Abstract
The replacement and growth of 311 primary feathers of eight captive male rock pigeons, Columba livia Gmelin, 1789 were monitored daily. Feather replacement was recorded in all months, but the primaries 1 to 5 (innermost primaries) were replaced mostly from September to December, whereas the primaries 6 to 10 (outermost primaries) were more frequently replaced from January to August. Each primary was held in plumage from six to fifteen months, but the lifetime of the outer feathers was longer than that of the inner feathers. A new primary emerges two or three days after its predecessor has been dropped, but the primaries replacing the feathers accidentally lost during bird handling emerge only after about eight days. The average growth period of a primary ranged from 21 to 37 days, with the larger and outermost feathers exhibiting a longer growth period. A constant average growth rate of 4 to 5 mm/day was found for all primaries until the last two days of growth, when the growth rate of the feathers became progressively slower. Bilateral symmetry in the primary replacement, when the same feather is replaced simultaneously in both wings, was not significant (22.2%) in the birds monitored in this study.
Feather growth; feather replacement; molt; primary feather
BIOLOGY
Replacement and growth of primary feathers in captive rock pigeons, Columba livia (Aves: Columbidae)
Francisco Mallet-Rodrigues
Laboratório de Ornitologia, Departamento de Zoologia, Instituto de Biologia, Universidade Federal do Rio de Janeiro. 21944-970 Rio de Janeiro, RJ, Brazil. E-mail: fmallet@bol.com.br
ABSTRACT
The replacement and growth of 311 primary feathers of eight captive male rock pigeons, Columba livia Gmelin, 1789 were monitored daily. Feather replacement was recorded in all months, but the primaries 1 to 5 (innermost primaries) were replaced mostly from September to December, whereas the primaries 6 to 10 (outermost primaries) were more frequently replaced from January to August. Each primary was held in plumage from six to fifteen months, but the lifetime of the outer feathers was longer than that of the inner feathers. A new primary emerges two or three days after its predecessor has been dropped, but the primaries replacing the feathers accidentally lost during bird handling emerge only after about eight days. The average growth period of a primary ranged from 21 to 37 days, with the larger and outermost feathers exhibiting a longer growth period. A constant average growth rate of 4 to 5 mm/day was found for all primaries until the last two days of growth, when the growth rate of the feathers became progressively slower. Bilateral symmetry in the primary replacement, when the same feather is replaced simultaneously in both wings, was not significant (22.2%) in the birds monitored in this study.
KEY WORDS: Feather growth; feather replacement; molt; primary feather.
Feathers are the diagnostic feature of the birds. Because old feathers are less efficient, particularly in flight, they need to be replaced when they become worn out (STRESEMANN & STRESEMANN 1966). Feathers are replaced at variable intervals depending on the species. Small birds molt all their feathers once, or even twice a year (PRYS-JONES 1991). Some large birds replace their entire plumage every two years, whereas other birds replace their feathers almost continuously throughout the year (EDWARDS & ROHWER 2005, EDWARDS 2008). These birds have multiple waves of primary replacement proceeding simultaneously on each wing. Multiple waves of feather replacement occur in two ways. In some birds such as albatrosses and falcons, the primaries are molted into two or more non-simultaneous replacement series on each wing (MILLER 1941, LANGSTON & ROHWER 1995, PYLE 2005, EDWARDS & ROHWER 2005), while in other birds the primaries of each wing undergo two or more distinct waves of feather replacement, at the same time and in the same direction (ROHWER & WANG 2010).
The normal process of feather replacement is influenced by a number of interacting factors such as hormonal variations, photoperiod and nutritional conditions (COOPER & HARRISON 1994). Considerable energy is spent during the molting process, from 15 to 30% above of the basal metabolic rate (ALTMAN 1982, MCKIBBEN & HARRISON 1986), because feathers contain 20 to 30% of the structural proteins of a bird (ALTMAN 1982). The primaries represent almost 1% of the body mass of the Eared Dove, Zenaida auriculata (Des Murs, 1847) (GRILLI & MONTALTI 2010). Thus, the loss of feathers during the molting process is reflected in a reduction in structural protein values. An increase in nutritional demand for protein for a new feather is essential to the molting process. Blood calcium levels also drop during the molt (ALTMAN 1982).
The replacement of flight feathers in most birds is sequential, bilaterally symmetrical, and follows a centrifugal pattern. In this manner, those feathers are replaced progressively over a period of several weeks, and only one or two flight feathers are missing from each wing and each side of the tail at any given time (STRESEMANN & STRESEMANN 1966, SICK 1997, MALLET-RODRIGUES & NORONHA 2001).
The loss of a feather from a follicle is followed by the growth of a new feather. Thus, the same follicle is capable of producing several generations of feathers throughout the life of the bird. Initially the growing feather consists of a tubular invagination in the follicle with its own blood and nerve supply. The new feather pushes the older feather out, forcing it to fall. The emerging feather is encased in a sheath of keratin and once it is mature, the blood supply is interrupted and the feather becomes an inert structure (WATSON 1963, SIMMONS 1964, LUCAS & STETTENHEIM 1972). The daily growth rate of a flight feather ranges from 2 mm/day to smaller feathers (WOLF et al. 2003) to more than 8 mm/day to larger feathers (INGOLFSSON 2008).
Primaries are the principal flight feathers on the wing of a bird. Most species have nine or ten primaries attached to the hand bones. The primary molt is an event of great importance in the life of birds, because the primaries are directly related to their ability to fly (STRESEMANN & STRESEMANN 1966).
Many studies have assessed the growth of feathers by measuring the width of daily growth bands (WHITE et al. 1991, WHITE & KENNEDY 1992, ROHWER & WANG 2010, JOVANI et al. 2011). Growth bands are distinct and fairly uniform bars across a flight feather indicating a 24-h period of feather growth (MICHENER & MICHENER 1938, WOOD 1950). The width of daily growth bars on a feather has also been used to assess the nutritional condition of birds while the feather was growing (GRUBB 1989, 1991).
The main objective of this study is to contribute to the knowledge of the replacement and growth patterns of primary feathers of birds by monitoring them on captive domestic rock pigeons, Columba livia Gmelin, 1789.
MATERIAL AND METHODS
Eight captive adult Rock Pigeon males were studied between 1998 and 2006 in the state of Rio de Janeiro, southeastern Brazil. These pigeons, fed twice a day, received a diet with varied seeds and water ad libitum. Male pigeons were identified by their mating display behavior, when they vocalize intensely and are engaged in a courtship that includes a mating dance.
Every day of each month of the study period the plumage of each bird was examined in search for molting primaries. Every morning, data on the dropping and growth of primary feathers during molt were carefully gathered. Primary feathers were analyzed individually. In order to measure the length of each primary, the feather was held flat on a millimeter ruler. Each feather was measured from the insertion of the calamus on the skin to its distal end. The ten primaries were numbered from the innermost (primary 1 or P1) outward (primary 10 or P10).
Analysis of variance (ANOVA) and Spearman rank correlation were used for the descriptive analysis. Statistical significance was accepted at p < 0.05.
RESULTS
During the study period, 306 molting primary feathers were monitored. Additionally, five accidentally replaced primaries were also studied. (Tab. I). Molting of primary feathers was recorded all year round, but nearly half of the molting primaries were replaced in October, November and December. P1 to P5 were almost completely molted from September to December, whereas P6 to P10 were mostly replaced from January to August (Tab. II).
The lifetime of 238 primaries was monitored in this study. Primaries were retained in the plumage from six to fifteen months. The outermost primaries had a longer average lifetime than the innermost primaries (F = 15.08, p < 0.05). While a P1 had an average lifetime of 8.2 months, a P10 was retained in the plumage during an average of 12.2 months (Tab. III).
Only fifteen (30%) of the fifty events of complete wing molt studied here had a perfect centrifugal sequence of primary replacement. In most birds, although the innermost primaries were replaced before the outermost primaries, changes in the centrifugal wave of feather replacement were often recorded in the pigeons.
Each old primary feather dropped when the feather adjacent to it, and previously replaced, had grown three-quarters (or approximately two weeks after feather emergence). The replacement time for primaries that had dropped during bird handling was longer (eight days) than the time taken to replace feathers that had molted naturally (Tab. I).
The tubular invagination of a new primary feather emerged between one and three days after the old feather had dropped. The time elapsed between the drop of an old feather and the emergence of a new feather did not differ significantly among the distinct primaries (F = 8.91, p < 0.05). Smaller feathers (innermost primaries) emerged, on average, two days after the old feather had dropped, whereas larger feathers (outermost primaries) emerged between two and three days after the fall of the old feather (Tab. I).
The average growth period of the primaries ranged from 21 to 37 days. A significant positive correlation was found between the period a feather takes to grow and its length (r = 0.94, p < 0.05). The average growth period of larger feathers was longer than that of smaller feathers (Fig. 1). While a P1 with an average length of 101.1 mm took 21 days to reach its final size, a P9 with an average length of 168.7 mm took approximately 36 days. However, the longest average growth period (37 days) was recorded for P10, a feather on average smaller than P8 and P9. The growth period of the feathers replacing the ones that had dropped accidentally was not different from the growth period of feathers that had fallen naturally during the molt (F = 0.20, p < 0.05). All primaries that had dropped accidentally grew within the expected growth period (Tab. I).
All primaries exhibited a constant growth rate until their last two days of growth, when their growth rate progressively decreased (Fig. 1). The average growth rate of the primaries ranged from 4 to 5 mm/day (Tab. IV).
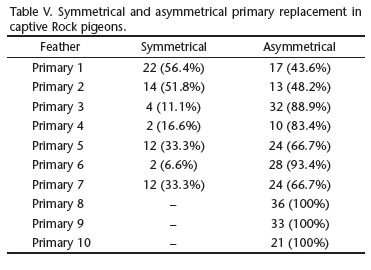
The primary molt was bilaterally asymmetrical in most cases. Symmetrical molt was found in only 68 (22.2%) of the 306 molting primaries, when the same primary was molting simultaneously in both wings. P1 and P2 were the only primaries exhibiting more symmetrical than asymmetrical replacement (Tab. V).
DISCUSSION
Data on the growth of wild bird feathers has been mainly obtained through studies of growth bands (WHITE et al. 1991, ROHWER & WANG 2010, JOVANI et al. 2011) and capture-recapture programs involving banded birds (GRUBB et al. 1991, MALLETRODRIGUES et al. 1995). Captive birds have also been used for the study of feather growth (CHILGREN 1978, WHITE & KENNEDY 1992, GRUBB & PRAVOSUDOV 1994), although captivity may affect the growth rate of feathers by causing stress and nutritional deficiencies (MURPHY & KING 1991, GRUBB 1991, WHITE & KENNEDY 1992).
The fact that feathers were replaced throughout the year reveals that (at least in captivity) the pigeons do not have a short timing of primary molt. However, primary molt in captive rock pigeons in the northern hemisphere has been recorded from July to December (VRIENDS & ERSKINE 2005), differing slightly from that obtained in our results, despite the differences in climate and season.
The basic pattern of centrifugal primary replacement (STRESEMANN & STRESEMANN 1966) was seldom recorded here. The predominance of a non-centrifugal pattern in the primary replacement may be a consequence of a lower selective pressure on the captive birds, because wild doves have their primary feathers replaced in a centrifugal pattern (MEDLAND 2003). Similarly, most primaries did not molt simultaneously on both wings, characterizing a predominantly asymmetrical pattern. It is possible that the selective pressure for an orderly sequence in the replacement of flight feathers is relaxed in conditions of captivity. Difference in the lifetime of flight feathers may be responsible for the irregular sequence of feather replacement found here. However, there is not enough evidence to conclude that the Rock Pigeon has multiple waves of primary replacement, as found in many large birds (LANGSTON & ROHWER 1995, PYLE 2005, EDWARDS & ROHWER 2005). Most birds with body mass lower than 1kg (like the Rock Pigeon) replace all of their primaries annually, whereas most species weighting more than 3kg molt their primaries over two or more years (ROHWER et al. 2009). Several species of Ptilinopus and Columbina doves often have two runs of new primaries that are separated by older primaries, but are not multiple waves of primary replacement (ROHWER & WANG 2010). Here, although the primary molt in the pigeons was not a perfect centrifugal sequence of successive feather replacement, some distinct primaries were replaced simultaneously on the same wing, reducing the duration of the molt.
In the molting process of the pigeons studied here, a new primary emerged on average two days after it had pushed up the older feather out of the follicle. However, primaries that had accidentally fallen or removed from the body of the bird were replaced over a longer period than the primaries that had naturally dropped during the molting period, as reported elsewhere (JUHN 1957, BAILEY 1952, MORTON 1962). This result was not surprising because after the traumatic loss of a feather the follicle needs more time to start producing a new feather and an additional supply of nutrients is required to make it.
The period of growth of a primary is strongly influenced by its full length. Small feathers have a shorter period of growth than large feathers, although both have the same growth rate. All primaries exhibit a constant growth rate until the last two or three days of growth. The primary growth rate found here is similar to that reported for the dove Streptopelia roseogrisea (Sundevall, 1857) (5.5 mm/day) (PREVOST apud ROHWER et al. 2009). The rate of feather growth in captive Common Starlings, Sturnus vulgaris Linnaeus, 1758 was also similar in all primary feathers (except for the shorter P9, which increased more slowly), but the primaries rate of mass gain was lower for P1 and higher for P9 (DAWSON 2003).
The timing of primary molt of birds such as the pigeon would be very long if their primaries were replaced one by one with no temporal overlap in feather growth. As each primary of a pigeon grows from three to five weeks (forty days according to SICK 1997) the primary molt would be completed between thirty to fifty weeks (7 to 12 months). However, each feather is dropped when its adjacent feather has grown three-quarters. Hence, the molt is faster and the bird has a shorter period of energy expenditure.
ACKNOWLEDGEMENTS
I am grateful to Luiz Pedreira Gonzaga and to the entire staff of the Laboratório de Ornitologia of the Universidade Federal do Rio de Janeiro for their support to my research. I am also indebted to an anonymous reviewer for his helpful comments on an earlier version of this article.
LITERATURE CITED
Submitted: 09.VIII.2011; Accepted: 02.I.2012.
Editorial responsibility: Diego Astúa de Moraes
- ALTMAN, R.B. 1982. Condition involving the integument system, p. 368-381. In: M.L. PETRAK (Ed.). Diseases of cage and aviary birds. Philadelphia, Lea and Febiger.
- BAILEY, R.E. 1952. The incubation patch of passerine birds. Condor 54: 121-136.
- CHILGREN, J.D. 1978. Effects of photoperiod and temperature on postnuptial molt in captive White-crowned Sparrows. Condor 80: 222-229.
- COOPER, J.E. & G.J HARRISON. 1994. Dermatology, p. 605-639. In: B.W. RITCHIE; G.J. HARRISON & L.R. HARRISON (Eds). Avian medicine: principles and application. Lake Worth, Wingers Publishing Inc.
- DAWSON, A. 2003. A detailed analysis of primary feather moult in the Common Starling Sturnus vulgaris new feather mass increases at a constant rate. Ibis 145 (2): 69-76.
- EDWARDS, A.E. 2008. Large-scale variation in flight feather molt as a mechanism enabling biennial breeding in albatrosses. Journal of Avian Biology 39: 144-151.
- EDWARDS, A.E. & S. ROHWER. 2005. Large-scale patterns of molt activation in the flight feathers of two albatross species. Condor 107: 835-848.
- GRILLI, M.G. & D. MONTALTI. 2010. On the study of the birds' plumage: The case of the Eared Dove (Zenaida auriculata). Revista Brasileira de Ornitologia 18 (4): 341-343.
- GRUBB JR, T.C. 1989. Ptilochronology: feathers growth bars as indicators of nutritional status. Auk 106: 314-320.
- GRUBB JR, T.C. 1991. A deficient diet narrows growth bars on induced feathers. Auk 108: 725-727.
- GRUBB JR, T.C. & V.V. PRAVOSUDOV. 1994. Ptilochronology: follicle history fails to influence growth of an induced feather. Condor 96: 214-217.
- GRUBB JR, T.C.; T.A. WAITE & A.J. WISEMAN. 1991. Ptilochronology: induced feather growth in Northern Cardinals varies with age, sex, ambient temperature, and day length. Wilson Bulletin 103 (3): 435-445.
- INGOLFSSON, A. 2008. The moult of remiges and rectrices in great Black-backed Gulls Larus marznus and Glaucous Gulls L. hyperboreus in Iceland. Ibis 112 (1): 83-92.
- JOVANI, R.; J. BLAS; C. NAVARRO & F. MOUGEOT. 2011. Feather growth bands and photoperiod. Journal of Avian Biology 42 (1): 1-4.
- JUHN, M. 1957. "Frightmolt" in a male Cardinal. Wilson Bull 69: 108-109.
- LANGSTON, N.E. & S. ROHWER. 1995. Unusual patterns of incomplete primary molt in Laysan and Black-footed Albatrosses. Condor 97: 1-19.
- LUCAS, A.M. & P.R. STETTENHEIM. 1972. Avian anatomy: integument. Washington, D.C., U.S. Government Printting Office, Agriculture Handbook 362.
- MALLET-RODRIGUES, F. & M.L.M. NORONHA. 2001. Molt pattern in Pyriglena leucoptera with considerations about the study of molt. Ararajuba 9 (1): 51-55.
- MALLET-RODRIGUES, F.; G.D.A. CASTIGLIONI & L.P. GONZAGA. 1995. Muda e seqüência de plumagens em Ramphocelus bresilius na restinga de Barra de Maricá, Estado do Rio de Janeiro (Passeriformes: Emberizidae). Ararajuba 3: 88-93.
- MCKIBBEN, J.S. & G.J. HARRISON. 1986. Clinical anatomy with emphasison the Amazon parrot, p. 31-66. In: G.J. HARRISON & L.R. HARRISON (Eds). Clinical avian medicine and surgery. Philadelphia, WB Saunders Co.
- MEDLAND, B. 2003. Notes on the moult of some Columbidae species in Malawi. African News 32 (2): 60-64.
- MICHENER, H. & J.R. MICHENER. 1938. Bars in flight feathers. Condor 40: 149-160.
- MILLER, A.H. 1941. The significance of molt centers among the secondary remiges in the Falconiformes. Condor 43: 113-115.
- MORTON, M.L. 1962. A plucking experiment with White-crowned Sparrows. Condor 64: 327-328.
- MURPHY, M.E. & J.R. KING. 1991. Ptilochronology: a critical evaluation of assumptions and utility. Auk 108: 695-704.
- PRYS-JONES, R.M. 1991. The occurrence of biannual primary molt in passerines. Bulletin of the British Ornithologist's Club 111: 150-152.
- PYLE, P. 2005. Remigial molt patterns in North American Falconiformes as related to age, sex, breeding status, and life-history strategies. Condor 107: 823-834.
- ROHWER, S. & L.-K. WANG. 2010. A quantitative analysis of flight feather replacement in the Moustached Tree Swift Hemiprocne mystacea, a tropical aerial forager. PLoS ONE 5 (7): e11586. doi:10.1371/journal.pone.0011586
- ROHWER, S.; R.E. RICKLEFS; V.G. ROHWER & M.M. COPPLE. 2009. Allometry of the duration of flight feather molt in birds. PLoS Biol 7 (6): e1000132. doi:10.1371/journal.pbio.1000132
- SICK, H. 1997. Ornitologia Brasileira. Rio de Janeiro, Editora Nova Fronteira.
- SIMMONS, K.E.L. 1964. Feather maintenance, p. 278-286. In: A.L. THOMSON (Ed.) A new dictionary of birds. New York, McGraw-Hill.
- STRESEMANN, E. & V. STRESEMANN. 1966. Die Mauser der Vögel. Journal fuer Ornithologie 107: 1-447.
- VRIENDS, M.M & T.E. ERSKINE. 2005. Pigeons. A Complete Pet Owner's Manual. Hauppauge, Barron's Educational Series.
- WATSON, G.E. 1963. The mechanism of feather replacement during natural molt. Auk 80: 486-495.
- WHITE, D.W. & E.D. KENNEDY. 1992. Growth of induced feathers in photostimulated American Tree Sparrows. Condor 94: 543-545.
- WHITE, D.W.; E.D. KENNEDY & P.C. STOUFFER. 1991. Feather regrowth in female European Starlings rearing broods of different sizes. Auk 108: 889-895.
- WOLF, P.; N. RABEHL & J. KAMPHUES. 2003. Investigations on feathering, feather growth and potential influences of nutrient supply on feathers' regrowth in small pet birds (canaries, budgerigars and lovebirds). Journal of Animal Physiology and Animal Nutrition 87 (3-4): 134-41.
- WOOD, H.B. 1950. Growth bars in feathers. Auk 67: 486-491.
Publication Dates
-
Publication in this collection
07 May 2012 -
Date of issue
Apr 2012
History
-
Received
09 Aug 2011 -
Accepted
02 Jan 2012