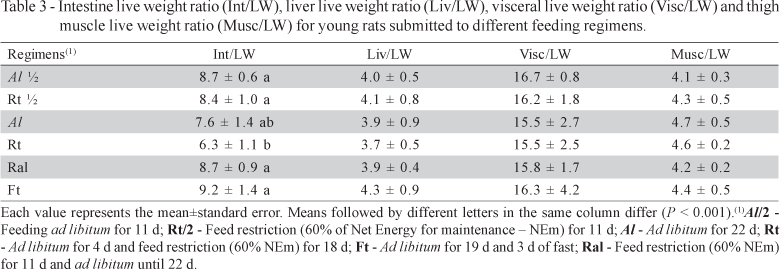
IW was higher (P < 0.07) for animals fed Al for the two evaluation times (i.e., Al/2 and Al). This same variable differed (P < 0.07) among the restricted regimens at the end of the period (i.e., Ral, Rt and Ft). In the Ft regimen, the internal organs which normally demand high energy, especially the intestine, presented lower weight as compared to other regimens. Fasting for 72 hours causes hypoplasia in intestinal villi and crypts, and decreases activity of several enzymes from epithelial cells (Holt et al., 1986). Although Rt did not show such an acute case in terms of compromising the final weight of internal organs, it was the regimen that showed the lowest (P < 0.001) intestine/body weight ratio (Table 3). On the other hand, Ral reverted partially the slow growth rate during restriction, which confirms that animals which had undergone severe food deprivation are able to counteract the downward regulation of intestinal villi height and crypt depth as well as in enterocyte enzymatic activity, when they were allowed to ad libitum feeding (Holt et al., 1979).
Rats under Al regimen also presented higher (P < 0.05) liver weight, at older age, than those under feed restriction, except for Ral (Table 2). In order to allow lower maintenance energy expenditure and increased feed efficiency under feed restriction regimens, the liver is one of the first organs to reduce its growth (Hicks et al., 1990; Levin et al., 1993). In severe feed restriction, there is a reduction of the liver growth hormone mRNA receptor (Brameld et al., 1996) and IGF-I gene expression (Straus & Takemoto, 1990), which may be correlated to reduced liver growth. On the other hand, in the Ral regimen liver might have experienced compensatory growth, which may have been a result of increased liver cell (hepatocytes) sizes that result from refeeding animals under feed restriction (Sainz & Bentley, 1997). The smaller liver observed for the rats submitted to Ft, which were 29% lighter than those under Al, agreed with a 23% reduction in liver size reported in rats fasting for 48 hours (Anand & Gruppuso, 2005). The Rt rats also had their liver weight decreased, even though the effect was more moderate, in the order of 20%.
Differences observed in liver and intestines were expected to reflect in the visceral weight. Furthermore, in animals under feed restriction other visceral organs were supposed to present growth decrease due to their high energy requirement. Indeed Al rats presented higher (P < 0.05) visceral weight than the restricted rats on both observation times (Table 2). However, Rt and Ral did not differ in visceral weight. This result suggests that even though Ral was sufficient to promote compensatory liver and intestine growth rate, there were other visceral organs that had significant contribution to total visceral weight, also affected by restriction, which did not recover its size after the refeeding period. Besides liver, there was a reduction in visceral weight especially in organs such as heart, gonads and kidney in rats under severe protein restriction (Reichling & German, 2000). The lowest visceral weight observed for the Ft regimen could be explained by lower RNA and protein contents followed by decreases in cell size that resulted from long fasting periods in rats, which have drastic impact in the total visceral weight (Burrin et al., 1988).
At the first observation time, restricted animals (Rt/2) already showed lower (P < 0.05) muscle weight (Table 2), which would be expected since young rats under feeding restriction had been shown to decrease rapidly muscle protein synthesis and increase degradation (Ogata et al., 1978). The animals from the Rt, Ral and Ft groups had lower (P < 0.05) muscle weight as compared to Al, but did not differ among them (Table 2). Although skeletal muscle may be spared for some time under feeding restriction, there was a negative impact on thigh muscles. These muscles are mostly phasic muscles, which could be the explanation for the observed results considering the reported differences in response of phasic and tonic muscles to feed restriction, phasic muscles having been more sensitive to undernutrition (Goldspink, 1978).
Dirks & Leeuwenburgh (2006) shows that experimental animals (e.g., rat) under feed or caloric restriction experience a significant decrease in both fat mass and lean body mass, even though the decrease in muscle mass/body weight ratio is attenuated with age (Dirks & Leeuwenburgh, 2004).
Myofibrillar Fragmentation Index and its kinetics
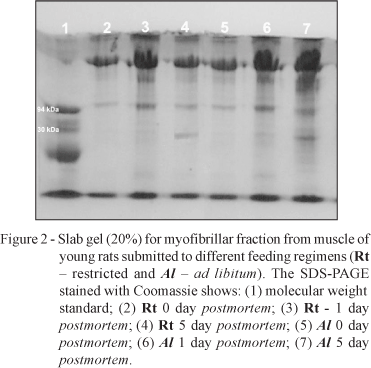
The spectrophotometric (540 nm) readings were higher on the muscle samples from 0 day postmortem and decreased with time postmortem under refrigeration. There is a possibility that very small myofibril fragments in later times postmortem might have scattered more light forward which ended up reaching the photo cell detector and so resulting in lower absorbance readings (lower turbidity). The phase-contrast microscopy showed an increased myofibrillar fragmentation with time postmortem (Figure 1). The increased fragmentation was also verified by the appearance of bands less than 90 kDa, especially in the range of 30 kDa, in SDS-PAGE (Figure 2). In order to better interpret these observations and not allow misinterpretations such as a decrease in fragmentation with time postmortem, The MFI (Table 4) was calculated, subtracting the standardized MFI (200 X Absorbance reading) from 100.
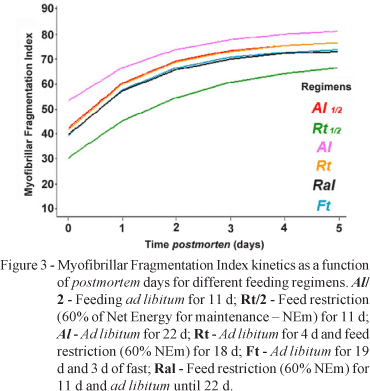
The feeding regimens had impact on the rate and extent of postmortem myofibrillar fragmentation on muscles under refrigeration (Figure 3). The estimated parameter k0, indicator of maximum MFI (Table 5), were greater (P < 0.05) for animals under Al regimen at the two observation times (Table 4). Among the restricted regimens there were no differences in k0. The lower extent of fragmentation associated with lower initial MFI (Table 4) in restriction and/or realimentation regimens may be related to a possible increase of calpastatin activity of muscles, which has been observed for animals submitted to undernutrition (Goicoechea & Conde, 1997; McDonagh et al., 2001). Calpastatin is one of the key muscular protease inhibitors that regulates myofibrillar protein turnover (Goll et al., 1991). High calpastatin activity causes low postmortem myofibrillar fragmentation in several animal models (Whipple et al., 1990b; Koohmaraie et al., 1992; Morgan, 1993; Delgado et al., 2001; McDonagh et al., 2001).
Although there were no differences in the extent of myofibrillar fragmentation between Rt and Ral regimens, there are reports showing trends of increased activity of µ-calpain, higher MFI values, and lower shear force in pigs that presented compensatory growth (Kristensen et al., 2002; Therkildsen et al., 2002). The lack of differentiation between restricted and compensating animals might be related to a rather slow response of the in vivo proteolytic system going from restricted to ad libitum feeding, which would require several days before an effect could be expected on myofibrillar fragilization (Therkildsen et al., 2002).
Another possibility for higher initial and final MFI for non-restricted animals (Al) may be related to fast glycolytic fiber (FG) transformation towards fast oxidative-glycolytic (FOG) and oxidative fibers (SO), observed in underfed animals. Solomon et al. (1988) reported smaller SO and FG as well as higher proportions of FOG and lower proportions of FG fibers in restrictively fed pigs as compared to pigs with ad libitum access to diet. The percentage area of FOG is negatively correlated to the myofibril fragmentation index (Whipple et al., 1990a). On the other hand, the parameter k1 was lower for Al in relation to the Rt, Ral and Ft regimens (Table 5), which suggests that most of the fragilization occurred within 24 hours postmortem in the muscle of the animals from the Al regimen and resulted in a greater extent of myofibrillar fragmentation. The restricted regimens did not differ among them.
The results for the k2 parameter were higher for restricted regimens as compared to Al. Considering the idea that higher calpastatin would be elicited for the restriction of feeding it would have had a negative impact on proteolysis. The problem would be to explain why the difference between initial MFI and maximal MFI (k1) was higher and why there were higher postmortem proteolytic rates (k2) on the restricted regimens. One hypothesis would be an increased amount of µ-calpain in vivo as result of feed restriction and muscle mass loss (Brooks et al., 1983; Thomson et al., 1997; Du et al., 2004). In case of greater amounts of calpain spared from autolysis due to calpastatin binding during the first postmortem hours, this would allow a more stable enzyme to proceed with myofibrillar protein proteolysis afterwards (Koohmaraie et al., 1991; Dransfield, 1993; Geesink & Koohmaraie, 1999a; Geesink & Koohmaraie, 1999b). The first postmortem hours are recognized as important for the extent of postmortem myofibrillar fragmentation (Taylor et al., 1995), probably because of optimal pH and ionic strength conditions for calpain activity which becomes limiting as time postmortem proceeds (Kendall et al., 1993).
In summary, in the feed restricted animal muscles there would be a limitation in the rate and extent of initial myofibrillar proteolysis and therefore a lower initial MFI. On the other hand, a faster rate of proteolysis may occur after 24 hours compared to non-restricted animal muscles. However, the suboptimal conditions for proteolytic activity would limit the compensation for lower initial fragmentation, resulting in a lower final MFI. Muscular growth is affected by alimentary restriction in a differentiated way, according to the animal growth phase the occurred changes have impact on the postmortem myofibrillar fragmentation indicating that a proteolytic system is involved.
ACKNOWLEDGEMENTS
To the Unimep bioterium for animal supply.
Received May 05, 2006
Accepted November 14, 2007
- ANAND, P.; GRUPPUSO, P.A. The regulation of hepatic protein synthesis during fasting in the rat. The Journal of Biological Chemistry, v.280, p.16427-16436, 2005.
- BRAMELD, J.M.; ATKINSON, J.L; SAUNDERS, J.C.; PELL, J.M.; BUTTERY, P.J.; GILMOUR, R.S. Effects of growth hormone administration and dietary protein intake on insulin-like growth factor I and growth hormone receptor mRNA expression in porcine liver, skeletal muscle, and adipose tissue. Journal of Animal Science, v.74, p.1832-1841, 1996.
- Brandstetter, A.m.; PICARD, B.; GEAY, Y. Muscle fiber characteristics in four muscles of growing male cattle. II. Effect of castration and feeding level. Livestock Production Science, v.53, p.25-36, 1998.
- Brooks, B.A.; GOLL, D.E.; PENG, Y.S.; GREWELING, J.A.; HENNECKE, G. Effect of starvation and refeeding on activity of a Ca2+-dependent protease in rat skeletal muscle. Journal of Nutrition, v.113, p.145-158., 1983.
- BURRIN, D.G.; BRITTON, R.A.; FERRELL, C.L. Visceral organ size and hepatocyte metabolic activity in fed and fasted rats. Journal of Nutrition, v.118, p.1547-1552, 1988.
- CARDOSO, L.A.; STOCK, M.J. Effect of clenbuterol on growth and body composition during food restriction in rats. Journal of Animal Science, v.74, p.22452252, 1996.
- CHOAT, W.G.; KREHBIEL, C.R.; BROWN, M.S.; DUFF, G.C.; WALKER, D.A.; GILL, D.R. Effects of restricted versus conventional dietary adaptation on feedlot performance, carcass characteristics, site and extent of digestion, digestion kinetics, and ruminal metabolism. Journal of Animal Science, v.80, p.27262739, 2002.
- Culler, R.D.; PARRISH, F.C.; SMITH, G.C.; CROSS, H.R. Relationship of myofibril fragmentation index to certain chemical, physical and sensory characteristics of bovine longissimus muscle. Journal of Food Science, v.43, p.117, 1978.
- Delgado, E.F.; GEESINK, G.H.; MARCHELLO, J.A.; GOLL, D.E.; KOOHMARAIE, M. Properties of myofibril-bound calpain activity in longissimus muscle of callipyge and normal sheep. Journal of Animal Science, v.79, p.2097-2107, 2001.
- DIRKS, A.; LEEUWENBURGH, C. Aging and lifelong caloric restriction result in adaptations os skeletal muscle apoptosis repressor, apoptosis inducing factor, x-linked inhibitor of apoptosis, caspase-3, and caspase-12. Free Radical Biology Medicine, v.36, p.27-39, 2004.
- DIRKS, A.; LEEUWENBURGH, C. Caloric restricition in humans: potential pitfalls and health concerns. Mechanisms of Ageing and Development, v.127, p.1-7, 2006.
- Dransfield, E. Modelling post-mortem tenderization. IV. Role of calpains and calpastatin in conditioning. Meat Science, v.34, p.217- 234, 1993.
- DU, M.; ZHU, M.J.; MEANS, W.J.; HESS, B.W.; FORD, S.P. Effect of nutrient restriction on calpain and calpastatin content of skeletal muscle from cows and fetuses. Journal of Animal Science, v.82, p.2541-2547, 2004.
- FARMER, C.; PETITCLERC, D.; SORENSEN, M.T.; VIGNOLA, M.; DOURMAD, J.Y. Impacts of dietary protein level and feed restriction during prepuberty on mammogenesis in gilts. Journal of Animal Science, v.82, p.2343-2351, 2004.
- Geesink, G.H.; Koohmaraie, M. Effect of calpastatin on degradation of myofibrillar proteins by µ-calpain under postmortem conditions. Journal of Animal Science, v.77, p.26852692, 1999a.
- Geesink, G.H.; Koohmaraie, M. Postmortem proteolysis and calpain/calpastatin activity in callipyge and normal lamb biceps femoris during extended postmortem storage. Journal of Animal Science, v.77, p.14901501, 1999b.
- Goicoechea, S.M.; Conde, R.D. Influence of protein nutrition on calpain activity of mouse kidney: modulation by calpastatin. Molecular and Cell Biochemistry, v.166, p.95-99, 1997.
- Goldspink, D.F. The influence of passive stretch on the growth and protein turnover of the denervated extensor digitorum longus muscle. Biochemical Journal, v.174, p.595-602, 1978.
- Goll, D.E.; TAYLOR, R.G.; CHRISTIANSEN, J.A.; THOMPSON, V.F. Role of proteinases and protein turnover in muscle growth and meat quality. Proceedings Recipe Meat Conference, v.44, p.25-33, 1991.
- HICKS, R.B.; OWENS, F.N.; GILL, D.R.; MARTIN, J.J.; STRASIA, C.A. Effects of controlled feed intake on performance and carcass characteristics of feedlot steers and heifers. Journal of Animal Science, v.68, p.233, 1990.
- HOLT, P.R.; DOMINGUEZ, A.A.; KWARTLER, J. Effect of sucrose feeding upon intestinal and hepatic lipid synthesis. American Journal of Clinical Nutrition, v.32, p.1792-1798, 1979.
- HOLT, P.R.; WU, S.; YEH, K.Y. Ileal hyperplastic response to starvation in the rat. American Journal of Physiological Gastrointestinal and Liver Physiology, v.251, p.124-131, 1986.
- HUANG, J.; FORSBERG, N.E. Role of calpain in skeletal-muscle protein degradation. Proceedings of the National Academy of Science, v.95, p.12100-12105, 1998.
- KENDALL, T.L.; KOOHMARAIE, M.; ARBONA, J.R.; WILLIAMS, S.E.; YOUNG, L.L. Effect of pH and ionic strength on bovine m-calpain and calpastatin activity Journal of Animal Science, v.71, p.96-104, 1993.
- KRISTENSEN, L.; THERKILDSEN, M.; RIIS, B.; SORENSEN, M.T.; OKSBJERG, N.; PURSLOW, P.P.; ERTBJERG, P. Dietary-induced changes of muscle growth rate in pigs: Effects on in vivo and postmortem muscle proteolysis and meat quality. Journal of Animal Science, v.80, p.2862-2871, 2002.
- KOOHMARAIE, M.; WHIPPLE, G.; CROUSE, J.D. Acceleration of postmortem tenderization in lamb and Brahman cross beef carcasses through infusion of calcium chloride. Journal of Animal Science, v.68, p.1278, 1990.
- KOOHMARAIE, M. Effect of pH, temperature, and inhibitors on autolvsis and catalvtic activity of bovine skeletal muscle ì-Calpain. Journal of Animal Science, v.70, p.3071-3080, 1992.
- Koohmaraie, M.; WHIPPLE, G.; KRETCHMAR, D.H.; CROUSE, J.D.; MERSMANN, H.J. Postmortem proteolysis in longissimus muscle from beef, lamb and pork carcasses. Journal of Animal Science, v.69, p.617-624, 1991.
- LEVIN, S.; SEMLER, D.; RUBEN, Z.. Effects of two weeks of feed restriction on some common toxicologic parameters in Sprague-Dawley Rats. Toxicology and Pathology, v.21, p.1-14, 1993.
- MAXWELL, L.C.; ENWEMEKA, C.S.; FERNANDES, G. Effects of exercise and food restriction on Rat skeletal muscles Tissue and Cell, v.24, p.491-498, 1992.
- McDonagh, M.B.; HERD, R.M.; RICHARDSON, E.C.; ODDY, V.H.; ARCHER, J.A.; ARTHUR, P.F. Meat quality and the calpain system of feedlot steers following a single generation of divergent selection for residual feed intake. Australian Journal of Experimental Agriculture, v.41, p.1013-1021, 2001.
- Morgan, J.B. Effect of castration on myofibrillar protein turnover, endogenous proteinase activities, and muscle growth in bovine skeletal muscle. Journal of Animal Science, v.71, p.408-414, 1993.
- Murphy, T.A.; Loerch, S.C. Effects of restricted feeding of growing steers on performance, carcass characteristics, and composition. Journal of Animal Science, v.72, p.2497-2507, 1994.
- OGATA, E.S.; FOUNG, S.K.; HOLLIDAY, M.A. The effects of starvation and refeeding on muscle protein synthesis and catabolism in the young rat. Journal of Nutrition, v.108, p.759-765, 1978.
- OWENS, F.N.; DUBESKI, P.; HANSON, C.F. Factors that alter the growth and development of ruminants. Journal of Animal Science, v.71, p.3138-3150, 1993.
- Pethes, G.; BOKORI, J.; RUDAS, P.; FRENYO, V.L.; FEKETE, S. Thyroxine, triiodothyronine, reverse-triiodothyronine, and other physiological characteristics of periparturient cows fed restricted energy. Journal of Dairy Science, v.68, p.1148-1154, 1985.
- POTOKAR-CANDEK, M.; LEFAUCHEUR, L.; ZLENDER, B.; BONNEAU, M. Effect of slaughter weight and/or age on histological characteristics of pig longissimus dorsi muscle as related to meat quality. Meat Science, v.52, p.195-203, 1999.
- Reichling, T.D.; German, R.Z. Bones, muscles and visceral organs of protein-malnourished rats (Rattus norvegicus) grow more slowly but for longer durations to reach normal final size. Journal of Nutrition, v.130, p.2326-2332, 2000.
- RILEY, D.G.; CHASE JR., C.C.; PRINGLE, T.D.; WEST, R.L.; JOHNSON, D.D.; OLSON, T.A.; HAMMOND, A.C.; COLEMAN, S.W. Effect of sire on µ- and m-calpain activity and rate of tenderization as indicated by myofibril fragmentation indices of steaks from Brahman cattle. Journal of Animal Science, v.81, p.2440-2447, 2003.
- SAINZ, R.D; BENTLEY, B.E. Visceral organ mass and cellularity in growth-restricted and refed beef steers. Journal of Animal Science, v.75, p.1229-1236, 1997.
- Solomon, M.B.; CAMPBELL, R.G.; STEELE, N.C.; CAPERNA, T.J.; McMURTRY, J.P. Effect of feed-intake and exogenous porcine somatotropin on longissimus muscle-fiber characteristics of pigs weighting 55 kilograms live weight. Journal of Animal Science, v.66, p.3279-3284, 1988.
- STRAUS, D.S.; TAKEMOTO, C.D. Effect of fasting on insulin-like growth factor-I (IGF-I) and growth hormone receptor mRNA levels and IGF-I gene transcription in rat liver. Molecular Endocrinology, v.4, p.91-100, 1990.
- TAYLOR, R.G.; GEESINK, G.H.; THOMPSON, V.F.; KOOHMARAIE, M.; GOLL, D.E. Is Z-Disk Degradation Responsible for Postmortem Tenderization? Journal of Animal Science, v.73, p.1351-1367, 1995.
- Therkildsen, M.; Larsen, L.M.; Vestergaard, M. Influence of growth rate and muscle type on muscle fibre type characteristics, protein synthesis capacity and activity of the calpain system in Friesian calves. Journal of Animal Science, v.74, p.253-264, 2002.
- THOMSON, B.C.; HOSKING, B.J.; SAINZ, R.D.; ODDY, V.H. The effect of nutritional status on protein degradation and components of the calpain system in skeletal muscle of weaned wether lambs. Journal of Agriculture Science, v.129, p.471-477, 1997.
- Wertz, A.E.; BERGER, L.L.; FAULKNER, D.B.; NASH, T.G. Intake restriction strategies and sources of energy and protein during the growing period affect nutrient disappearance, feedlot performance, and carcass characteristics of crossbred heifers. Journal of Animal Science, v.79, p.1598-1610, 2001.
- WHIPPLE, G.; KOOHMARAIE, M.; DIKEMAN, M.E., CROUSE, J.D. Predicting beef-longissimus tenderness from various biochemical nd histological muscle traits. Journal of Animal Science, v.68, p.4193-4199, 1990a.
- WHIPPLE, G.; KOOHMARAIE, M.; DIKEMAN, M.E.; CROUSE; HUNT, M.C.; KLEMM, R.D. Evaluation of attributes that affect longissimus muscle tenderness in Bos taurus and Bos indicus cattle. Journal of Animal Science, v.68, p.2716-2728, 1990b.
Publication Dates
-
Publication in this collection
31 Mar 2008 -
Date of issue
Apr 2008
History
-
Accepted
14 Nov 2007 -
Received
05 May 2006