Abstract
The impact of the discovery of Temnocephala haswelli Ponce de Léon, 1989, described as ectosymbionts of ampullariid apple snails outside of Uruguay, motivated us to collect a large number of specimens of Pomacea canaliculata (Lamarck, 1822) from several localities in the southern portion of the state of Rio Grande do Sul. This species was recorded three times after its description: in a study of chromosomes, in a study about the ultrastructure of the collar receptor cells, and in a study of the Haswell glands, all conducted in Uruguay. A total of 301 specimens of P. canaliculata were collected from 1999 to 2007. Temnocephalans found in the pallial cavity were identified as T. haswelli, which occurred in single infestations or concurrently with Temnocephala iheringi Haswell, 1893. Helminths usually showed a light-orange body pigmentation and conspicuous, intense red-eye pigment. Many taxonomic characters evidenced by several techniques were documented photographically for the first time. The typical curved cirrus, approximately 90°, typical of the species, showed some variation in the width of the shaft base, whereas the first longitudinal row of spines of the introvert appeared with shorter spines. The vagina was found to be thick-walled, but not very muscular, and to have a single, large and slightly asymmetrical sphincter, with the posterior portion of slightly larger diameter. Eggs were observed in the umbilicus and along the suture, but predominantly in the body whorl of the shell. Egg peduncles were found to be very short or, most of the time, the eggs were sessile, always with a long apical filament. The rounded shape of the dorsolateral 'excretory' syncytial epidermal plates had external margins reaching the ventrolateral region of the body and eccentric nephridiopores. This is the first record of the species outside Uruguay and in Brazil.
Ectocommensals; mollusks; Neotropical Region; South America; taxonomy
TAXONOMY AND NOMENCLATURE
First report of Temnocephala haswelli (Platyhelminthes: Temnocephalida) in Pomacea canaliculata (Mollusca: Ampullariidae) from Brazil: description update based on specimens from the state of Rio Grande do Sul, Brazil
Samantha A. Seixas; José F. R. Amato11 Corresponding author. E-mail: josefelipeamato@gmail.com Editorial responsibility: Marcus V. Domingues; Suzana B. Amato
Departamento de Zoologia, Instituto de Biociências, Universidade Federal do Rio Grande do Sul. Caixa Postal 15014, 91501-970 Porto Alegre, Rio Grande do Sul, Brazil. E-mail: samantha_bio@yahoo.com.br; sbamato@ufrgs.br
ABSTRACT
The impact of the discovery of Temnocephala haswelli Ponce de Léon, 1989, described as ectosymbionts of ampullariid apple snails outside of Uruguay, motivated us to collect a large number of specimens of Pomacea canaliculata (Lamarck, 1822) from several localities in the southern portion of the state of Rio Grande do Sul. This species was recorded three times after its description: in a study of chromosomes, in a study about the ultrastructure of the collar receptor cells, and in a study of the Haswell glands, all conducted in Uruguay. A total of 301 specimens of P. canaliculata were collected from 1999 to 2007. Temnocephalans found in the pallial cavity were identified as T. haswelli, which occurred in single infestations or concurrently with Temnocephala iheringi Haswell, 1893. Helminths usually showed a light-orange body pigmentation and conspicuous, intense red-eye pigment. Many taxonomic characters evidenced by several techniques were documented photographically for the first time. The typical curved cirrus, approximately 90°, typical of the species, showed some variation in the width of the shaft base, whereas the first longitudinal row of spines of the introvert appeared with shorter spines. The vagina was found to be thick-walled, but not very muscular, and to have a single, large and slightly asymmetrical sphincter, with the posterior portion of slightly larger diameter. Eggs were observed in the umbilicus and along the suture, but predominantly in the body whorl of the shell. Egg peduncles were found to be very short or, most of the time, the eggs were sessile, always with a long apical filament. The rounded shape of the dorsolateral 'excretory' syncytial epidermal plates had external margins reaching the ventrolateral region of the body and eccentric nephridiopores. This is the first record of the species outside Uruguay and in Brazil.
Key words: Ectocommensals; mollusks; Neotropical Region; South America; taxonomy.
Four species have been described as ectosymbionts of ampullariid apple snails (HASWELL 1893, PONCE DE LEÓN 1979, 1989, DAMBORENEA & BRUSA 2008). Temnocephala iheringi Haswell, 1893, the first of these species to be reported, was originally described from Brazil; it is the most studied species of temnocephalans from apple snails and has been recorded from different genera/species of ampullariid snails: Pomacea lineata (Spix in Wagner, 1827) (PEREIRA & CUOCOLO 1941), Asolene platae (Maton, 1811) (HYMAN 1955), Pomacea canaliculata (Lamarck, 1822) (DAMBORENEA 1992, DAMBORENEA & CANNON 2001), and Pomella megastoma (Sowerby, 1825) (DAMBORENEA et al. 1997), in Brazil and Argentina. Recently, SEIXAS et al. (2010) redescribed T. iheringi inferring about the type locality and the type host of this species. Temnocephala rochensis Ponce de León, 1979 was the second species described as an ectosymbiont of P. canaliculata, and, so far, has been recorded only once, from Uruguay. Temnocephala haswelli Ponce de León, 1989, is the third species described as ectosymbiont of P. canaliculata, also in Uruguay, and was recorded three times after its description in a cytogenetic study along with T. iheringi (GONZÁLEZ et al. 1987), on a study about the ultrastructure of the collar receptor cells (PONCE DE LEÓN & VOLONTERIO 2003), and in a study about the Haswell glands also with T. iheringi (VOLONTERIO & PONCE DE LEÓN 2004), also conducted in Uruguay. The fourth species - Temnocephala lamothei Damborenea & Brusa, 2008 -, has been described recently as ectosymbiont of P. megastoma, from Argentina (DAMBORENEA & BRUSA 2008).
The present work records for the first time T. haswelli in Brazil and outside of Uruguay. A complete study (showing several specific characters) of this species has not yet been published. Thus, we present a complete characterization of the species following the methodology of AMATO et al. (2007) and SEIXAS et al. (2010).
MATERIAL AND METHODS
A total of 301 specimens of P. canaliculata were collected between 1999 and 2007, using dip nets and/or large sand sieves, and transported live to the Laboratório de Helmintologia, Universidade Federal do Rio Grande do Sul (UFRGS). Live temnocephalans were obtained from hosts collected from: an artificial lake in the area of Parque Marinha do Brasil (30º03'34.2"S, 051º13'49.7"W); Sava Clube (30º06'09"S, 051º15'57.5"W), Lago Guaíba, and Rio Jacuí, at Ilha da Pintada (30º02'23"S, 051º25'49"W), all within the municipality of Porto Alegre; Praia Florida (30º15'54"S, 051º32'25"W), municipality of Guaíba; ditches around rice plots 5 km West of Interstate Road BR-290, near the locality of Arrozeira (30º01'36"S, 051º22'42"W), municipality of Eldorado do Sul; and Ponta do Ceroula (30º15'07.51"S, 051º16'49.55"W), municipality of Barra do Ribeiro. All localities are in the Guaíba River Basin, Lago Guaíba Sub-water Basin. Rio Cachoeira 'Barragem do Cerrito' (Tributary of Rio Maquiné), district of Barra do Ouro (29º34'15"S, 050º16'51"W), municipality of Maquiné, in the Coastal River Basin, Tramandaí Sub-water basin. All the above municipalities are in the state of Rio Grande do Sul, Brazil. These areas are included in the Atlantic Rain Forest (Brazilian Atlantic Forest Biome).
Some helminths taken from live hosts were studied and photomicrographed alive or fixed and stained according to SEIXAS et al. (2010) for internal morphometry. To preserve the eyespots' red pigmentation, body shape, and for the purpose of scanning electron microscopy studies (SEM), some specimens were flooded with hot, phosphate-buffered 10% formalin (HF) (AMATO et al. 2005, 2006). The morphology of the dorsolateral 'excretory' syncytial epidermal plates (DLSPs) and the distribution of rhabditogen and disc glands followed AMATO et al. (2007). Photomacrographs of hosts showing egg distribution and live helminth pigmentation were taken with a Zeiss Stemi SV-6 stereomicroscope (Figs 1-2, 4, and 5). Photomicrographs were taken with a Zeiss Axiolab microscope with phase contrast (or just the phase contrast condenser), a Leica DMR Hc microscope with Nomarski's differential interference contrast (DIC) prisms, and a Jeol (JSM-6060) SEM. Drawings were made with a drawing tube on a Nikon E-200 microscope. The photographic images and scanned line drawings were prepared using Adobe's Photoshop CS2® and CorelDRAW X4®. Measurements are in micrometers (µm) unless otherwise indicated; ranges are followed by the arithmetic mean, the number of specimens measured for a given character (when different than 30), and the standard deviation values (between parentheses). A number of internal organs were no longer measured and the terminology used followed SEIXAS et al. (2010). Voucher specimens fixed in AFA or silver nitrate (SN), as well as slides containing individual cirri in Faure's mounting medium (F) were deposited in the Coleção Helmintológica do Instituto Oswaldo Cruz (CHIOC), Rio de Janeiro, RJ, Brazil. Other voucher specimens stained in Delafield's hematoxylin, were deposited in the Colección de la Sección Helmintológica, División Zoología Invertebrados, Museo de La Plata (MLP), La Plata, Buenos Aires, Argentina. Some mollusk hosts were deposited in the Coleção Malacológica do Instituto Oswaldo Cruz (CMIOC). The remaining host specimens and temnocephalans are in the host and helminth collections of the UFRGS Coleção Helmintológica do Laboratório de Helmintologia, Porto Alegre, RS, Brazil.
TAXONOMY
Temnocephala haswelli Ponce de León, 1989
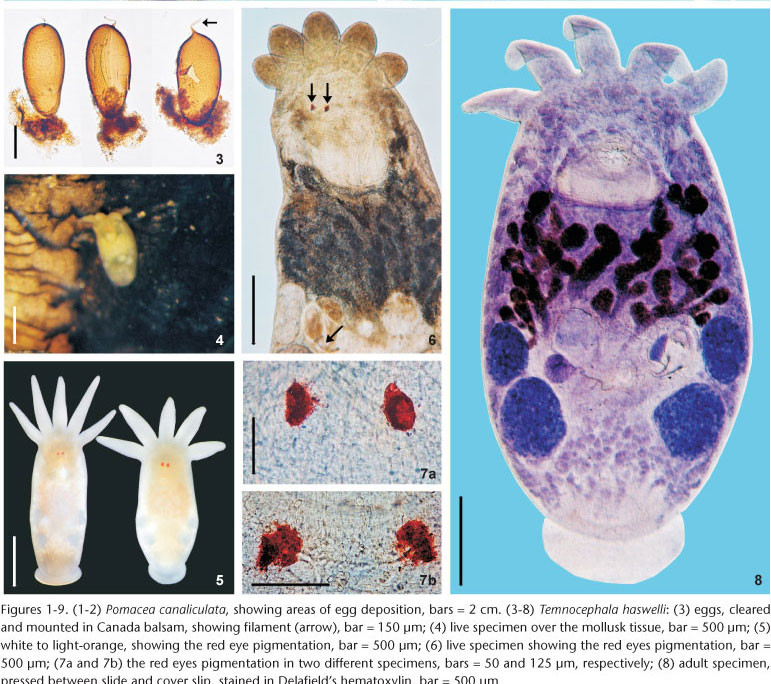
Description. Based on 218 specimens collected; 144 whole mounted adult specimens; 74 juveniles; 6 specimens mounted on stubs for SEM; 15 dissected cirri mounted in F; 12 mounted specimens fixed in SN; 30 specimens fixed in AFA under slight cover slip pressure measured:
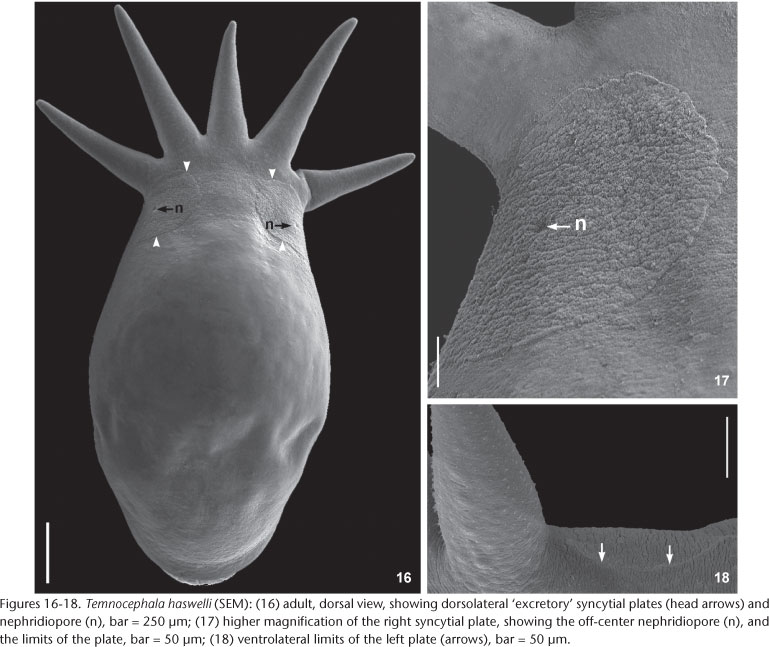
External characteristics. Body, without tentacles (Figs 8 and 13) 1461.5-3476 (2173, 462) long, 987.5-1935.5 (1329, 224) wide; adhesive disk ventral, subterminal, partially covered by body (Figs 8 and 11-13) 350-940 (546, 115) long, 470-1150 (677, 153) wide; disc peduncle 100-350 (177, n = 26, 55) long, 190-450 (308, 80) wide; ratio between of maximum cirrus length/adhesive disk diameter 3.15: 1. Body pigmentation absent (Fig. 6) or light-orange (Fig. 4) observed in live adults and juveniles. Eyespots' red pigmentation present (observations made on live specimens and fixed in HF) (Figs 5-7a, and 7b). Two dorsolateral, rounded epidermal 'excretory' syncytial plates, slightly wider than long (Figs 16 and 17); external margin reaching ventrolateral margin of body (Fig. 18), extending from basis of first and fifth tentacles, respectively; left plate 150-340 (263, n = 12, 49) long, 170-300 (254, n = 12, 38) wide; right plate 180-360 (254, n = 12, 58) long, 190-330 (255, n = 12, 48) wide; ratio between length of DLSPs/total body length, without tentacles, 5.35: 1. Nephridiopore (excretory pore) usually central, sometimes appearing off-centered (Figs 16 and 17).
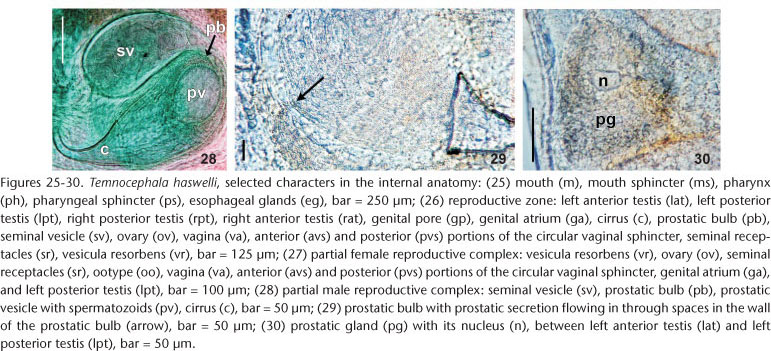
Alimentary system. Mouth surrounded by small sphincter; pharynx wider than long (Fig. 25), 257-573 (382, 156) long, 237-652 (445, 143) wide, with large sphincter; esophageal glands surrounding esophagus at base (Figs 9 and 25); intestine saccular (Figs 11 and 13), inconspicuous septations in juveniles and adults; intestinal walls thick.
Glands. Rhabditogen glands small, numerous, with granular appearance, forming bunches (average 18 cells), in lateral fields of body (Fig. 11), 37.5-80 (51, 10) in diameter, extending from sides of intestinal sac, to the anterior testes, ducts conspicuous (Fig. 11), best observed in juvenile specimens cleared in lactophenol, before complete development of vitellaria. Two groups of three Haswell glands (Fig. 13), showing little affinity with hematoxylin, in front of the brain transverse band (Fig. 13), diameter of the largest cell 50-132.5 (74, n = 29, 17). Disc glands between adhesive disc and genital complex, forming two lateral bunches extending from posterior testes to margin of adhesive disc (Fig. 12), including two pairs, of large, round, more central cells (paranephrocites?), 37.5-87.5 (55, 12) in diameter (Fig. 12). Prostatic glands observed between anterior and posterior testes of the same side (Fig. 30); prostatic secretion observed entering prostatic bulb through the prostatic bulb wall (Fig. 29).
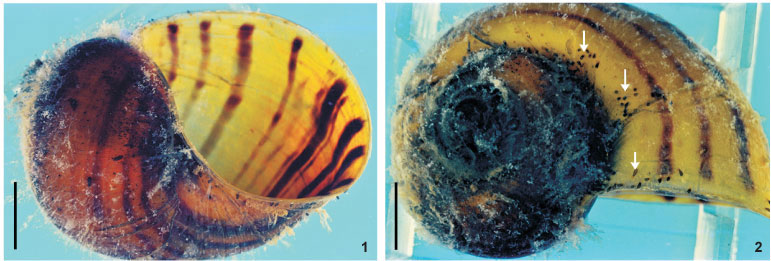
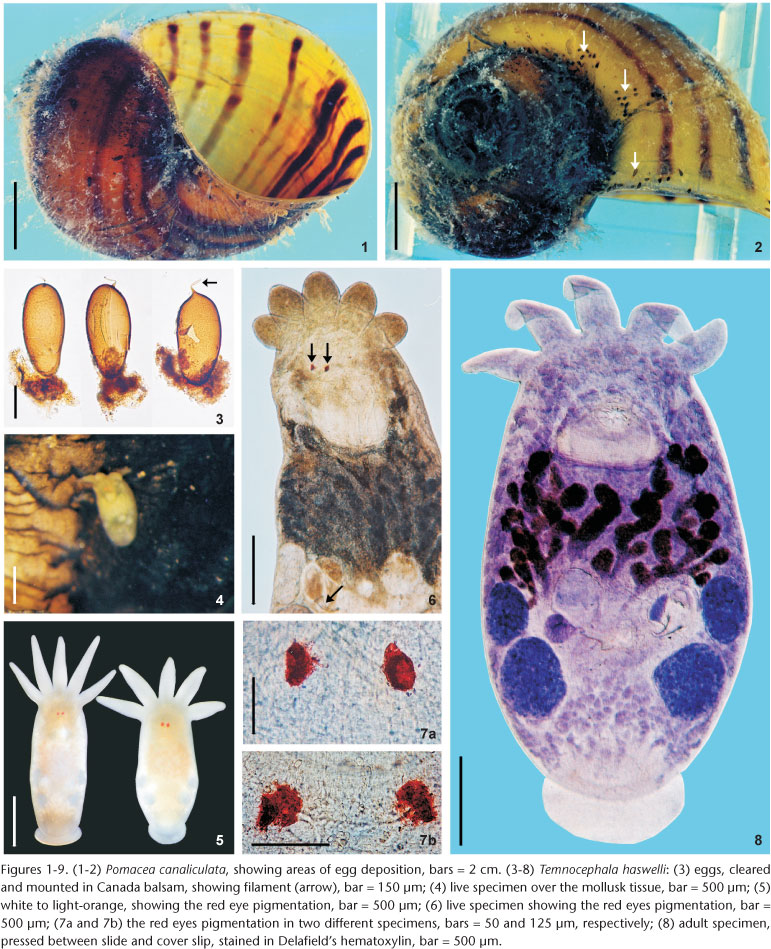
Reproductive system. Female. Vitellaria arborescent, completely covering intestine dorsally (Figs 8 and 13), ventral region partially covered in lateral margins; vagina short, wide, thick-walled, not very muscular (Figs 14 and 27), 40-120 (85, 17) long, 40-100 (71, 15) maximum width; sphincter large, slightly asymmetrical (Figs 14 and 27) 65-107.5 (86, 11) in diameter; diameter of anterior portion 25-40 (30, 4), diameter of posterior portion 27.5-55 (39, 7); vesicula resorbens thick-walled, usually full of sperm (Figs 14 and 27), 140-400 (227, 60) long, 150-320 (234, 51) wide; wall thickness 2.5-22.5 (8, 5). Eggs sessile or with tiny peduncles (Fig. 3), fixed in umbilicus, suture, and in spire, sometimes in larger number in the body whorl of the mollusks (Figs 1 and 2), 360-430 (400, n = 10, 21) long, 170-200 (189, n = 10, 10) wide; filament long, sub-apical or apical (Fig. 3) 50-140 (97, n = 10, 32) long; peduncle, when present, 37,5-72,5 (53, n = 3, 18) long; opercular plates inconspicuous.
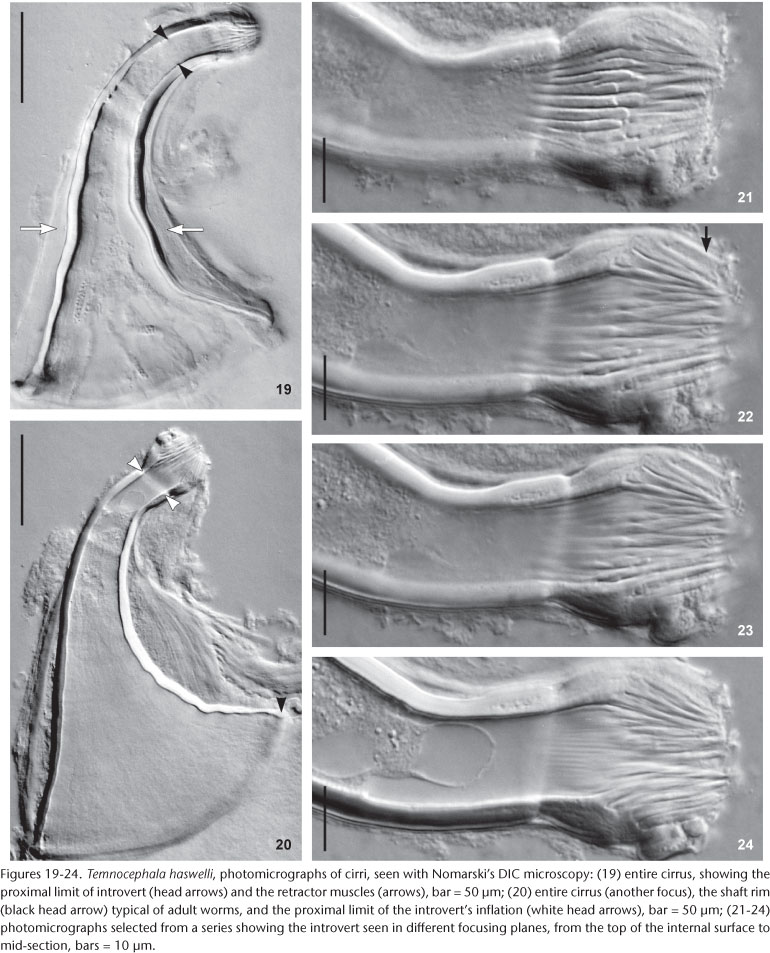
Male. Seminal vesicle short, wide, thin-walled (Figs 15 and 28) 122-287.5 (204, 25) long, 60-120 (86, 25) wide; wall thickness 5-17.5 (10, 4); prostatic bulb with muscular wall (Figs 15 and 28), 115-267.5 (180, 25) long, 87.5-217.5 (151, 25) wide; wall thickness 5-22.5 (11, 4); prostatic vesicle present (Figs 15 and 28). Cirrus curved (curvature approximately 90°) (Figs 15 and 19-20) 197.5-230 (215, n = 10, 12) long, shaft 140-185 (163, n = 10, 14) long, shaft base 105-145 (122, n = 10, 12) wide; introvert 45-57.5 (52, n = 10, 4) long, 20-22.5 (21, n = 10, 1) wide at base; maximum introvert width at level of swelling, 25-32.5 (27, n = 10, 2). Introvert swelling with approximately 18 longitudinal rows of long, stout spines, approximately 4 spines per row (Figs 21-24), first longitudinal row with shorter spines (Fig. 22). Ratio between total body length, without tentacles/total length of cirrus 10: 1; ratio between total length of cirrus/maximum width of shaft's base 1.7: 1; ratio between total length of cirrus/total length of introvert 4.1: 1.
Taxonomic summary
Type host and locality. Pomacea canaliculata (Lamarck, 1822) (Gastropoda: Caenogastropoda: Ampullariidae), Río Canelón Chico, Canelones, Uruguay (PONCE DE LEÓN 1989).
Other localities (present work). Parque Marinha do Brasil, Ilha da Pintada, and Sava Clube, Lago Guaíba, Porto Alegre, RS, Brazil; Ponta do Ceroula, Barra do Ribeiro, RS, Brazil; Praia Florida, Lago Guaíba, Guaíba, RS, Brazil; Arrozeira, Eldorado do Sul, RS, Brazil; Barra do Ouro, Maquiné, RS, Brazil.
Site of infestation. Adults and juveniles were found in the mantle cavity, eggs in umbilicus, suture, and spire, sometimes in larger numbers in the body whorl of the host shell; never present on operculum.
Overall prevalence. 79.8%.
Helminth specimens deposited. CHIOC Nº 37246, 37239a-d - voucher specimens fixed in AFA, stained in Delafield's hematoxylin, CHIOC Nº 37243 - voucher specimens (juvenile) fixed in AFA, stained in Delafield's hematoxylin; CHIOC Nº 37244a-b, 37245 - voucher specimens fixed in AFA, stained in aceto-carmine/fast green, CHIOC Nº 37241 - cirrus from voucher specimen, in F, CHIOC Nº 37242 - unhatched eggs. MLP Nº 5921 - 5926 - voucher specimens fixed in AFA and stained in Delafield's hematoxylin.
Host specimens deposited. Representative specimens of P. canaliculata collected at Sava Clube, Lago Guaíba, and euthanized according to the indicated procedures by malacologists, were identified using molecular methodology, as a species included in the complex P. canaliculata, being described by Dr Silvana A. Thiengo (pers. comm.) were deposited in the CMIOC under the number 5820.
Remarks. Like the majority of Neotropical species, T. haswelli has red-eye pigment, which in this species is conspicuous and intense (Figs 5-7b). The DLSP's are rounded, with external margins on the ventrolateral region of the body (Figs 16-18). The nephridiopores are eccentric and displaced to the inner region (Figs 16 and 17). As the female parts of the reproductive system of this species do not stain well with hematoxylin, measurements and photomicrographs were taken of specimens stained with aceto-carmine\fast green (Figs 26 and 27). Ootype and vagina (Figs 14 and 27) with thick walls, not very muscular, being thicker proximally. The vaginal sphincter is more difficult to observe, in some specimens it is symmetrical, but in the majority it is slightly asymmetrical (30 µm in average in the anterior portion, 39.5 µm in the posterior portion), with the anterior portion thinner, showing considerable variation within the sample (25-40 µm). Almost all eggs observed were sessile while others had minute peduncles. Temnocephala haswelli was found in single infestations, allowing the documentation of its eggs. The cirrus showed important intra-specific variation, on the curvature as well as on the width of the shaft's base (70-100 µm). The size of spines in the introvert of T. haswelli is not so uniform; first row has smaller spines than all the rest (Figs 22, arrow).
DISCUSSION
Temnocephala haswelli was described based on 23 specimens collected from Río Canelón Chico, in Uruguay (PONCE DE LEÓN 1989). Three papers published about T. haswelli also included information on T. iheringi (GONZÁLEZ et al. 1987, PONCE DE LEÓN & VOLONTERIO 2003, VOLONTERIO & PONCE DE LEÓN 2004). PONCE DE LEÓN (1989) described the body pigmentation as a variation from white to yellow, similar to that reported in the present work, in which this variation went from white to light-orange. There are differences in body size (without tentacles) in relation to the specimens of the present work when compared to the original description, but despite being of smaller size the specimens from Brazil (2.17 mm in Brazil and 3.82 mm in Uruguay) have the length as well as the shape of the cirri similar to those characteristic of the species (average length of cirri: 215 µm in Brazil and 200 µm in Uruguay). The red-eye pigment is conspicuous and intense in T. haswelli (Figs 5-7a, and 7b), whereas the vitellaria are very characteristic, which, according to PONCE DE LEÓN (1989), is arborescent with long branches which do not exceed 4-8 in number in the dorsal side of the body, agreeing with the observations made in the present work (Fig. 8), the vitellaria have, in average, four branches, with two more limited branches to the margins of the ventral region. Staining with aceto-carmine/fast green the cement glandular become evident around the genital pore, as PONCE DE LEÓN (1989), pointed out in the original description.
The eggs of T. haswelli were well studied herein (measurements, shape, filament position, and opercular plates) because we were able to collect mono-specific infestations of P. canaliculata with this species, although the opercular plates were sometimes difficult to see in all eggs. Measurements showed considerable differences when compared to those of the original description. PONCE DE LEÓN (1989) measured eggs of the interior of the "ootype" (= genital atrium) in his studies, but this method is likely to give inaccurate measurements. The measurement presented as being of the peduncle, is probably a measurement of the sub-apical or apical filament. This becomes clear when the average length of the peduncle (115 µm) is compared to the average of the length of the filament in Brazilian specimens (97.5 µm), which are quite similar. These values contrast with the average length of peduncles (53.3 µm), which, besides being minute, are only present in 30% of the eggs observed in the present study, as they are largely sessile. Although the original description indicates the limits of the rhabditogen glands and disk glands, two pairs of the large disk glands (paranephrocites?) are shown for the first time in the present work (Fig. 12).
ACKNOWLEDGEMENTS
The authors are grateful to Jorge E. de Araújo Mariath and Rinaldo P. dos Santos, Laboratório de Anatomia Vegetal (LaVEG), Instituto de Biociências, UFRGS, for the permission to use the Leica DMR Hc microscope to make the DIC photomicrographies; to Centro de Microscopia Eletrônica (CME), UFRGS; to Maria C. Damborenea, Curator, Colección Helmintologica Museo de La Plata, División Zoología Invertebrados for receiving Brazilian specimens of T. haswelli, collected and prepared by us, for deposit; to Silvana A. Thiengo for sending us the deposit number of P. canaliculata specimens in the Coleção Malacológica do Instituto Oswaldo Cruz and for calling our attention that molecular biology studies are under way to clarify the existence of a possible canaliculata complex of species; to Philip Jon Scholl for kindly reviewing the English version of the manuscript; and to Luiz Carlos C. Daudt, Lauren B. Pordany and Viviane T. dos Santos for their help in the field.
LITERATURE CITED
Submitted: 22.IX.2009; Accepted: 03.III.2010.
- AMATO, J.F.R.; S.B. AMATO & S.A. SEIXAS. 2005. Temnocephala lutzi Monticelli (Platyhelminthes, Temnocephalida) ectosymbiont on two species of Trichodactylus Latreille (Crustacea, Decapoda, Trichodactylidae) from southern Brazil. Revista Brasileira de Zoologia 22 (4): 1085-1094.
- AMATO, J.F.R.; S.B. AMATO & S.A. SEIXAS. 2006. A new species of Temnocephala Blanchard (Platyhelminthes, Temnocephalida) ectosymbiont on Trichodactylus fluviatilis Latreille (Crustacea, Decapoda, Trichodactylidae) from southern Brazil. Revista Brasileira de Zoologia 23 (3): 796-806.
- AMATO, J.F.R.; S.A. SEIXAS & S.B. AMATO. 2007. A new species of Temnocephala Blanchard (Platyhelminthes, Temnocephalida) ectosymbiont on creeping water bugs, Cryphocricos granulosus De Carlo (Hemiptera, Naucoridae) from southern Brazil. Revista Brasileira de Zoologia 24 (4): 1043-1051.
- DAMBORENEA, M.C. 1992. Espécies de Temnocephala (Platy-helminthes, Temnocephalidae) de crustaceos y moluscos de la Argentina. Iheringia, Série Zoologia, (72): 3-21.
- DAMBORENEA, M.C. & F. BRUSA. 2008. A new species of Temnocephala (Platyhelminthes, Temnocephalida) commensal of Pomella megastoma (Mollusca, Ampullariidae) from Misiones, Argentina. Revista Mexicana de Biodiversidad 79 (Suppl.): 1S-7S.
- DAMBORENEA, M.C. & L.R.G. CANNON. 2001. On neotropical Temnocephala (Platyhelminthes). Journal of Natural History 35 (12): 1103-1118.
- DAMBORENEA, M.C.; I.I. CÉSAR & L.C. ARMENDÁRIZ. 1997. Especies de Temnocephala (Platyhelminthes, Temnocephalidae) de la Isla Martín García, Buenos Aires, Argentina. Neotropica 43 (109-110): 123-124.
- GONZÁLEZ, L.E.; R. PONCE DE LEÓN & E.S. DE VAIO. 1987. Chromo-somes differences between two species of Temnoce-phala (Platyhelminthes). Cytobios 49 (197): 85-88.
- HASWELL, W.A. 1893. A monograph of the Temnocephaleae, p. 93-152. In: J.J. FLETCHER (Ed.). Proceedings of the Linnean Society of New South Wales. The Macleay Memorial Volume, p. 93-152.
- HYMAN, L.H. 1955. Miscellaneous marine and terrestrial flatworms from South America. American Museum Novitates 1742: 1-33.
- PEREIRA, C. & R. CUOCOLO. 1941. Estudos sobre "Temnocephalidae Monticelli, 1899", com estabelecimento de dois novos gêneros australianos e descrição de duas novas espécies neotrópicas. Arquivos do Instituto Biológico 12 (9): 101-127.
- PONCE DE LEÓN, R. 1979. Espécies americanas de Temnocephalidea Benham (Platyhelmintha), I. Descripcion de Temnocephala rochensis n. sp. de la camara paleal de Pomacea canaliculata (Lamarck). Revista de Biologia del Uruguay 7 (1): 39-48.
- PONCE DE LEÓN, R. 1989. Description of Temnocephala haswelli n. sp. (Platyhelminthes) from the mantle cavity of Pomacea canaliculata (Lamarck). Journal of Parasitology 75 (4): 524-526.
- PONCE DE LEÓN, R. & O. VOLONTERIO. 2003. First description of collar receptors in Temnocephalidae (Platyhelminthes). Acta Zoologica 84: 155-160.
- SEIXAS, S.A.; J.F.R. AMATO & S.B. AMATO. 2010. Redescription of Temnocephala iheringi (Platyhelminthes: Temnocephalida) based on specimens from Pomacea canaliculata (Mollusca: Ampullariidae) of the State of Rio Grande do Sul: the possible type host and type locality. Zoologia 27 (2): 245-257.
- VOLONTERIO, O. & R. PONCE DE LEÓN. 2004. The first ultrastructural description of the Haswell cells in Temnocephalidae (Platyhelminthes, Temnocephalida), with insights into their function. Parasitology Research 92 (5): 355-360.
Publication Dates
-
Publication in this collection
12 July 2010 -
Date of issue
June 2010
History
-
Received
22 Sept 2009 -
Accepted
03 Mar 2010