ABSTRACT
Efficiently obtaining high-quality data on animal populations and communities is paramount for ecological and conservation studies. In many instances these data come from live-trapping, the success of which depends on various factors, such as the interaction between the trap's mechanisms and the morphological or ecological characteristics of the animals, and weather conditions that can affect both trap efficiency and animal behavior. Integrative approaches that address the simultaneous effects of these factors on capture-recapture success are rare. Here we contribute to close this knowledge gap by focusing on a large capture-recapture dataset from three 2-ha grids monitored for approximately two years (totaling 55.000 traps-night) in the Morro Grande Forest Reserve, São Paulo, Brazil. The dataset contains data on 3608 captures of 1273 individuals from 24 species of Atlantic forest small mammals. We evaluated if mortality rates and the capture-recapture success of small mammals varied between two types of trap (Sherman and pitfall), and if the capture success of each type varied with age and sex of individuals, and with weather conditions. Our findings highlight that trap efficiency depends not only on the quantities considered (species, individuals or recaptures), but also on animal characteristics and weather conditions. Large pitfall traps should be used whenever the focus is on biodiversity and community parameters, since they captured more individuals and species. Studies focusing on demographic parameters require the combined use of pitfall and Sherman traps. While pitfall traps captured a larger number of individuals and a higher proportion of juveniles, Sherman traps provided higher recapture rates for most species.
KEY WORDS.
Abundance; biodiversity survey; demography; richness; trapping protocol.
Obtaining high-quality data on animal populations and communities in an efficient manner is paramount to a variety of ecological studies. Studies focusing on hypothesis testing in population and community ecology, and those aiming at monitoring populations and communities for practical purposes, rely on efficient sampling (Williams et al. 2002Williams BK, Nichols JD, Conroy MJ (2002) Analysis and management of animal populations: modeling, estimation, and decision making. London, Academic Press, 817p.,Magurran & Macgill 2011Magurran AE, Mcgill BJ (2011) Biological Diversity: Frontiers in Measurement and Assessment: Frontiers in Measurement and Assessment. Oxford, Oxford University Press, 368p.). Efficient sampling techniques facilitate the use of sampling designs that result in large samples, which are important for advancing both ecology and conservation (Dray et al. 2012Dray S, Pélissier R, Couteron P, Fortin MJ Legendre P, Peres-Neto PR, Bellier E, Bivand R, Blanchet FG, Cáceres M, Dufour AB, Heegaard E, Jombart T, Munoz F, Oksanen J, Thioulouse J, Wagner HH (2012) Community ecology in the age of multivariate multiscale spatial analysis. Ecological Monographies 82: 257-275.).
The study of populations and communities of a large number of animal species, especially those with a cryptic life-style, requires capture-recapture data from traps. Trapping success depends on the interaction between the type of trap (i.e., its capture mechanism), the morphological characteristics of the species in focus, the ecology and behavior of individuals and species, as well as weather conditions, which can affect both the efficiency of traps and animal behavior. Thus, the choice of trap determines the quality of the data obtained, and therefore should be carefully considered when planning field sampling protocols.
Non-volant small mammals are usually captured in two main types of traps: (1) Sherman traps (and other types of box or cage traps), used in association with baits, the success of which depends on the exploratory behavior of individuals, and (2) pitfall traps, where capture is the result of guiding the movements of individuals into buried buckets (Flowerdew et al. 2004Flowerdew JR, Hore RF, Poulton SMC, Sparks TH (2004) Live trapping to monitor small mammals in Britain. Journal of Comparative Physiology [B] 34: 31-50. doi: 10.1046/j.0305-1838.2003.00025.x
https://doi.org/10.1046/j.0305-1838.2003...
,Nicolas & Colyn 2006Nicolas V, Colyn M (2006) Relative efficiency of three types of small mammal traps in an African rainforest. Belgian Journal of Zoology 136: 107-111.). Due to these different capture mechanisms, the two types of traps differ in their efficiency (Nicolas & Colyn 2006Nicolas V, Colyn M (2006) Relative efficiency of three types of small mammal traps in an African rainforest. Belgian Journal of Zoology 136: 107-111.,Dizney et al. 2008Dizney L, Jones PD, Ruedas LA (2008) Efficacy of Three Types of Live Traps Used for Surveying Small Mammals in the Pacific Northwest. Northwest Naturalist 89: 171-180.,Torre et al. 2010Torre I, Guixé D, Sort F (2010) Comparing Three Live Trapping Methods for Small Mammal Sampling in Cultivated Areas of Ne Spain. Hystrix 21: 147-155. doi: 10.4404/hystrix-21.2-4462
https://doi.org/10.4404/hystrix-21.2-446...
). Particularly, sex and age of individuals can interact with trap mechanisms to determine capture-recapture success. For example, the males of several small mammal species move longer distances than their female counterparts (Pires & Fernandez 1999Pires AS, Fernandez FAS (1999) Use of space by the marsupial Micoureus demerarae in small Atlantic forest fragments in south-eastern Brazil. Journal of Tropical Ecology 15: 279-290. doi: 10.1017/S0266467499000814
https://doi.org/10.1017/S026646749900081...
,Püttker et al. 2006Püttker T, Meyer-Lucht Y, Sommer S (2006) Movement distances of five rodent and two marsupial species in forest fragments of the coastal atlantic rainforest, Brazil. Biotropica 22: 131-139.), and hence they may encounter drift-fences of pitfall traps more often. Juveniles, on the other hand, may have less exploratory ability to find the trap's bait, and they may be too light to trigger box/cage traps such as Sherman traps (Cáceres et al. 2011Cáceres NC, Nápoli RP, Hannibal W (2011) Differential trapping success for small mammals using pitfall and standard cage traps in a woodland savannah region of southwestern Brazil. Mammalia 75: 45-52. doi: 10.1515/MAMM.2010.069
https://doi.org/10.1515/MAMM.2010.069...
,Nicolas & Colyn 2006Nicolas V, Colyn M (2006) Relative efficiency of three types of small mammal traps in an African rainforest. Belgian Journal of Zoology 136: 107-111.,Dizney et al. 2008Dizney L, Jones PD, Ruedas LA (2008) Efficacy of Three Types of Live Traps Used for Surveying Small Mammals in the Pacific Northwest. Northwest Naturalist 89: 171-180.).
Determining the best trapping method, however, depends on the objectives of the study. Particularly, community studies require as many individuals from as many species as possible (Magurran & McGill 2011Magurran AE, Mcgill BJ (2011) Biological Diversity: Frontiers in Measurement and Assessment: Frontiers in Measurement and Assessment. Oxford, Oxford University Press, 368p.), while population studies require a representative subset of a population (i.e., different age classes and sexes), as well as achieving as many recaptures as possible (Williams et al. 2002Williams BK, Nichols JD, Conroy MJ (2002) Analysis and management of animal populations: modeling, estimation, and decision making. London, Academic Press, 817p.). Pitfall traps, which are able to capture more than one individual per night, should capture more individuals, and therefore a larger number of species compared to Sherman traps. In pitfall traps, captures should also be less dependent on the exploratory behavior of individuals or species and thus result in a better representation of age and sex. On the other hand, animals captured in pitfalls are exposed to predators and to unwanted interactions between the individuals captured in the same trap. Hence, when mortality rates from unwanted interactions and predation are negligible, pitfall traps are expected to be important in community studies (to estimate species richness and composition) as well as in population studies, given that they capture different age classes and sexes (Williams et al. 2002Williams BK, Nichols JD, Conroy MJ (2002) Analysis and management of animal populations: modeling, estimation, and decision making. London, Academic Press, 817p.). Although previous studies have shown that pitfall traps tend to capture more juveniles (Nicolas & Colyn 2006Nicolas V, Colyn M (2006) Relative efficiency of three types of small mammal traps in an African rainforest. Belgian Journal of Zoology 136: 107-111.,Dizney et al. 2008Dizney L, Jones PD, Ruedas LA (2008) Efficacy of Three Types of Live Traps Used for Surveying Small Mammals in the Pacific Northwest. Northwest Naturalist 89: 171-180.,Cáceres et al. 2011Cáceres NC, Nápoli RP, Hannibal W (2011) Differential trapping success for small mammals using pitfall and standard cage traps in a woodland savannah region of southwestern Brazil. Mammalia 75: 45-52. doi: 10.1515/MAMM.2010.069
https://doi.org/10.1515/MAMM.2010.069...
), no study has evaluated differences in capture success between the sexes. Moreover, results pertaining the number of individuals and species are incongruent: some studies indicate that more individuals and species are captured in pitfall traps (Umetsu et al. 2006Umetsu F, Naxara L, Pardini R (2006) Evaluating the efficiency of pitfall traps for sampling small mammals in the Neotropics. Journal of Mammalogy 87: 757-765. doi: 10.1644/05-MAMM-A-285R2.1
https://doi.org/10.1644/05-MAMM-A-285R2....
,Cáceres et al. 2011Cáceres NC, Nápoli RP, Hannibal W (2011) Differential trapping success for small mammals using pitfall and standard cage traps in a woodland savannah region of southwestern Brazil. Mammalia 75: 45-52. doi: 10.1515/MAMM.2010.069
https://doi.org/10.1515/MAMM.2010.069...
), whereas others have obtained superior results using Sherman traps (Nicolas & Colyn 2006Nicolas V, Colyn M (2006) Relative efficiency of three types of small mammal traps in an African rainforest. Belgian Journal of Zoology 136: 107-111.,Santos-Filho et al. 2006Santos-Filho M, Silva DJ, Sanaiotti TM (2006) Efficiency of four Trap Types in Sampling Small Mammals in Forest Fragments, Mato Grosso, Brazil. Mastozoología Neotropical 13: 217-225.).
In contrast to pitfall traps, baited box traps such as Sherman traps provide food and shelter, thereby reducing stress and trauma (White et al. 1982White GC, Anderson DR, Burnham KP, Otis DL (1982) Capture-Recapture and removal methods for sampling closed populations. Los Alamos, National Laboratory, 13p.), which may reduce the mortality of captured animals and favor recaptures. In view of this, Sherman traps would be essential in population studies. However, despite the fact that several studies assessed the number of captures of different traps (e.g.,Nicolas & Colyn 2006Nicolas V, Colyn M (2006) Relative efficiency of three types of small mammal traps in an African rainforest. Belgian Journal of Zoology 136: 107-111.,Santos-Filho et al. 2006Santos-Filho M, Silva DJ, Sanaiotti TM (2006) Efficiency of four Trap Types in Sampling Small Mammals in Forest Fragments, Mato Grosso, Brazil. Mastozoología Neotropical 13: 217-225.,Umetsu et al. 2006Umetsu F, Naxara L, Pardini R (2006) Evaluating the efficiency of pitfall traps for sampling small mammals in the Neotropics. Journal of Mammalogy 87: 757-765. doi: 10.1644/05-MAMM-A-285R2.1
https://doi.org/10.1644/05-MAMM-A-285R2....
,Dizney et al. 2008Dizney L, Jones PD, Ruedas LA (2008) Efficacy of Three Types of Live Traps Used for Surveying Small Mammals in the Pacific Northwest. Northwest Naturalist 89: 171-180.,Cáceres et al. 2011Cáceres NC, Nápoli RP, Hannibal W (2011) Differential trapping success for small mammals using pitfall and standard cage traps in a woodland savannah region of southwestern Brazil. Mammalia 75: 45-52. doi: 10.1515/MAMM.2010.069
https://doi.org/10.1515/MAMM.2010.069...
), none considered the number of recaptures or mortality rates. This is most likely because many available studies are short-term, are based on few traps set in rows over a small area, and thus provide limited data for evaluating recaptures and mortality rates (e.g.Nicolas & Colyn 2006Nicolas V, Colyn M (2006) Relative efficiency of three types of small mammal traps in an African rainforest. Belgian Journal of Zoology 136: 107-111.,Umetsu et al. 2006Umetsu F, Naxara L, Pardini R (2006) Evaluating the efficiency of pitfall traps for sampling small mammals in the Neotropics. Journal of Mammalogy 87: 757-765. doi: 10.1644/05-MAMM-A-285R2.1
https://doi.org/10.1644/05-MAMM-A-285R2....
,Cáceres et al. 2011Cáceres NC, Nápoli RP, Hannibal W (2011) Differential trapping success for small mammals using pitfall and standard cage traps in a woodland savannah region of southwestern Brazil. Mammalia 75: 45-52. doi: 10.1515/MAMM.2010.069
https://doi.org/10.1515/MAMM.2010.069...
).
Weather conditions, particularly rainfall and temperature, can affect capture probability by affecting either the activity of animals (thus the chance of encountering a trap) or the effectiveness of the trap's mechanism. Some studies found a positive relationship between temperature and activity of small mammals (Vickery & Bider 1981Vickery WL, Bider JR (1981) The Influence of Weather on Rodent Activity. Journal of Mammalogy 62: 140-145.,Vieira et al. 2010Vieira EM, Baumgarten LC, Paise G, Becker RG (2010) Seasonal patterns and influence of temperature on the daily activity of the diurnal neotropical rodent Necromys lasiurus. Canadian Journal of Zoology 88: 259-265. doi: 10.1139/Z09-142
https://doi.org/10.1139/Z09-142...
). Some species lapse into a state of torpor at low temperatures in combination with low rainfall (Bozinovic et al. 2005Bozinovic F, Ruiz G, Rosenmann M (2005) Energetics, thermoregu lation and torpor in the Chilean mouse-opossum Thylamys elegans (Didelphidae). Revista Chilena de História Natural 78: 199-120.). Extremely high temperatures can also affect the water balance of small mammals, and torpor can happen in this situation (Geiser & Baudinette 1987Geiser F, Baudinette RV (1987) Seasonality of torpor and thermoregulation in three dasyurid marsupials. Journal of Comparative Physiology [B] 157: 335-344.,Bozinovic et al. 2004Bozinovic F, Ruiz G, Rosenmann M (2004) Energetics and torpor of a South American "living fossil", the microbiotheriid Dromiciops gliroides. Journal of Comparative Physiology [B] 174: 293-297. doi: 10.1007/s00360-004-0414-8
https://doi.org/10.1007/s00360-004-0414-...
). Weather conditions affect the activity of small mammals, since their high surface-volume ratio facilitates the loss of heat and water through respiration (Vickery & Bider 1981Vickery WL, Bider JR (1981) The Influence of Weather on Rodent Activity. Journal of Mammalogy 62: 140-145.,Halle 2000Halle S (2000) Ecological relevance of daily activity patterns, p. 67-90. In: Halle S, Stenseth NC (Eds.). Activity patterns in small mammals: an ecological approach. Berlin, Springer-Verlag.). Likewise, weather conditions may interfere with the efficiency of both Sherman and pitfall traps: Sherman traps may close during storms, or the accumulation of water in the bottom of pitfall buckets may hamper the escape of trapped animals (Voss et al. 2001Voss RS, Lunde DP, Simmons NB (2001) The Mammals of Paracou, French Guiana: A Neotropical Lowland Rainforest Fauna - Part 2. Nonvolant Species. Bulletin of the American Museum of Natural History 263: 1-236.). Further, drift-fences of pitfall traps may be more effective when weather conditions favor animal activity (e.g., depending on temperature). However, few studies have evaluated the influence of weather conditions on small mammal captures.
Here we investigated the simultaneous effects of individual characteristics (sex and age), and weather conditions (rainfall and minimum temperature) on the capture efficiency of Sherman and pitfall traps. Our analysis is based on a large capture-recapture dataset on Atlantic forest small mammals, trapped in three 2-ha grids for almost two years in one of the largest tracks of continuous forest in southeastern Brazil. We aim to provide information to help defining efficient field protocols for sampling Neotropical small mammals, to be used in studies focusing on estimating community and/or population parameters. Specifically we investigated: (1) if mortality rates, and the number of species, individuals and recaptures varied between the two trap types, and (2) if individual characteristics (age and sex) and (3) weather conditions (minimum temperature and rainfall) influenced the number of captures and interacted with the types of trap. We predicted that pitfall traps would result in higher mortality rates and the capture of more individuals and species, and that Sherman traps would provide more recaptures. We also predicted that Sherman trap captures would be more affected by the sex and age of individuals than pitfall trap captures. Finally, we predicted that both traps would provide more captures in warm, humid days, when animals are more active.
MATERIAL AND METHODS
The study was carried out at the Morro Grande Forest Reserve (23°39'-23°48'S, 47°01'-46°55'W, Cotia, São Paulo,Figs. 1-2), a 9400-ha reserve connected to the largest tract of Atlantic Forest in Brazil. The reserve is covered by Lower Montane Atlantic Rain Forest in different regeneration stages (Metzger et al. 2006Metzger JP, Alves LF, Goulart W, Teixeira AMG, Simões SJC, Catharino EL (2006) An important biological area, but still poorly known: the Morro Grande Forest Reserve. Biota Neotropica 6. doi: 10.1590/S1676-06032006000200003
https://doi.org/10.1590/S1676-0603200600...
). The altitude there varies from 850 to 1100 m above sea level. Mean maximum temperature is 27°C and mean minimum temperature is 11°C. Mean annual rainfall is 1339 mm, with the wet and warm season from September to March (Metzger et al. 2006Metzger JP, Alves LF, Goulart W, Teixeira AMG, Simões SJC, Catharino EL (2006) An important biological area, but still poorly known: the Morro Grande Forest Reserve. Biota Neotropica 6. doi: 10.1590/S1676-06032006000200003
https://doi.org/10.1590/S1676-0603200600...
). The non-volant small mammal fauna is very diverse, including rare and endemic Atlantic Forest species (Pardini & Umetsu 2006Pardini R, Umetsu F (2006) Non-volant small mammals from the Morro Grande Forest Reserve - distribution of species and diversity in an Atlantic Forest area. Biota Neotropica 6(2): bn00606022006. Available online at:Available online at:http://www.biotaneotropica.org.br/v6n2/pt/abstract?article+bn00606022006
[Accessed: 10/02/2008]
http://www.biotaneotropica.org.br/v6n2/p...
).
(1) Location of the Morro Grande Forest Reserve (highlighted in a rectangle) in São Paulo State (black), Brazil, near to São Paulo City. Black dots represent the 2-ha trapping grids. Gray areas: forest; white areas: non-forest. (2) Trapping grids used to capture small mammals. Rectangles: Sherman traps; circles: pitfall traps connected by drift-fences (lines).
We placed three 2-ha trapping grids (100 m x 200 m) ~2 km apart at the Reserve (Figs. 1-2). Each grid comprised 11, 100-m long parallel lines, 20 m apart from each other, each composed of 11 trap stations every 10 m. Six alternated lines had only 11 Sherman traps (37.5 x 10.0 x 12.0 cm or 23.0 x 7.5 x 8.5 cm) on the ground. The other five lines had, in addition to the Sherman traps, 11 pitfall traps (60 L buckets, with a height of 54 cm and a diameter of 40 cm each, buried at the ground, and connected by 10-m long and 50-cm high drift-fences, forming a 100-m long fence) at the same trap stations, totaling 121 Sherman traps and 55 pitfall traps per grid (Figs. 1-2). The efficiency of pitfall traps has been shown to depend on the size of their buckets and it is thus important to notice that we used large (60 L) ones, followingRibeiro-Junior et al. (2011Ribeiro-Júnior MA, Rossi RV, Miranda CL, Ávila-Pires TCS (2011) Influence of pitfall trap size and design on herpetofauna and small mammal studies in a Neotropical Forest. Zoologia 28(1): 80-91. doi: 10.1590/S1984-46702011000100012
https://doi.org/10.1590/S1984-4670201100...
). In order to reduce mortality rates, we (1) drilled small holes at the bottom of the buckets to prevent water accumulation, (2) placed Styrofoam at the bottom to provide a dry fluctuating surface in case of water accumulation, (3) placed food in the buckets, and (4) installed bucket lids at 50 cm height to function as an umbrella. All traps received daily baits consisting of mashed bananas, cornmeal, peanut butter and sardines. This mixture of baits has been shown to be effective in attracting Neotropical small mammals (Astúa de Moraes et al. 2006Astúa de Moraes D, Moura RT, Grelle CEV, Fonseca MT (2006) Influence of baits, trap type and position for small mammal capture in a Brazilian lowland Atlantic Forest. Boletim do Museu de Biologia Mello Leitão 19: 31-44.,Hice & Velazco 2013Hice CLH, Velazco PM (2013) Relative Effectiveness of Several Bait and Trap Types for Assessing Terrestrial Small Mammal Communities in Neotropical Rainforest. Occasional Papers, Museum of Texas Tech University 316: 1-15.).
We performed 21 five-night capture sessions between March 2008 and October 2009, when both types of traps were used simultaneously. Intervals between sessions varied from 16 to 30 days (mean of 23.4 days). Captured individuals were marked with aluminum tags with unique codes (small animal tags OLT - A. Hartenstein GmbH, Würzburg/Versbach, Germany), allowing individual identification. For each capture we recorded the species name, sex, reproductive condition, and body weight and, in the case of marsupials, the tooth eruption pattern. The reproductive activity of females was determined by pregnancy or the presence of swollen teats, and of males by testicles in the scrotal position (in rodents only). Rodents were classified into age classes according to body mass, assuming the minimum weight of a reproductive individual (separately for each sex) as the threshold between juveniles and adults. Marsupials were put into age classes based on the eruption patterns of their teeth (Tribe 1990Tribe CJ (1990) Dental age classes in Marmosa incana and other didelphoids. Journal of Mammalogy 71: 566-569. doi: 10.2307/1381795
https://doi.org/10.2307/1381795...
,Macedo et al. 2006Macedo J, Loretto D, Vieira MV, Cerqueira R (2006) Classes de desenvolvimento em marsupiais: um método para animais vivos. Mastozoología Neotropical 13: 133-136.).
Voucher specimens of all species were collected in a pilot sample, identified by experts, and are kept in the Department of Zoology, University of São Paulo. Trapping, handling and collection of specimens were approved by IBAMA - Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA/SP: 11577-1, 11577-2, 11577-4) and conformed to the guidelines sanctioned by the American Society of Mammalogists Animal Care and Use Committee.
During all sessions, daily rainfall (averaged between two pluviometers placed close to the grids on the Reserve's main road) and minimum daily temperature (from hourly records obtained with data loggers placed in each grid) were recorded. We chose to use the minimum daily temperature because a previous study, carried out in a close site in the Atlantic forest (Umetsu et al. 2006Umetsu F, Naxara L, Pardini R (2006) Evaluating the efficiency of pitfall traps for sampling small mammals in the Neotropics. Journal of Mammalogy 87: 757-765. doi: 10.1644/05-MAMM-A-285R2.1
https://doi.org/10.1644/05-MAMM-A-285R2....
), showed that this variable had a stronger effect on the number of small mammal captures than mean or maximum temperatures. Indeed, maximum temperatures in the study region are mild and extremely high temperatures, which can negatively affect small mammal activity, are very uncommon.
To evaluate how the number of species accumulate as a function of sampling effort, we used mean accumulation curves of the number of species per capture session and per individual for each type of trap, using the vegan package in R environment (Oksanen et al. 2012Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Wagner H (2012) Vegan: Community Ecology Package. R package version 2.0-7. Available online at:Available online at:http://CRAN.R-project.org/package=vegan
[Accessed:18/08/2012]
http://CRAN.R-project.org/package=vegan...
).
In order to answer our main questions - on the effects of the type of trap on mortality rates, number of species, individuals and recaptures, and on the effects of individual characteristics and weather condition on the number of captures - we used Generalized Linear Mixed-Effects models (GLMM), allowing the incorporation of time and space dependence through the use of random factors (Bolker et al. 2008Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS (2008) Generalized linear mixed models: a practical guide for ecology and evolution. Trends in ecology and evolution 24: 127-135. doi: 10.1016/j.tree.2008.10.008
https://doi.org/10.1016/j.tree.2008.10.0...
,Online Supplementary Material). Candidate models were compared using the Akaike information criterion (AIC). AIC values of each model were subtracted from the AIC value of the best model (Δi), and models with Δi ≤ 2 were considered equally plausible (Burnham & Anderson 2002Burnham KP, Anderson DR (2002) Model selection and multimode inference: A practical Information-Theoretic Approach. Colorado, Springer, 2nd ed., 488p.). All analyses were run in R environment, version 2.13.0 (R Development Core Team 2011R Development Core Team (2011) R: A language and environment for statistical computing. Vienna, R Foundation for Statistical Computing, ISBN 3-900051-07-0.) using lmer4 package (Bates et al. 2011Bates D, Maechler M, Bolker B (2011) Lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-42.http://CRAN.R-project.org/package=lme4[Accessed: 05/10/2009]
http://CRAN.R-project.org/package=lme4...
), and scripts are found inOnline Supplementary Material1.
Effects of the type of trap on mortality rates, number of species, individuals and recaptures
The dependent variables in these analyses were mortality rates (dead individuals in relation to either the number of captured individuals or the number of captures), number of species (richness), number of individuals, and number of recaptures (total captures except the first capture). They were computed for each type of trap and each capture session in each grid. With the exception of richness, which was computed based on the entire data set, the remaining dependent variables were computed considering only six species with enough captures (species with more than 120 captures;Table 1). The candidate model set for each of the five dependent variables included two models: a constant model (no fixed factor) as a reference, and a model considering trap type as the fixed factor (Table 2). In all candidate models, grids and capture sessions were considered random intercept factors, and species was considered random intercept and slope factor (except for richness, since in this case data are aggregated across all species). Models for recaptures also included the individuals as an extra intercept random factor. Mortality rates were modeled using a binomial distribution as it considers the number of dead individuals in relation to either the number of captured individuals or the number of captures. All other three dependent variables were modeled using Poisson error distribution. For the number of individuals, as the variance was greater than the mean, we accounted for overdispersion by adding an observation-level random effect (a new grouping variable with a separate level for every observation in the data set), as a way to add more variance to the distribution (Harrison 2014Harrison XA (2014) Using observation-level random effects to model overdispersion in count data in ecology and evolution. PeerJ 2: e616. doi: 10.7717/peerj.616
https://doi.org/10.7717/peerj.616...
). The resulting lognormal-Poisson distribution is similar to a negative binomial distribution.
Number of captures and number of individuals (in parentheses) obtained in each type of trap and in total for each small mammal species from the Morro Grande Forest Reserve. Numbers for pitfall traps are from the set of five trap lines per grid, and for Sherman traps are from the set of five trap lines per grid intercalated with pitfall lines or from the set of five trap lines per grid adjacent to pitfall traps. Due to the impossibility to separate the cryptic species Monodelphis americana and M. scalops in the field, individuals from both species were considered together.
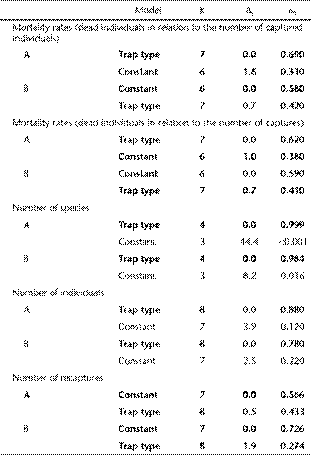
Results of model selection for mortality rates, and for the number of species, of individuals and of recaptures of small mammals as a function of the type of trap. Two data-subsets were used for Sherman traps: only captures from Sherman placed adjacent to pitfall lines (A) or from Sherman traps intercalated with pitfall lines (B). Check the text for random factors. Selected models (Δi < 2) are in bold. K: number of parameters; Δi: difference in AIC value compared to the best model; ωi: Akaike weight.
Since the sampled area differed between the two types of traps (all trap lines had Sherman traps and only five trap lines had pitfall traps), all five dependent variables for Sherman traps were computed using two data-subsets: (A) considering only the five lines containing both types of trap, or (B) considering only the five lines containing exclusively Sherman traps (excluding the sixth of these lines), thus standardizing the area sampled between trap types. Models for each dependent variable were run twice (once for each data-subset), allowing us to evaluate the influence of the proximity of pitfall lines on the efficiency of Sherman traps. Our study design, however, does not allow for an assessment of the effects of Sherman traps on the efficiency of pitfall lines.
Effects of individual characteristics and weather condition on the number of captures
Two similar candidate model sets were constructed to answer these two questions, considering the data from the six species with enough captures for a robust analysis (species with more than 120 captures;Table 1). To investigate the effects of individual characteristics (age and sex) and their interaction with the type of trap, the dependent variable was the total number of captures (i.e., sum of first capture and recaptures) in each grid and capture session. Fourteen candidate models were compared, considering a constant model (no fixed factors) as a reference, three simple models (with each of the three fixed factors alone: type of trap, sex and age), all four possible additive combinations of fixed factors, and six interaction models (excluding only the interaction among the three fixed factors due to the difficulty of interpreting such complex interactions,Table 3). To investigate the effects of weather condition (minimum temperature and rainfall) and its interaction with trap type, the dependent variable was again the number of total captures (sum of first capture and recaptures), but in this case for each grid and day of sampling (since weather conditions were measured daily). The candidate model set in this case follows the same logic of the one for individual characteristics (Table 3). In all candidate models, grids and capture sessions were considered random intercept factors and species was considered random intercept and slope factor. Models for weather condition also included day as an extra intercept random factor. The two dependent variables were modeled using Poisson error distribution. However, because in both of them variance was greater than the mean, we accounted for overdispersion by adding an observation-level random effect (Harrison 2014Harrison XA (2014) Using observation-level random effects to model overdispersion in count data in ecology and evolution. PeerJ 2: e616. doi: 10.7717/peerj.616
https://doi.org/10.7717/peerj.616...
), as we did for the number of individuals. Since our results on the effects of the type of trap on the number of species, individuals and recaptures indicated a negative effect of the presence of pitfall traps on captures in adjacent Sherman traps, for these analyses we considered only the data-subset for Sherman traps from the five lines containing exclusively these traps.
Results of model selection for the number of captures of small mammals as a function of the type of trap, individual characteristics (age and sex), and weather condition (rainfall and minimum temperature), considering only captures from Sherman traps placed intercalated with pitfall lines. Check the text for random factors. Selected models Δi < 2) are in bold. K: number of parameters; Δi: difference in AIC value compared to the best model; ωi: Akaike weight.
RESULTS
In total, we obtained 3607 captures of 1273 individuals from 24 species. Of these, 1557 captures of 927 individuals from 23 species (14 rodents and 9 marsupials) were obtained in 17325 pitfall-nights (9% capture success), and 2050 captures of 346 individuals from 15 species (8 rodents and 7 marsupials) in 34650 Sherman-nights (6% capture success; see alsoTable 1). The mortality rate in pitfall traps was 3.97%, and in Sherman traps 2.07%, of the total number of captured individuals (both types of traps together throughout the study). All species captured in Sherman traps, except for one, were also captured in pitfall traps (Table 1). For any given sampling effort, pitfall traps captured more species than Sherman traps, and the accumulated number of species leveled off sooner in Sherman than in pitfall traps (Fig. 3).
Mean accumulation curves of the number of species (mean and standard deviation) as a function of sampling effort (either the number of capture sessions or the number of captured individuals) for pitfall and Sherman traps at the Morro Grande Forest Reserve.
Effects of the type of trap on mortality rates, number of species, individuals and recaptures
Considering both data-subsets from Sherman traps, the model including the type of trap as a fixed factor was more plausible than the constant model for both the number of species and individuals (Table 2), which were greater in pitfalls than in Sherman traps (Fig. 4). However, the effect of the type of trap was stronger when considering data-subset A (captures in Sherman traps adjacent to pitfall traps), especially for the number of species (Fig. 4,Table 2), indicating that Sherman traps captured fewer species when adjacent to pitfalls.
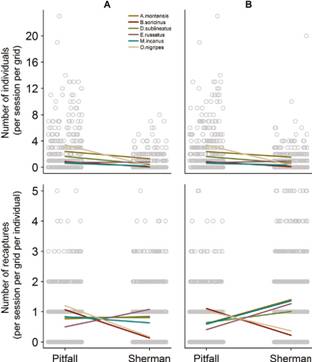
Number of species, individuals and recaptures in pitfall and Sherman traps. Overlapping dots are horizontally scattered to improve visualization. Two data-subsets were used for Sherman traps: only captures from Sherman traps placed adjacent to pitfall lines (column A) or from Sherman traps intercalated with pitfall lines (column B). Expected means for each type of trap estimated from constant models (dashed lines) and from models with trap type as a fixed factor (solid lines).
On the other hand, for mortality rates (either in relation to the number of captured individuals or the number of captures) both models were equally plausible considering both data-subsets from Sherman traps (Fig. 5,Table 2), indicating no difference in mortality rates between traps. Similarly, in the number of recaptures both models were equally plausible considering both data-subsets from Sherman traps (Fig. 4,Table 2). However, while Brucepattersonius soricinus Hershkovitz, 1998 and Oligoryzomys nigripes Olfers, 1818 had more recaptures in pitfall traps, all four remaining species had markedly more recaptures in Sherman traps, especially when considering results from Sherman traps not-adjacent to pitfall lines (Fig. 6).
Mortality rates in relation to either the number of captured individuals or the number of captures in pitfall and Sherman traps. Overlapping dots are horizontally scattered to improve visualization. Two data-subsets were used for Sherman traps: only captures from Sherman traps placed adjacent to pitfall lines (column A) or from Sherman traps intercalated with pitfall lines (column B). Expected means for each type of trap estimated from constant models (dashed lines) and from models with trap type as a fixed factor (solid lines).
Number of individuals and number of recaptures in pitfall and Sherman traps. Overlapping dots are horizontally scattered to improve visualization. Two data-subsets were used for Sherman traps: only captures from Sherman traps placed adjacent to pitfall lines (column A) or from Sherman traps intercalated with pitfall lines (column B). Colored lines: random effects of species.
Effects of individual characteristics (sex and age)
The only selected model (with a high Akaike weight) was the model containing age, trap type and their interaction, while the constant model was the last-ranked model (Table 3). All models that included age were better than those including sex but not age, corroborating that capture is more influenced by age than sex (Table 3). Adults were more frequently captured than juveniles in both traps (Fig. 7). However, the only selected model indicates that the effect of age depended on trap type (interactions) (Fig. 7). The coefficient of this interaction indicates that differences in the number of captures between adults and juveniles were stronger in Sherman traps, with proportionately more captures of juveniles in pitfall traps (Fig. 7).
Graphical representation of the interactions between trap type (pitfall traps: circles; Sherman traps: squares) and age in determining the number of captures. Overlapping dots are horizontally scattered to improve visualization. On the left, the whole dataset is shown; on the right, a zoom for better visualization of the lines that show the expected means for each trap type and age estimated from the best model inTable 3(solid line: pitfall traps; dashed line: Sherman traps).
Effects of weather condition (minimum temperature and rainfall)
Two models were equally plausible: the most complete model (additive effect of the three fixed factors and the interaction of both minimum temperature and rainfall with the type of trap), and the model considering the additive effect of the three fixed factors, but only the interaction between type of trap and minimum temperature (Table 3). Again, selected models presented high Akaike weights and the constant model was among the worst models. The coefficients of the interaction terms of the best model indicate that increased minimum temperature negatively affected captures, particularly by Sherman traps, while rainfall positively affected captures by both traps, with a stronger effect in pitfall than Sherman traps (Fig. 8).
Graphical representation of the interactions between trap type (pitfall traps: circles; Sherman traps: squares) and rainfall or minimum temperature in determining the number of captures. Overlapping dots are horizontally scattered to improve visualization. Expected means for each trap type and rainfall or minimum temperature estimated from the best model inTable 3(solid line: pitfall traps; dashed line: Sherman traps).
DISCUSSION
Effects of the type of trap on mortality rates, number of species, individuals and recaptures
As expected, our results suggest that pitfall traps are more efficient than Sherman traps to estimate the number of species and the number of individuals. The greater richness and numbers of individuals in pitfall traps are likely a result of the interception mechanism that makes pitfall less selective than Sherman traps. In contrast, the capture success of Sherman traps relies on the successful attraction of the animals and thus on the exploratory behavior of individuals. Moreover, pitfall traps allow the capture of more than one individual and thus more than one species simultaneously, which happened frequently in this study.
It is noteworthy that pitfall traps, even with half the number of buckets compared to the number of Sherman traps, captured 53% more species. Indeed, the number of captured species was greater in pitfall traps than in Sherman traps not only in average across grids (i.e., five trap lines) and sessions, but also in total for any given sampling effort. A recent study carried out in the Neotropics indicates that the efficiency in capturing small mammals in large pitfall traps is higher compared to small pitfall traps (Ribeiro-Junior et al. 2011Ribeiro-Júnior MA, Rossi RV, Miranda CL, Ávila-Pires TCS (2011) Influence of pitfall trap size and design on herpetofauna and small mammal studies in a Neotropical Forest. Zoologia 28(1): 80-91. doi: 10.1590/S1984-46702011000100012
https://doi.org/10.1590/S1984-4670201100...
). Consistent with this, previous studies carried out in the Neotropics, and which also used large buckets (≥ 60 L), obtained more species in pitfall traps (Umetsu et al. 2006Umetsu F, Naxara L, Pardini R (2006) Evaluating the efficiency of pitfall traps for sampling small mammals in the Neotropics. Journal of Mammalogy 87: 757-765. doi: 10.1644/05-MAMM-A-285R2.1
https://doi.org/10.1644/05-MAMM-A-285R2....
,Cáceres et al. 2011Cáceres NC, Nápoli RP, Hannibal W (2011) Differential trapping success for small mammals using pitfall and standard cage traps in a woodland savannah region of southwestern Brazil. Mammalia 75: 45-52. doi: 10.1515/MAMM.2010.069
https://doi.org/10.1515/MAMM.2010.069...
), while studies that used smaller buckets captured more species in Sherman traps in the Neotropics (Santos-Filho et al. 2006Santos-Filho M, Silva DJ, Sanaiotti TM (2006) Efficiency of four Trap Types in Sampling Small Mammals in Forest Fragments, Mato Grosso, Brazil. Mastozoología Neotropical 13: 217-225.) and elsewhere (Nicolas & Colyn 2006Nicolas V, Colyn M (2006) Relative efficiency of three types of small mammal traps in an African rainforest. Belgian Journal of Zoology 136: 107-111.,Dizney et al. 2008Dizney L, Jones PD, Ruedas LA (2008) Efficacy of Three Types of Live Traps Used for Surveying Small Mammals in the Pacific Northwest. Northwest Naturalist 89: 171-180.). However, is it important to note that only one of those studies included replicates and statistically compared the mean number of species and individuals between types of traps (Umetsu et al. 2006Umetsu F, Naxara L, Pardini R (2006) Evaluating the efficiency of pitfall traps for sampling small mammals in the Neotropics. Journal of Mammalogy 87: 757-765. doi: 10.1644/05-MAMM-A-285R2.1
https://doi.org/10.1644/05-MAMM-A-285R2....
).
Our results also suggest that pitfall traps are better at detecting rare species: all seven species that were captured less than five times, including a recently described new species (the arboreal rodent Drymoreomys albimaculatus;Pecequillo et al. 2011Pecequillo AR, Weksler M, Costa LP (2011) A new genus and species of rodent from the Brazilian Atlantic Forest (Rodentia: Cricetidae: Sigmodontinae: Oryzomyini), with comments on oryzomyine biogeography. Zoological Journal of Linnean Society of London 161: 357-390. doi: 10.1111/j.1096-3642.2010.00643.x
https://doi.org/10.1111/j.1096-3642.2010...
), were captured only by pitfalls. In contrast, only one species was exclusively captured in Sherman traps, the semi-aquatic rodent Nectomys squamipes Brants, 1827. Indeed, pitfall traps captured all arboreal and scansorial small mammal species that had been recorded by previous inventories of the Reserve, which included Sherman traps placed in the understory (Pardini & Umetsu 2006Pardini R, Umetsu F (2006) Non-volant small mammals from the Morro Grande Forest Reserve - distribution of species and diversity in an Atlantic Forest area. Biota Neotropica 6(2): bn00606022006. Available online at:Available online at:http://www.biotaneotropica.org.br/v6n2/pt/abstract?article+bn00606022006
[Accessed: 10/02/2008]
http://www.biotaneotropica.org.br/v6n2/p...
). This result suggests that Sherman trap captures may not complement pitfall trap captures when assessing community richness and composition, as previously suggested (Lyra-Jorge & Pivello 2001Lyra-Jorge MC, Pivello VR (2001) Combining live trap and pitfall to survey terrestrial small mammals in savanna and forest habitats in Brazil. Mammalia 65: 524-530.). In contrast, our study clearly indicates the critical importance of using large pitfall traps in biodiversity surveys and monitoring, as well as community ecology studies that aim at estimating richness and composition of small mammal communities in the Neotropics.
Our findings also show that mortality rates are not necessarily higher in pitfalls compared to Sherman traps. Although individuals captured in pitfall traps are usually more exposed to predation, aggressive interactions among them, and weather conditions, compared to Sherman traps, it is possible to obtain similar mortality rates between these two types of traps by taking some simple measures that avoid water accumulation, protect animals from rain and sun, and avoid starvation in pitfalls.
On the other hand, although model selection indicated that the type of trap does not influence the total number of recaptures, most species had more recaptures in Sherman traps, as expected. Only the two smallest species were recaptured more often in pitfall traps (weight: B. soricinus mean = 29.3 g, maximum = 37.0g; and O. nigripes mean = 18.4g, maximum = 30.0g), most likely because the small size of these species decreases the efficiency of the trigger mechanism of Sherman traps, as observed in other studies (Astúa de Moraes et al. 2006Astúa de Moraes D, Moura RT, Grelle CEV, Fonseca MT (2006) Influence of baits, trap type and position for small mammal capture in a Brazilian lowland Atlantic Forest. Boletim do Museu de Biologia Mello Leitão 19: 31-44.,Nicolas & Colyn 2006Nicolas V, Colyn M (2006) Relative efficiency of three types of small mammal traps in an African rainforest. Belgian Journal of Zoology 136: 107-111.,Dizney et al. 2008Dizney L, Jones PD, Ruedas LA (2008) Efficacy of Three Types of Live Traps Used for Surveying Small Mammals in the Pacific Northwest. Northwest Naturalist 89: 171-180.,Cáceres et al. 2011Cáceres NC, Nápoli RP, Hannibal W (2011) Differential trapping success for small mammals using pitfall and standard cage traps in a woodland savannah region of southwestern Brazil. Mammalia 75: 45-52. doi: 10.1515/MAMM.2010.069
https://doi.org/10.1515/MAMM.2010.069...
). The greater number of recaptures in Sherman traps may indicate that animals learn to avoid or seek certain types of traps after their first experience of being captured. Sherman traps provide protection and most likely reduce stress and trauma (White et al. 1982White GC, Anderson DR, Burnham KP, Otis DL (1982) Capture-Recapture and removal methods for sampling closed populations. Los Alamos, National Laboratory, 13p.), while individuals in pitfall traps are exposed to other animals and to weather conditions. Our results thus suggest that Sherman traps are of foremost importance for both basic and applied population studies that require a large number of individual recaptures to obtain accurate and precise estimates of demographic parameters, such as abundance, and rates of survival, recruitment and population growth (Williams et al. 2002Williams BK, Nichols JD, Conroy MJ (2002) Analysis and management of animal populations: modeling, estimation, and decision making. London, Academic Press, 817p.).
Finally, the comparison between the number of recaptures and the number of species, considering the Sherman traps placed in the same or in alternate lines to the pitfall lines, suggests that pitfall lines reduce the efficiency of the nearby Sherman traps. Thus, when planning sampling protocols, one should avoid placing different types of traps too close together. It is important to highlight that our sampling design did not allow us to evaluate how distant Sherman traps should be from pitfall lines to maximize the efficiency of both traps, nor the effects of Sherman lines on pitfall traps, two aspects that should be addresses in future studies.
Effect of individual characteristics (sex and age)
Besides affecting the number of species, individuals and recaptures, the type of trap interacts with the age of individuals to determine capture success. Although both types of traps captured more adults than juveniles, the strength of this bias depended on the type of trap. Pitfall traps captured more juveniles in relation to adults than Sherman traps, resulting in a less biased capture ratio between age classes.
Adults tend to be more abundant than juveniles in small mammal populations (e.g.,Gentile et al. 2000Gentile R, D'Andrea PS, Cerqueira R, Maroja LS (2000) Population dynamics and reproduction of marsupials and rodents in a Brazilian rural area: a five-year study. Studies on Neotro pical Fauna and Environment 35: 1-9. doi: 10.1076/0165-0521(200004)35:1;1-M;FT001
https://doi.org/10.1076/0165-0521(200004...
,Feliciano et al. 2002Feliciano BR, Fernandez FAS, Freitas D, Figueiredo MSL (2002) Population dynamics of small rodents in grassland between Atlantic Forest Fragments in southeastern Brazil. Mammalian Biology 67: 304-314. doi: 10.1078/1616-5047-00045
https://doi.org/10.1078/1616-5047-00045...
,Barros et al. 2008Barros CS, Crouzeilles R, Fernandez FAS (2008) Reproduction of the opossums Micoureus paraguayanus and Philander frenata in a fragmented Atlantic Forest landscape in Brazil: is seasonal reproduction a general rule for Neotropical marsu pials? Mammalian Biology 73: 463-467. doi: 10.1016/j.mambio.2007.08.002
https://doi.org/10.1016/j.mambio.2007.08...
), and a higher number of captured adults is expected. However, Sherman traps captured proportionally fewer juveniles than pitfall traps, most likely as a consequence of differences in exploratory behavior or body size between age classes. Adults exhibit well-developed exploratory behavior and are heavier, characteristics that are important either to find the bait or to trigger the door-closing mechanism of Sherman traps (Nicolas & Colyn 2006Nicolas V, Colyn M (2006) Relative efficiency of three types of small mammal traps in an African rainforest. Belgian Journal of Zoology 136: 107-111.,Dizney et al. 2008Dizney L, Jones PD, Ruedas LA (2008) Efficacy of Three Types of Live Traps Used for Surveying Small Mammals in the Pacific Northwest. Northwest Naturalist 89: 171-180.,Cáceres et al. 2011Cáceres NC, Nápoli RP, Hannibal W (2011) Differential trapping success for small mammals using pitfall and standard cage traps in a woodland savannah region of southwestern Brazil. Mammalia 75: 45-52. doi: 10.1515/MAMM.2010.069
https://doi.org/10.1515/MAMM.2010.069...
). On the other hand, our findings suggest that neither one of the two trap types results in a sample bias with respect to sex.
These results highlight the importance of including pitfalls together with Sherman traps in population studies. A greater efficiency in capturing juveniles is particularly important when studying reproduction or estimating emigration, immigration and dispersal rates (Williams et al. 2002Williams BK, Nichols JD, Conroy MJ (2002) Analysis and management of animal populations: modeling, estimation, and decision making. London, Academic Press, 817p.).
Effect of weather condition (minimum temperature and rainfall)
Our results show that the type of trap interacts also with weather condition to determine capture success. In contrast to our expectation that minimum daily temperature should be positively associated with the activity of small mammals (Vickery & Bider 1981Vickery WL, Bider JR (1981) The Influence of Weather on Rodent Activity. Journal of Mammalogy 62: 140-145.,Paise & Vieira 2006Paise G, Vieira EM (2006) Daily activity of a neotropical rodent (Oxymycterus nasutus): seasonal changes and influence of environmental factors. Journal of Mammalogy 87: 733-739. doi: 10.1644/05-MAMM-A-158R5.1
https://doi.org/10.1644/05-MAMM-A-158R5....
,Merritt 2010Merritt JF (2010) The Biology of small mammals. Baltimore, Johns Hopkins University Press, 336p.,Vieira et al. 2010Vieira EM, Baumgarten LC, Paise G, Becker RG (2010) Seasonal patterns and influence of temperature on the daily activity of the diurnal neotropical rodent Necromys lasiurus. Canadian Journal of Zoology 88: 259-265. doi: 10.1139/Z09-142
https://doi.org/10.1139/Z09-142...
) and thus with the number of captures, we observed that captures decreased with increasing minimum daily temperature, especially in Sherman traps. It is possible that the minimum daily temperatures in our study area were not sufficiently low to negatively affect the activity of small mammals and to substantially alter their encounter with drift-fences of pitfall traps, but they may have been low enough to lead the animals to enter the Sherman traps seeking shelter.
Increasing rainfall positively affected captures in both traps, as expected, but the effect was stronger in pitfall traps. This result is congruent with the idea that both the activity of animals (which may affect strongly pitfall traps) and the search for shelter (which may affect Sherman traps) should increase with increasing rainfall. The stronger effect of rainfall on captures in pitfall traps may be related to the fact that the accumulation of water in the buckets prevents the escape of trapped animals (Voss et al. 2001Voss RS, Lunde DP, Simmons NB (2001) The Mammals of Paracou, French Guiana: A Neotropical Lowland Rainforest Fauna - Part 2. Nonvolant Species. Bulletin of the American Museum of Natural History 263: 1-236.,Nicolas & Colyn 2006Nicolas V, Colyn M (2006) Relative efficiency of three types of small mammal traps in an African rainforest. Belgian Journal of Zoology 136: 107-111.,Santos-Filho et al. 2006Santos-Filho M, Silva DJ, Sanaiotti TM (2006) Efficiency of four Trap Types in Sampling Small Mammals in Forest Fragments, Mato Grosso, Brazil. Mastozoología Neotropical 13: 217-225.). Alternatively, rain could affect the way animals move, by driving them close to objects for shelter, thus increasing the efficiency of pitfall drift-fences. These results suggest that pitfall traps are more efficient in rainy days, while Sherman traps are more effective during cold, rainy days.
Implications for efficient field protocols for sampling Neotropical small mammals
Our large spatial and temporal sampling effort allowed a high number of captures of several species and thus the simultaneous evaluation of the effects of distinct factors on the capture success of small mammals. Our results reinforce the differences in the efficiency of Sherman and pitfall traps. More importantly, they also highlight that for Neotropical small mammals trap efficiency is highly dependent not only on the quantities considered (species, individuals or recaptures), but also on animal characteristics and weather conditions. The observed differences between traps and interactions with age, minimum temperature and rainfall suggest that large pitfall traps should be used whenever the focus is on biodiversity and other community parameters, and are particularly crucial in species-rich tropical communities with a high proportion of rare species. These pitfall traps should be used preferentially in the wet season, when their efficiency should be higher. Implementing simple measures to protect animals captured in pitfalls from weather conditions seems to reduce mortality rates to similar levels to those observed in Sherman traps. On the other hand, population studies focusing on demographic parameters require the combined use of pitfall and Sherman traps. While pitfall traps ensure the capture of a larger number of individuals and a higher proportion of juveniles, Sherman traps provide higher recapture rates for most species. However, Sherman and pitfall traps should be placed far apart, since pitfall lines seem to reduce captures by the Sherman traps nearby. In summary, our study calls for caution when comparing results obtained with different traps or in different seasons, given the considerable differences between Sherman and pitfall traps in the number of individuals, and particularly in the number of species captured. Our results also highlight the value of standardized surveys for both basic and applied studies in ecology and conservation. Finally, it is important that future studies consider the cost-effectiveness of using different types of traps, by confronting the benefits in terms of the number of species, individuals or recaptures, with the costs of both acquiring (most likely higher for Sherman traps) and installing (most likely higher for pitfall) the two types of traps.
ACKNOWLEDGMENTS
We thank all field assistants, especially R. Bovendorp and T.K. Martins; C. Castanho for invaluable help with graphics and models; T.B. Quental, A.A. de Oliveira, P.R. Guimarães, and C. Banks-Leite for comments on previous versions of the manuscript; SABESP (Companhia de Saneamento Básico do Estado de São Paulo) for logistic support; colleagues from our lab, especially T.K. Martins, for valuable discussions; and CNPq - Conselho Nacional de Desenvolvimento Científico e Tecnológico/BMBF - German Federal Ministry of Education and Research (690144/01-6), CAPES - Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), and FAPESP - Fundação de Amparo à Pesquisa do Estado de São Paulo (05/56555-4) for financial support and scholarships.
LITERATURE CITED
- Astúa de Moraes D, Moura RT, Grelle CEV, Fonseca MT (2006) Influence of baits, trap type and position for small mammal capture in a Brazilian lowland Atlantic Forest. Boletim do Museu de Biologia Mello Leitão 19: 31-44.
- Barros CS, Crouzeilles R, Fernandez FAS (2008) Reproduction of the opossums Micoureus paraguayanus and Philander frenata in a fragmented Atlantic Forest landscape in Brazil: is seasonal reproduction a general rule for Neotropical marsu pials? Mammalian Biology 73: 463-467. doi: 10.1016/j.mambio.2007.08.002
» https://doi.org/10.1016/j.mambio.2007.08.002 - Bates D, Maechler M, Bolker B (2011) Lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-42.http://CRAN.R-project.org/package=lme4[Accessed: 05/10/2009]
» http://CRAN.R-project.org/package=lme4 - Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS (2008) Generalized linear mixed models: a practical guide for ecology and evolution. Trends in ecology and evolution 24: 127-135. doi: 10.1016/j.tree.2008.10.008
» https://doi.org/10.1016/j.tree.2008.10.008 - Bozinovic F, Ruiz G, Rosenmann M (2004) Energetics and torpor of a South American "living fossil", the microbiotheriid Dromiciops gliroides Journal of Comparative Physiology [B] 174: 293-297. doi: 10.1007/s00360-004-0414-8
» https://doi.org/10.1007/s00360-004-0414-8 - Bozinovic F, Ruiz G, Rosenmann M (2005) Energetics, thermoregu lation and torpor in the Chilean mouse-opossum Thylamys elegans (Didelphidae). Revista Chilena de História Natural 78: 199-120.
- Burnham KP, Anderson DR (2002) Model selection and multimode inference: A practical Information-Theoretic Approach. Colorado, Springer, 2nd ed., 488p.
- Cáceres NC, Nápoli RP, Hannibal W (2011) Differential trapping success for small mammals using pitfall and standard cage traps in a woodland savannah region of southwestern Brazil. Mammalia 75: 45-52. doi: 10.1515/MAMM.2010.069
» https://doi.org/10.1515/MAMM.2010.069 - Dizney L, Jones PD, Ruedas LA (2008) Efficacy of Three Types of Live Traps Used for Surveying Small Mammals in the Pacific Northwest. Northwest Naturalist 89: 171-180.
- Dray S, Pélissier R, Couteron P, Fortin MJ Legendre P, Peres-Neto PR, Bellier E, Bivand R, Blanchet FG, Cáceres M, Dufour AB, Heegaard E, Jombart T, Munoz F, Oksanen J, Thioulouse J, Wagner HH (2012) Community ecology in the age of multivariate multiscale spatial analysis. Ecological Monographies 82: 257-275.
- Feliciano BR, Fernandez FAS, Freitas D, Figueiredo MSL (2002) Population dynamics of small rodents in grassland between Atlantic Forest Fragments in southeastern Brazil. Mammalian Biology 67: 304-314. doi: 10.1078/1616-5047-00045
» https://doi.org/10.1078/1616-5047-00045 - Flowerdew JR, Hore RF, Poulton SMC, Sparks TH (2004) Live trapping to monitor small mammals in Britain. Journal of Comparative Physiology [B] 34: 31-50. doi: 10.1046/j.0305-1838.2003.00025.x
» https://doi.org/10.1046/j.0305-1838.2003.00025.x - Geiser F, Baudinette RV (1987) Seasonality of torpor and thermoregulation in three dasyurid marsupials. Journal of Comparative Physiology [B] 157: 335-344.
- Gentile R, D'Andrea PS, Cerqueira R, Maroja LS (2000) Population dynamics and reproduction of marsupials and rodents in a Brazilian rural area: a five-year study. Studies on Neotro pical Fauna and Environment 35: 1-9. doi: 10.1076/0165-0521(200004)35:1;1-M;FT001
» https://doi.org/10.1076/0165-0521(200004)35:1;1-M;FT001 - Halle S (2000) Ecological relevance of daily activity patterns, p. 67-90. In: Halle S, Stenseth NC (Eds.). Activity patterns in small mammals: an ecological approach. Berlin, Springer-Verlag.
- Harrison XA (2014) Using observation-level random effects to model overdispersion in count data in ecology and evolution. PeerJ 2: e616. doi: 10.7717/peerj.616
» https://doi.org/10.7717/peerj.616 - Hice CLH, Velazco PM (2013) Relative Effectiveness of Several Bait and Trap Types for Assessing Terrestrial Small Mammal Communities in Neotropical Rainforest. Occasional Papers, Museum of Texas Tech University 316: 1-15.
- Lyra-Jorge MC, Pivello VR (2001) Combining live trap and pitfall to survey terrestrial small mammals in savanna and forest habitats in Brazil. Mammalia 65: 524-530.
- Macedo J, Loretto D, Vieira MV, Cerqueira R (2006) Classes de desenvolvimento em marsupiais: um método para animais vivos. Mastozoología Neotropical 13: 133-136.
- Magurran AE, Mcgill BJ (2011) Biological Diversity: Frontiers in Measurement and Assessment: Frontiers in Measurement and Assessment. Oxford, Oxford University Press, 368p.
- Merritt JF (2010) The Biology of small mammals. Baltimore, Johns Hopkins University Press, 336p.
- Metzger JP, Alves LF, Goulart W, Teixeira AMG, Simões SJC, Catharino EL (2006) An important biological area, but still poorly known: the Morro Grande Forest Reserve. Biota Neotropica 6. doi: 10.1590/S1676-06032006000200003
» https://doi.org/10.1590/S1676-06032006000200003 - Nicolas V, Colyn M (2006) Relative efficiency of three types of small mammal traps in an African rainforest. Belgian Journal of Zoology 136: 107-111.
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Wagner H (2012) Vegan: Community Ecology Package. R package version 2.0-7. Available online at:Available online at:http://CRAN.R-project.org/package=vegan [Accessed:18/08/2012]
» http://CRAN.R-project.org/package=vegan - Paise G, Vieira EM (2006) Daily activity of a neotropical rodent (Oxymycterus nasutus): seasonal changes and influence of environmental factors. Journal of Mammalogy 87: 733-739. doi: 10.1644/05-MAMM-A-158R5.1
» https://doi.org/10.1644/05-MAMM-A-158R5.1 - Pardini R, Umetsu F (2006) Non-volant small mammals from the Morro Grande Forest Reserve - distribution of species and diversity in an Atlantic Forest area. Biota Neotropica 6(2): bn00606022006. Available online at:Available online at:http://www.biotaneotropica.org.br/v6n2/pt/abstract?article+bn00606022006 [Accessed: 10/02/2008]
» http://www.biotaneotropica.org.br/v6n2/pt/abstract?article+bn00606022006 - Pecequillo AR, Weksler M, Costa LP (2011) A new genus and species of rodent from the Brazilian Atlantic Forest (Rodentia: Cricetidae: Sigmodontinae: Oryzomyini), with comments on oryzomyine biogeography. Zoological Journal of Linnean Society of London 161: 357-390. doi: 10.1111/j.1096-3642.2010.00643.x
» https://doi.org/10.1111/j.1096-3642.2010.00643.x - Pires AS, Fernandez FAS (1999) Use of space by the marsupial Micoureus demerarae in small Atlantic forest fragments in south-eastern Brazil. Journal of Tropical Ecology 15: 279-290. doi: 10.1017/S0266467499000814
» https://doi.org/10.1017/S0266467499000814 - Püttker T, Meyer-Lucht Y, Sommer S (2006) Movement distances of five rodent and two marsupial species in forest fragments of the coastal atlantic rainforest, Brazil. Biotropica 22: 131-139.
- R Development Core Team (2011) R: A language and environment for statistical computing. Vienna, R Foundation for Statistical Computing, ISBN 3-900051-07-0.
- Ribeiro-Júnior MA, Rossi RV, Miranda CL, Ávila-Pires TCS (2011) Influence of pitfall trap size and design on herpetofauna and small mammal studies in a Neotropical Forest. Zoologia 28(1): 80-91. doi: 10.1590/S1984-46702011000100012
» https://doi.org/10.1590/S1984-46702011000100012 - Santos-Filho M, Silva DJ, Sanaiotti TM (2006) Efficiency of four Trap Types in Sampling Small Mammals in Forest Fragments, Mato Grosso, Brazil. Mastozoología Neotropical 13: 217-225.
- Torre I, Guixé D, Sort F (2010) Comparing Three Live Trapping Methods for Small Mammal Sampling in Cultivated Areas of Ne Spain. Hystrix 21: 147-155. doi: 10.4404/hystrix-21.2-4462
» https://doi.org/10.4404/hystrix-21.2-4462 - Tribe CJ (1990) Dental age classes in Marmosa incana and other didelphoids. Journal of Mammalogy 71: 566-569. doi: 10.2307/1381795
» https://doi.org/10.2307/1381795 - Umetsu F, Naxara L, Pardini R (2006) Evaluating the efficiency of pitfall traps for sampling small mammals in the Neotropics. Journal of Mammalogy 87: 757-765. doi: 10.1644/05-MAMM-A-285R2.1
» https://doi.org/10.1644/05-MAMM-A-285R2.1 - Vickery WL, Bider JR (1981) The Influence of Weather on Rodent Activity. Journal of Mammalogy 62: 140-145.
- Vieira EM, Baumgarten LC, Paise G, Becker RG (2010) Seasonal patterns and influence of temperature on the daily activity of the diurnal neotropical rodent Necromys lasiurus Canadian Journal of Zoology 88: 259-265. doi: 10.1139/Z09-142
» https://doi.org/10.1139/Z09-142 - Voss RS, Lunde DP, Simmons NB (2001) The Mammals of Paracou, French Guiana: A Neotropical Lowland Rainforest Fauna - Part 2. Nonvolant Species. Bulletin of the American Museum of Natural History 263: 1-236.
- White GC, Anderson DR, Burnham KP, Otis DL (1982) Capture-Recapture and removal methods for sampling closed populations. Los Alamos, National Laboratory, 13p.
- Williams BK, Nichols JD, Conroy MJ (2002) Analysis and management of animal populations: modeling, estimation, and decision making. London, Academic Press, 817p.
Publication Dates
-
Publication in this collection
31 Oct 2015
History
-
Received
05 Aug 2014 -
Reviewed
27 Apr 2015 -
Accepted
13 July 2015