Abstracts
Lasiodiplodia theobromae is an important fungal pathogen of higher plants from tropical and sub-tropical regions. The fungus infects divergent hosts in a wide range of environmental conditions, suggesting that it is highly variable. The aim of this study was to develop new polymorphic microsatellite markers from a Brazilian isolate of L. theobromae that can be used in population studies of this and related fungi. The nine microsatellite markers developed included six that revealed allelic polymorphisms among nine isolates of the disease collected from infected plants in Brazil. Preliminary evaluation of the markers suggested substantial genetic variability among Brazilian L. theobromae populations. These markers have potential utility for evolutionary and epidemiologic studies of this fungus.
simple sequence repeat; molecular marker
Lasiodiplodia theobromae é um importante patógeno de vegetais superiores nas regiões tropicais e sub-tropicais, sendo capaz de infectar diferentes hospedeiros sob as mais variadas condições ambientais, o que sugere uma grande variabilidade genética. O objetivo deste estudo foi o de desenvolver marcadores moleculares microsatélites polimórficos de um isolado brasileiro que possa ser usado na caracterização da população de L. theobromae e fungos correlatos. Nove microsatélites foram identificados no genoma deste isolado, o que permitiu o desenvolvimento de seis marcadores polimórficos em uma população de nove isolados patogênicos de plantas tropicais do Brasil. Testes preliminares revelaram uma acentuada variabilidade genética entre os isolados testados, confirmando a grande variabilidade da população brasileira deste fungo. Estes marcadores poderão ser usados em estudos evolutivos em epidemiológicos desta espécie.
seqüência simples repetida (SSR); marcador molecular
NOTAS CIENTÍFICAS
Development and characterisation of microsatellite markers for the fungus Lasiodiplodia theobromae
Desenvolvimento e caracterização de marcadores microsatélites para o fungo Lasiodiplodia theobromae
José Emilson CardosoI; Michael James WilkinsonII
IEmbrapa Agroindústria Tropical, CP 3761, CEP 60 511-110, Fortaleza,CE, e-mail emilson@cnpat.embrapa.br
IIThe School of Plant Sciences, The University of Reading, Reading, UK
ABSTRACT
Lasiodiplodia theobromae is an important fungal pathogen of higher plants from tropical and sub-tropical regions. The fungus infects divergent hosts in a wide range of environmental conditions, suggesting that it is highly variable. The aim of this study was to develop new polymorphic microsatellite markers from a Brazilian isolate of L. theobromae that can be used in population studies of this and related fungi. The nine microsatellite markers developed included six that revealed allelic polymorphisms among nine isolates of the disease collected from infected plants in Brazil. Preliminary evaluation of the markers suggested substantial genetic variability among Brazilian L. theobromae populations. These markers have potential utility for evolutionary and epidemiologic studies of this fungus.
Additional keywords: simple sequence repeat, molecular marker.
RESUMO
Lasiodiplodia theobromae é um importante patógeno de vegetais superiores nas regiões tropicais e sub-tropicais, sendo capaz de infectar diferentes hospedeiros sob as mais variadas condições ambientais, o que sugere uma grande variabilidade genética. O objetivo deste estudo foi o de desenvolver marcadores moleculares microsatélites polimórficos de um isolado brasileiro que possa ser usado na caracterização da população de L. theobromae e fungos correlatos. Nove microsatélites foram identificados no genoma deste isolado, o que permitiu o desenvolvimento de seis marcadores polimórficos em uma população de nove isolados patogênicos de plantas tropicais do Brasil. Testes preliminares revelaram uma acentuada variabilidade genética entre os isolados testados, confirmando a grande variabilidade da população brasileira deste fungo. Estes marcadores poderão ser usados em estudos evolutivos em epidemiológicos desta espécie.
Palavras chave: seqüência simples repetida (SSR), marcador molecular.
Lasiodiplodia theobromae (Sin. Botryodiplodia theobromae, teleomorphic form Botryosphaeria Rhosdina (Berk. & M.A. Curtis)Arx AMX is one of the most important fungal plant pathogens in northern Brazil. This fungus is both ubiquitous and plurivorous and is able to infect over 500 plant species; causing symptoms ranging from seed rot to the discolouration of timber (7, 8). Infection is generally limited to wounded or stressed-weakened plants (1, 4, 5, 6). The species reproduces mainly by asexual mitospores, with the sexual stage rarely being observed under field conditions (7).
The current taxonomic treatment of L. theobromae is based entirely on culture characteristics and asexual reproductive structures (e. g. pycnidium, conidiophore, and conidiospores) (7). The extensive list of synonyms that apply to the species illustrates the confusion that exists over its taxonomic and phylogenetic status. Therefore, it is important to determine whether different genetic variants have evolved to specialise towards the infection of particular hosts or if the entire aggregate is characterised by genotypes that all possess the capacity for a broad host range. In order to distinguish between these alternative scenarios, it is first important to develop the capability to discriminate between closely related pathotypes.
Simple Sequence Repeat (SSR) or microsatellite analysis is widely acknowledged as the method of choice for molecular studies of population genetic structure, kinship, genotype diagnosis and genetic evolution. Whilst some SSR markers have recently been developed for species closely related to L. theobromae from South Africa (2, 3, 9), the aim of this study was to develop polymorphic microsatellite markers from Brazilian isolates that can be used in population studies of L. theobromae and related fungi.
A mycelial mat (200mg) was cultured from an isolate (Ao1) from a gummosis-infected cashew plant from northeastern Brazil (Piauí state) and grown in liquid still culture of potato dextrose broth (PDB) for 15 days. The culture was then macerated and its DNA extracted using the DNeasy kit (QiagenR) according to the manufacturer's protocol. A genomic library was constructed by first double-digesting fungal genomic DNA using restriction enzymes Sau3AI and Hind III to generate fragments typically 600-800bp and then ligation of the products directly into the pGEM vector using the manufacturer's instructions (Promega, Madison, Wis, USA) before transforming the plasmids into Escherichia coli (Stratagene), by heat shock. Clones with inserts containing SSRs were selected by colony blotting with a variety of P32 radioactively labelled oligonucleotide probes (e g CA15, AGG15, GA15), and exposing to X-ray film overnight at -80ºC.
Inserts of 'positive' colonies were amplified by PCR using the plasmid-specific M13 universal forward and reverse primers. The reaction mix for the PCR comprised of 1.0ìl 10x reaction Buffer, 0.3ìl MgCl2, 0.2ìl deoxynucleotide triphosphate (dNTP), 0.1ìl Taq DNA polymerase, 0.3ìl each primer pairs as described, 1 ìl bacterial suspension, and 6.80ìl deionised water. PCRs were performed on a Gene Amp® PCR System 2700 (Applied Biosystems) thermal cycler using an initial 94°C denaturing step for 1 min followed by 35 cycles of 1 minute to the 94ºC, 1 minute at 55ºC (annealing) and 4 minutes at 72ºC(extension), and finally 10 minutes at 72ºC. PCR amplicons from specific clones were size-estimated after electrophoresis through a 1.5% (w/v) agarose (midi) gel at 120V for around 2h 30minutes.
Strong amplicons in the expected size range were purified using QIAprep(-Qiagen) protocol and subjected to cycle sequencing after PCR using the M13 primers and BigDye terminator (Perkin-Elmer Applied Biosystems) cycle sequencing kit (4.0ìl BigDye 3.1, 1.6ìl 10ìM M13, SSR primers, and 4.4ìl of template DNA). The reaction was programmed for 30s denaturation at 95ºC, followed by 30 cycles of 30 s (annealing) at 50ºC, and 4 min at 60ºC (extension). Sequencing was performed on a ABI PrismTM (Perkin-Elmer Applied Biosystems) and base sequence was analysed using ChromasPro 2.3 software (http://www.technelysium.com.au/chromas.html). A total of 41 clones were sequenced, 11 sequences containing microsatellites were identified and flanking primers were designed to amplify these regions by using the Primer3 software (Whitehead Institute Biomedical Research, available at: http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi.).
Ten SSRs were found to contain 6 to 15 uninterrupted repeats and so specific primer pairs were designed to target the flanking regions of these loci by reference to the insert sequence. A gradient thermocycler (Whatman-Biometra) was then used to determine the optimal annealing temperature for each SSR primer pair; genomic DNA of isolate Ao1 acted as template for all PCRs. Nine of the ten primer pairs generated amplicons, with annealing temperatures ranging from 52 to 62º C (Table 1). Primers targeting locus 7 failed to generate any PCR products.
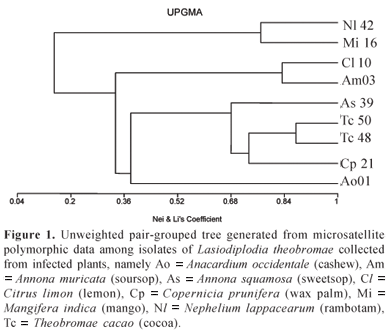
These SSR markers were then evaluated for their ability to reveal allelic polymorphisms between genotypes of the fungus using the following nine Brazilian isolates of L. theobromae collected from divergent plant hosts: Ao (Anacardium occidentale, cashew), Am (Annona muricata. soursop), Cp (Copernicius prunifera, wax palm), Mi (Mangifera indica, mango), Cl (Citrus limon, lemon), As (Annona squamosa, sweetsop), Nl (Nephelium lappacearum, rambotam), and two isolates of Tc (Theobromae cacao, cocoa). For this, one of the primer pair was fluorescently labelled with HEX or FAM (CMWG), submitted to PCR and fractionated by capillary electrophoresis on a ABI PrismTM, automated sequencer. The size of all amplicons detected was determined using the Genotyper® package software.
In spite of the limited number of samples included in this test, the PCR products yielded by seven primer pairs included polymorphisms in allele length among genotypes (Table 1). Data from Genotyper were then compiled into Excel files (Microsoft),with a data matrix of characters being compiled for each isolate by scoring the presence or absence of each allele at each locus. These data were then used to generate an Unweighted pair-group method with arithmetic mean (UPGMA) genetic distance tree based on Nei and Li's unbiased genetic distance and using the statistical package MVSP (Multi-Variate Statistical Package v.3.1) (Kovach Computing Services, Anglesey, Wales).
The resultant dendrogram revealed variability among all nine isolates. Interestingly the two isolates collected from Theobroma cacao showed markedly lower levels of genetic divergence than that between isolates from distantly related hosts (Fig. 1).
Thus, the primers developed in this study can be used to determine the genetic diversity of L. theobromae isolates and will also have value to provide diagnostic tests to distinguish ecotypes of this fungus.
ACNOWLEDGEMENTS
We thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, MCT-Brazil) and the Empresa Brasileira de Pesquisa Agropecuária (Embrapa) - Brazil for financial assistance . We also thank F.C.O. Freire, F.M.P. Viana, E.D.N.L. Martins and A.A. dos Santos for providing fungus isolates and Adam Croixford, Sara Hughes, Moy Robson, J.J.V.Cavalcanti, and Joel Allainguillame for technical assistance.
Data de chegada:09/11/2006.
Aceito para publicação em: 08/10/2007
Autor para correspondência: José Emilson Cardoso.
- 1. Britton, K.O.; Hendrix F.F. Population dynamics of Botryosphaeria spp. in peach gummosis canker. Plant Disease, Saint Paul, v.70, p.134-136,1986
- 2. Burgess, T.; Wingfield M.J.; Wingfield B.D. Simple sequence repeat markers distinguish among morphotypes of Sphaeropsis sapinea Applied and Environmental Microbiology, Washington, v. 67, p. 354-362, 2001.
- 3. Burgess, T.; Wingfield M.J.; Wingfield B.D. Development and characterization of microsatellite loci for the tropical tree pathogen Botryosphaeria rhodina Molecular Ecology Notes, Berlin, v.3, p.91-94, 2003.
- 4. Cardoso, J.E.; Vidal, J.C.; Santos, A.A.; Freire, F.C.O.; Viana, F.M.P. First report of black branch dieback of cashew caused by Lasiodiplodia theobromae in Brasil. Plant Disease, Saint Paul, v. 86, p.558, 2002.
- 5. Cardoso, J.E. Freire, F.C.O.; Sá, F.T. Disseminação e controle da resinose em troncos de cajueiro decepados para substituição de copa. Fitopatologia brasileira, Brasília, v. 23, p. 48-50, 1998.
- 6. Freire, F.C.O.; Cardoso, J.E.; Santos, A.A.; Viana, F.M.P. Diseases of cashew (Anacardium occidentale L.) in Brazil. Crop Protection, London, v. 21, p. 89-494, 2002.
- 7. Punnithaligam, E. Botryodioplodia theobromae cmi. Descriptions of Pathogenic Fungi and Bacteria. London: CMI. n.519, p.1-3. 1976.
- 8. Punnithaligam, E. Plant diseases attributed to Botryodiplodia theobromae Pat. Bibliotheca Mycologia, London, v.71, p.1-123, 1980.
- 9. Slippers, B.; Burgess, T.; Wingfield, B.D.; Crous, P.W.; Coutinho, T.A.; Wingfield, M.J. Development of simple sequence repeat markers for Botryosphaeria spp. with Fusicoccum anamorphs. Molecular Ecology Notes, Berlin, v.4, p. 675-677, 2004.
Publication Dates
-
Publication in this collection
26 May 2008 -
Date of issue
Feb 2008
History
-
Accepted
08 Oct 2007 -
Received
09 Nov 2006