Abstracts
We describe the applicability of the E test (AB Biodisk Solna, Sweden), a new method for determining minimum inhibitory concentrations of antimicrobial agents against bacteria. This report is based on the literature review and on our own experience using the E test for susceptibility testing of the Xanthomonas maltophilia, Streptococcus pneumoniae and Streptococcus viridans group against eight different drugs.
Antibiogram; E test; susceptibility
Descrevemos a aplicabilidade do E test (AB Biodisk, Solna, Suécia), um novo método para a determinação das concentrações inibitórias mínimas de agentes antimicrobianos contra bactérias. Apresentamos uma revisão da literatura e nossa experiência na utilização do E test na determinação da suscetibilidade de Xanthomonas maltophilia, Streptococcus viridians contra oito diferentes drogas.
ORIGINAL ARTICLE
E Test: a novel technique for antimicrobial susceptibility testing
Helio Silva SaderI; Antonio Carlos Campos PignatariII
IDepartment of Infectious and Parasitic Diseases of the Escola Paulista de Medicina and Clinical Microbiology at the Anti-Infectives Research Center, Department of Pathology, University of Iowa
IIClinical Microbiology Laboratory of the Department of Infectious and Parasitic Diseases of the Escola Paulista de Medicina
Address for correspondence Address for correspondence: Dr. Antonio Carlos Campos Pignatari Disciplina de Doenças Infecciosas e Parasitárias, Escola Paulista de Medicina, Rua Botucatu, 740 - São Paulo - SP Brasil - CEP 04023-062
ABSTRACT
We describe the applicability of the E test (AB Biodisk Solna, Sweden), a new method for determining minimum inhibitory concentrations of antimicrobial agents against bacteria. This report is based on the literature review and on our own experience using the E test for susceptibility testing of the Xanthomonas maltophilia, Streptococcus pneumoniae and Streptococcus viridans group against eight different drugs.
Uniterms: Antibiogram, E test, susceptibility.
RESUMO
Descrevemos a aplicabilidade do E test (AB Biodisk, Solna, Suécia), um novo método para a determinação das concentrações inibitórias mínimas de agentes antimicrobianos contra bactérias. Apresentamos uma revisão da literatura e nossa experiência na utilização do E test na determinação da suscetibilidade de Xanthomonas maltophilia, Streptococcus viridians contra oito diferentes drogas.
Measurement of the susceptibility of microorgan isms to antimicrobial agents is of great impor tance in the rational use of antimicrobial agents, in the evaluation of new drugs, and in epidemiological studies. However, the results of such measurements are not an absolute value because they are influenced sometimes markedly, by the test conditions used. Differences in each factor such as inoculum time, may all affect the amount of antimicrobial agent required to inhibit the organism in vitro (7). These factors make the standardization of susceptibility testing crucial.
Antimicrobial susceptibility testing may be done by a variety of techniques. The most frequently used method in Brazil is disk diffusion (3, 12). This is a test yields a qualitative result, such as classification of the organisms as being either susceptible, intermediate or resistant.
A major advantage of this procedure is its flexibility in the number and kind of antimicrobial agents that can be tested, and the easiness of setting up individual tests. It is technically simple and requires careful attention to de-tails, leading to reproducible results. The deficiencies of the disk diffusion test are its non-quantitative interpretation and its inapplicability to many fastidious organisms and anaerobes.
Other methods for susceptibility testing include: broth microdilution (13), agar dilution (13), and, more recently automated or mechanized susceptibility testing techniques (10). The convenience afforded by the availability of dilution susceptibility testing in broth microdilution trays has led the widespread use of microdilution methods in the United States and other developed countries.
The use of microdilution trays prepared in house allows simultaneous testing of several antimicrobial agents against individual organisms and also provides a reliable standardized reference method for susceptibility testing. This method is well standardized by the national Commit-tee for Clinical Laboratory Standards (NCCLS) (13) in the United States. However, the work involved in preparing the trays and the substantial costs of purchasing the laboratory hardware for in-house preparation may detract from the convenience of this method. Dilution testing by agar method is also standardized method and a reliable susceptibility testing technique that may be used as a reference for evaluating the accuracy of other testing systems. Additionally, the simultaneous testing of several isolates is possible (usually 20 to 37) and microbial contamination or heterogeneity is more readily detected than broth methods. The major disadvantages of the agar dilution are the time-consuming and the labor-intensive tasks of preparing the plates and inocula, especially as the number of different antimicrobial agents to be tested against each isolate increases.
The susceptibility testing instruments now available offer different levels of automation. Some instruments only interpret growth endpoints whereas, others incubate broth microdilution trays or special cuvettes and perform serial or final interpretations of growth in the presence of anti-microbial agents. Current instruments utilize either the principle of turbidity detection of bacterial growth in a liquid medium or detection of hydrolysis of a fluorogenic substrate incorporated in a special liquid medium (10). Al-though current microbiology instruments offer both potential for improved intra- and interlaboratory reproducibility and significantly reduces the time required to per-form the tests, the accuracy of the results has been lower than that of manual reference systems, particularly if the instrument has a short incubation period. An incubation period of only 3 to 5 hours may not be adequate for expression of all bacterial resistance mechanisms; e,g., inducible B -lactamase-mediated resistance among gram-negative bacilli to some enzyme-labile symbol 66 \f "Symbol" \s 10 -lactam antibiotics (10). The range of drug dilution offered by these methods is usually very narrow.
The E test is a new in vitro method developed to determine the minimum inhibitory concentration (MIC in symbol 117 \f "Symbol" \s 10 g/ml) of individual antimicrobial agents on agar medium (4). The E test overcomes some disadvantages of disk diffusion and broth dilution procedures. Additionally, it is able to retain some of the favorable principles of agar dilution testing by simply producing quantitative MICs.
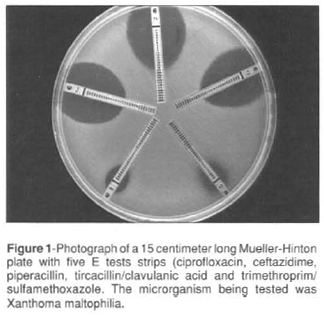
The E test consists of a thin reagent strip that carries a continuous concentration gradient of stabilized and dried drug (figure 1). The gradient range is equivalent to 15 log2 dilutions by a conventional reference MIC procedure (13). The E test then uses the principle of agar diffusion to perform quantitative testing (4). In order to determine an MIC with the E test, the surface of an agar plate is swab inoculated with an adjusted bacterial suspension in the same manner as a disk diffusion test. One or more E test strips for the antimicrobial agents to be tested are then placed on the inoculatated agar surface.
After an overnight incubation, the interaction of the antimicrobial agent gradient and the test bacterial inoculum gives rise to elliptical inhibitory zones (figure 1).
The results are read in the intersection of the ellipse with a MIC scale on the strip (figure 2).
The E test is of particular interest to the clinical laboratory because it allows the determination of the susceptibility (MIC) of an organism to one or more selected antibiotics rather than to a predetermined panel of antimicrobial agents. Since October 1991, when it was released by the Food and Drugs Administration (FDA) for use in the United States, the E test has been evaluated in several clinical studies (2, 5,11,14).
It has shown to be particularly useful for testing fastidious and unusual bacteria that can not be easily tested by more traditional methods, e.g., Streptococcus penumoniae, Hemophilus influenzae, Neisseria gonorrhoeae and anaerobic bacteria (8, 9).
In the study of Jorgensen et al, 100 Streptococcus penumoniae and 50 Hemophilus influenzae that demonstrated various resistance mechanisms and levels of anti-microbial suscetibility were examined by E tests performed on agar media currently recommended by the NCCLS (HTM and Muller-Hinton sheep blood agars, respectively). The E test MICs for a total of 10 antimicrobial agents were compared with broth microdilution MICs which were determined by following NCCLS recommendations. In general, E test MICs, for both species were quickly and easily interpreted and agreed within one log2 MIC increment in 89% of tests with Hemophilus influenzae and in 80% of pneumococcal tests. Only 0,7% of the tests have had major errors. The majority of disagreements occurred with trimethoprim/sulfamethoxazole. Therefore, the E test was found to be a reliable alternative method for the de-termination of MICs of these two fastidious bacterial strains. The E test was also compared with agar dilution using other fastidious and resistant bacterial (15). The E test showed a quantitative accuracy (+- 2 log 2 dilutions) of 99% for N gonorrheae and 95% for Enterococcus spp.
Baker et al (1) of the USA Centers for Disease Control compared the E test with disk diffusion, broth microdilution, and agar dilution tests by using a challenge set of 195 gram-positive and gram-negative bacteria for 14 antimicrobial agents, and the E test agreement was greater than 95%. The E test would be particularly useful in cases for which an MIC of penicillin is required for suspected penicillin-resistant pneumococci, for determining the level of oxacillin resistance in staphylococci, and for measuring the MICs of ampicillin and vancomycin for Enterococcus faecium isolates from severe infections.
We have used the E test for suscetibility testing eight Xanthomas maltophilia isolated from an outbreak in hemodialysis center in São Paulo. We tested the suscetibility for ciprofloxacin, ceftazidime, piperacillin, ticarcillin/clavulanic acid and trimethorpim/ sulfamehtoxazole on a Muller-Hinton agar plate (figure 1).The E test inhibition ellipses were clearly demarcated, and the points of intersection of the zone edge with the strips were generally easy to interpret (figure 2).
We have also used the E test for suscetibility testing of S pneumoniae and resistant viridans group Streptococcus isolates to penicillin, chloramphenicol, ciprofloxacin, erythromycin, trimethroprim/sulfamethoxazole by using blood agar plates. We did not have any problems for interpreting the results.
CONCLUSIONS
The E test represents a new innovative approach for the determination of antimicrobial suscetibility which is potentially applicable to a wide array of drugs and micro-organisms. Like the agar disk diffusion method, the E test is easy to execute. Nonetheless, the E test goes one step further by providing quantitative wide-range MICs in a simple and easily reproducible manner.
The E test approach may be well suited for the testing of certain fastidious bacteria or bacteria that is difficult to test. Further studies are required to fully explore the potential of the new method employed in antimicrobial suscetibility testing.
Acnowledgements: The authors are grateful to Dr. Ronald N. Jones from the Anti-Infectives Research Center, University of Iowa, USA, for reviewing this manuscript and providing technical support for this study.
- 1. BAKER, C.N.; STOKER, S.A.; CULVER, D.H. & THORNSBERRY, C. - Comparison of the E test to agar dilution, broth microdilution, and agar diffusion susceptibility testing thecniques by using a special challange set of bacteria. J Clin Microbiol, 29: 533-538, 1991.
- 2. BAKER, C.N. - The E test and Campylobacter jejuni. Diagn Microbiol Inf Dis, 15: 469-472, 1992.
- 3. BAUER, A.W.; KIRBY W.M.M.;SHERRIS, J.C. & TURK, M. - Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol, 45: 493-496, 1966.
- 4. BOLMSTROM, A.; ARVIDSON, S.; ERICSSON, M. & KARISSON, A.- A novel technique for direct quantification of antimicrobial susceptibility of microorganisms (abst 1209). Los Angeles ICAAC, 1988.
- 5. BROWN, D.F.J. - The E test challenged with selected strains. Diag Microbiol Inf Dis, 15: 465-468, 1992.
- 6. CITRON, D.M.; OSTOVARI, M.I.; KARISSON, A. & GOLDSTEIN, E.J.C. - Evaluation of E test for susceptibility testing of anaerobic bacteria. J Clin Microbiol, 29: 2197-2203, 1991.
- 7. ERICSSON, M.H.; SHERRIS, J.C., ed. Antibiotic sensititity testing. A report of an international collaborative study. Acta Pathol Microbiol Scand B (Suppl 217): 1-90, 1971.
- 8. JACOBS, M.R.; BAJAKSOUZIAN, S.; APPELBAUM, P.C. & BOLMOSTROM, A. - Evaluation of the E test for susceptibility testing of Pneumococci. Diag Microbiol Inf Dis, 15: 473-478, 1992.
- 9. JORGENSEN, J.H.; HOWELL, A.W. & MAHER, L.A. - Quantitative antimicrobiol susceptibility testing of Haemophilus influenza and Streptococcus pneumoniae by using the E test. J Clin Microbiol, 29: 109-114, 1991.
- 10. JORGENSEN, J.H. - Antibacterial susceptibility tests: auto-mated or instrument-based methods. In BALLOWS, A.; HAUSLER, W.J.Jr.; HERRMANN, K.L.; ISENBERG, H.D.; SHADOMY, H.J. (ed). Manual of Clinical Microbiology, 5`h Ed. American Society for Microbiology, Washington, D.C., p. 1166-1172, 1991.
- 11. KNAPP, C.C. & WASHINGTON, J.A. - Comparison of the E test and microdilution for detection of B-lactam-resistant mutants that are stably derepressed for type I B-lactamase. J Clin Microbiol, 30: 214:215, 1992.
-
12National Committee for Clinical Laboratory Standards (NCCLS). Performance standards for antimicrobial disk diffusion test standard, M2-T4, 2nd ed. National Committee for Clinical Laboratory Standards. Villanova, PA, 1990.
-
13National Committee for Clinical Laboratory Standards: Standards methods for dilution antimicrobial susceptibility test for bacteria that grow aerobicaly, Standard, M7-A2, 2nd ed. National Committe for Clinical Laboratory Standars, Villanova, PA, 1990.
- 14. RAUTELIN, H.; RENKONEN, I & RENKONEN, O.V. - Evaluation in testing susceptibility of Pseudomonas aeruginosa to tobramycin. Eur J Clin Microbiol Inf Did, 11: 177-180, 1992.
- 15. SANCHEZ, M.L.; BARRET, M.S. & JONES, R.N. - The E test applied to susceptibility tests of Gonococci, multiply-resistant Enterococci, and Enterobacteriaceae producing potent B-lactamases. Diagn Microbiol Infect Dis, 15: 459-463, 1992.
Publication Dates
-
Publication in this collection
03 July 2009 -
Date of issue
Dec 1994