Abstract
Urticating setae are exclusive to New World tarantulas and are found in approximately 90% of the New World species. Six morphological types have been proposed and, in several species, two morphological types can be found in the same individual. In the past few years, there has been growing concern to learn more about urticating setae, but many questions still remain unanswered. After studying individuals from several theraphosid species, we endeavored to find more about the segregation of the different types of setae into different abdominal regions, and the possible existence of patterns; the morphological variability of urticating setae types and their limits; whether there is variability in the length of urticating setae across the abdominal area; and whether spiders use different types of urticating setae differently. We found that the two types of urticating setae, which can be found together in most theraphosine species, are segregated into distinct areas on the spider's abdomen: type III occurs on the median and posterior areas with either type I or IV surrounding the patch of type III setae. Morphological intermediates between types I and III, as well as between III and IV, were found. We propose that type III urticating setae have evolved through modifications of body setae on specific areas of abdomen dorsum and subsequently gave independent origin to areas having either type I or IV. A parallel evolution seems to have occurred in some aviculariine genera in which type II setae evolved also from body setae from specific areas of abdomen dorsum. Concerning the length of the setae, we observed that towards the median and posterior areas of the abdomen the length of the urticating setae increases. These long setae are cast by the spider as part of an active defensive behavior against vertebrate predators. We propose that spiders use the various types of urticating setae differently and according to their different targets: type I setae, when incorporated either into the molting web or eggsac, is more effective against invertebrates (ants or phorid fly larvae) than type III. The latter seems to be used mainly against vertebrate predators.
Ant; defensive behavior; Phoridae; urticaria; urticating hair
MORPHOLOGY AND PHYSIOLOGY
Morphology, evolution and usage of urticating setae by tarantulas (Araneae: Theraphosidae)
Rogério BertaniI,* * Corresponding author. ;José Paulo Leite GuadanucciII
ILaboratório Especial de Ecologia e Evolução, Instituto Butantan. Avenida Vital Brazil 1500, 05503-900 São Paulo SP, Brazil. E-mail: rbert@butantan.gov.br
IILaboratório de Zoologia de Invertebrados, Universidade Federal dos Vales do Jequitinhonha e Mucuri, Campus JK. Rodovia MGT 367, km 583, 39100-000 Diamantina, MG, Brazil. E-mail: joseguadanucci@gmail.com
ABSTRACT
Urticating setae are exclusive to New World tarantulas and are found in approximately 90% of the New World species. Six morphological types have been proposed and, in several species, two morphological types can be found in the same individual. In the past few years, there has been growing concern to learn more about urticating setae, but many questions still remain unanswered. After studying individuals from several theraphosid species, we endeavored to find more about the segregation of the different types of setae into different abdominal regions, and the possible existence of patterns; the morphological variability of urticating setae types and their limits; whether there is variability in the length of urticating setae across the abdominal area; and whether spiders use different types of urticating setae differently. We found that the two types of urticating setae, which can be found together in most theraphosine species, are segregated into distinct areas on the spider's abdomen: type III occurs on the median and posterior areas with either type I or IV surrounding the patch of type III setae. Morphological intermediates between types I and III, as well as between III and IV, were found. We propose that type III urticating setae have evolved through modifications of body setae on specific areas of abdomen dorsum and subsequently gave independent origin to areas having either type I or IV. A parallel evolution seems to have occurred in some aviculariine genera in which type II setae evolved also from body setae from specific areas of abdomen dorsum. Concerning the length of the setae, we observed that towards the median and posterior areas of the abdomen the length of the urticating setae increases. These long setae are cast by the spider as part of an active defensive behavior against vertebrate predators. We propose that spiders use the various types of urticating setae differently and according to their different targets: type I setae, when incorporated either into the molting web or eggsac, is more effective against invertebrates (ants or phorid fly larvae) than type III. The latter seems to be used mainly against vertebrate predators.
Key words: Ant; defensive behavior; Phoridae; urticaria; urticating hair.
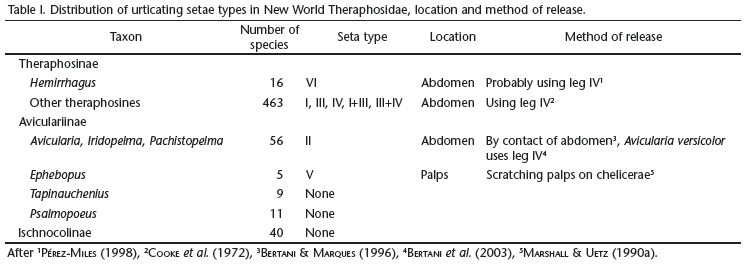
Among the array of defensive behaviors exhibited by spiders, the use of urticating setae by tarantulas (Theraphosidae) is noteworthy (Cooke et al. 1972). Theraphosids are distributed throughout the tropical and many subtropical regions of the world, and it is intriguing to find that these arrow-like setae only occur in the American fauna (Cooke et al. 1972). Moreover, urticating setae are found in roughly 540 of the 600 theraphosid species described for the New World. Representatives of all known species of the subfamily Theraphosinae, as well as species of the aviculariine genera Avicularia Lamarck, 1818, Iridopelma Pocock, 1901, Pachistopelma Pocock, 1901 and Ephebopus Simon, 1892 have urticating setae. The aviculariines Tapinauchenius Ausserer, 1871 and Psalmopoeus Pocock, 1895 and the ischnocoline species are the only New World theraphosids that lack these setae (Table I). Illustrations of urticating setae reported for ischnocolines (Schmidt 2003) are in fact non urticating body setae that normally cover the spider's cuticle.
Urticating setae can be located either on the distal prolateral surface of the palpal femur, as in the aviculariine genus Ephebopus (Marshall & Uetz 1990a, Foelix et al. 2009); or on the dorsum of the abdomen, as in theraphosines and aviculariine species of genera Avicularia, Iridopelma and Pachistopelma. These spiders release these setae in a number of ways. Individuals of theraphosine species (Cooke et al. 1972, Pérez-Miles & Prandi 1991, Bertani & Marques 1996) and the aviculariine Avicularia versicolor (Walckenaer, 1837) (Bertani et al. 2003) release setae by scratching legs IV against the abdominal urticating setae patch; in most species of the aviculariine Avicularia, Pachistopelma, and Iridopelma the release is by direct contact of the abdominal setae patch with the target (Bertani & Marques 1996); whereas individuals of the aviculariine genus Ephebopus scratch the palps against the basal segments of the chelicerae (Marshall & Uetz 1990a, Foelix et al. 2009) (Table I).
The effects of humans and laboratory animals coming into contact with such setae are well documented [Langsdorff 1812, Bates 1863 (apud Cooke et al. 1972), Bücherl 1951], whereas the morphology of the setae was poorly known until Cooke et al. (1972) compared setae from many theraphosid species. Cooke et al. (1972) described four morphological types of setae, and numbered them from I to IV (Fig. 1). They found that type II was exclusive to some aviculariine species, whereas types I, III and IV were found in genera currently included in Theraphosinae (Table I). They also showed that two types of setae can occur simultaneously in the same specimen, and suggested that different seta types are segregated into distinct regions of the spider's abdomen. The use of urticating hair setae morphology in taxonomy was also proposed by Cooke et al. (1972). Marshall & Uetz (1990a) described an additional type of seta (V) (Fig. 1), which was found on the distal prolateral surface of the pedipalpal femur of Ephebopus (Aviculariinae). Additionally, Pérez-Miles (1998) described the seta type VI (Fig. 1), found on the abdomen of Hemirrhagus cervinus (Simon, 1891) (Theraphosinae).
All urticating setae have a penetrating tip and barbs that aid embedding them. Except for types V and VI, which are attached into a socket, all other setae are attached to the spider's cuticle via a stalk, which represents the break-off region (Fig. 1).
Although the main use of the urticating setae seems to be defense against vertebrates (Cooke et al. 1972), some authors (e.g., Marshall & Uetz 1990b, Pérez-Miles & Costa 1994) found that these setae can be incorporated into the web to be used in the molting process, as well as onto the eggsac (Bücherl 1951, Melchers 1964). In the latter case, the setae appear to protect the spider and/or the eggsac against fly larvae (Diptera: Phoridae) (Marshall & Uetz 1990b).
Even though the amount of information on urticating setae has increased considerably after Cooke et al. (1972), many questions still remain unanswered. Herein, after the study of individuals from several theraphosid species, we endeavored to test whether urticating setae are segregated into different abdominal regions, as proposed by Cooke et al. (1972) and the possible existence of patterns; we also investigated the morphological variation of urticating setae types and their limits; the variations in the length of urticating setae across the abdominal area; and, the possible differential usage of the different types of urticating setae.
MATERIAL AND METHODS
Specimens are housed at the American Museum of Natural History, New York (AMNH); Instituto Butantan, São Paulo (IBSP); Museu Nacional da Universidade Federal do Rio de Janeiro, Rio de Janeiro (MNRJ); Richard C. West private collection, Victoria (RCW); and Senckenberg Museum, Frankfurt (SMF).
Urticating setae from six different regions of the abdomen [medians: anterior (MA), median (MM), and posterior (MP); laterals: anterior (LA), median (LM), and posterior (LP)] (Figs 2-3) were removed with a sharp forceps. They were examined under a compound microscope and lineal measurements were obtained using an ocular micrometer. The mean of four sampled setae per region was used in the analysis. Because of the presence of a conspicuous patch on the abdomen of many species having types III and IV setae, as in Grammostola sp., a different investigation of the distribution of the types of setae was carried out. Therefore, seven regions were explored (Fig. 4), three within the distinct patches on the center of the abdomen; and four in the region surrounding it.
For the analysis of the length of the urticating setae, samples were taken from 15 males and 15 females of Vitalius sorocabae (Mello-Leitão, 1923); 15 males and 13 females of Grammostola sp.; and 11 males and 15 females of Avicularia avicularia (Linnaeus, 1758). Statistical analysis of length differences was carried out using ANOVA, with a confidence level of 95%. For Grammostola sp. the setae were separated in two groups for analysis, one having type III setae and the other having type IV. The following specimens were examined: Avicularia avicularia (Linnaeus, 1758) - Males: Brazil, State of Pará: IBSP 7898 (2 specimens), IBSP 8565, IBSP 8566, IBSP 8570, IBSP 8572, IBSP 8575, IBSP 8579, IBSP 8580, U. H. E. Tucuruí, Tucuruí; IBSP 8578, Santarém. Females: Brazil, State of Pará: IBSP 7898, IBSP 7899, IBSP 8567, IBSP 8568, IBSP 8569, IBSP 8573, IBSP 8574, IBSP 8577, IBSP 8842, IBSP 8845, IBSP 8848, IBSP 8850, IBSP 8851, U. H. E. Tucuruí, Tucuruí; IBSP 8571, Belém; IBSP 8576, Itaituba. Vitalius sorocabae (Mello-Leitão, 1923) - Males: Brazil, State of São Paulo: IBSP 5232, Avaré; IBSP 5216, Anhembi; IBSP 4978, Cezário Lange; IBSP 5073, IBSP 5214, IBSP 5216, Ibiúna; IBSP 5226, Osasco; IBSP 5224, São Miguel Arcanjo; IBSP 5125, IBSP 5204, IBSP 5209, IBSP 5213, São Paulo; IBSP 3323, IBSP 5205, Sorocaba; IBSP 5215, Tietê. Females: Brazil, State of São Paulo: IBSP 5418, Carapicuíba; IBSP 5454, Cerquilho; IBSP 5442, IBSP 5425, Ibiúna; IBSP 5392, Itapeva; IBSP 5441, Jandira; IBSP 6943, São Manuel; IBSP 5429, IBSP 5439, IBSP 5451 São Paulo; IBSP 5453, São Roque; IBSP 6924, IBSP 6926, Sorocaba; IBSP 5420, IBSP 5427, Tatuí. Grammostola sp. Males: Brazil, State of Minas Gerais: IBSP 4020, Ouro Preto; IBSP 4603, IBSP 9669, Camanducaia; IBSP 4288, Camanducaia, Monte Verde. State of São Paulo: IBSP 4390, Cunha; IBSP 8033, Serra da Bocaina; IBSP 4301, Bragança Paulista; IBSP 4279, IBSP 8034, Arujá; IBSP 4509, Itu; IBSP 4582, São José do Barreiro; IBSP 9671, Campos do Jordão. State of Paraná: IBSP 4590 U.H.E. Foz do Areia. State of Santa Catarina: IBSP 9670, Videira; IBSP 1124, Colônia Ouro Verde. Females: Brazil, State of São Paulo: IBSP 2424, IBSP 2425, Amador Bueno; IBSP 1482, Pary; IBSP 4820, Sete Barras. State of Paraná: IBSP 2009, Araucária; IBSP 4911 (2 females), U. H. E. Foz do Areia; IBSP 2533, Mallet; State of Santa Catarina: IBSP 2188, Barra do Pinheiro; State of Minas Gerais: IBSP 4603, Camanducaia; IBSP 3545, IBSP 9667, Camanducaia, Monte Verde; IBSP 3595, Itajubá.
For the analysis of urticating setae types, we examined 132 specimens belonging to 33 genera and 60 species: Aviculariinae - Avicularia avicularia. Brazil, State of Pará, Tucuruí, U. H. E. Tucuruí. 1 male, IBSP 8575, 1 female IBSP 8574. Avicularia juruensis Mello-Leitão, 1923. Brazil, State of Rondônia, Porto Velho. 1 male, IBSP 2483. Brazil, State of Amazonas, Humaitá. 1 female, IBSP 2097. Avicularia versicolor (Walckenaer, 1837). Martinique. 1 male, AMNH, Beatty leg., 1967; 1 male, Morne Corbet, 1500 m, forest trees, 18 March 1967, H. Beatty; 2 exuviae of females. IBSP 9635. Pachistopelma bromelicola Bertani, 2012. Brazil, State of Sergipe, Areia Branca, 1 male, IBSP 8716; 1 female, IBSP 8698. Iridopelma hirsutum Pocock, 1901. Brazil, State of Paraíba, João Pessoa, 1 male, IBSP 8077; Lagarto, 1 female, IBSP 8026. Ephebopus murinus (Walckenaer, 1837). Brazil, State of Pará, Tucuruí, U.H.E. Tucuruí, 1 male IBSP, 9650; 1 female, IBSP 9658. Ephebopus uatuman Lucas, Silva & Bertani, 1992. Brazil, State of Amazonas, Presidente Figueiredo, U.H.E. Balbina, 1 male, IBSP 7858; 1 female, IBSP 7857. Theraphosinae - Acanthoscurria geniculata (C.L. Koch, 1841). Brazil, State of Pará, Tucuruí, U.H.E. Tucuruí, 1 male, IBSP 7022; Redenção, 1 female, IBSP 7023. Acanthoscurria paulensis Mello-Leitão, 1923. Brazil, State of Minas Gerais, Sacramento, 1 male, IBSP 4247; State of São Paulo, São Carlos, 1 female, IBSP 8062. Acanthoscurria sternalis Pocock, 1903. Argentina, Jujuy, 1 male, MNRJ. Aphonopelma chalcodes Chamberlin, 1940. United States, State of Califórnia, Fresno County, 1 male, IBSP 7047. Aphonopelma seemani (F. O. P.-Cambridge, 1897). Central America, 1 male, IBSP 7019; 1 female, IBSP 7020. Brachypelma emilia (White, 1856). Mexico, 1 male, IBSP 7027; 1 female, IBSP 7028. Brachypelma klaasi (Schmidt & Krause, 1994). Mexico, Chamela Sit. Biológica, 1 male, IBSP 7050; 1 female, IBSP 9646. Brachypelma smithi (F.O.P.-Cambridge, 1897). Mexico, 1 male, IBSP 4728; 1 female, IBSP 3448. Chromatopelma cyaneopubescens (Strand, 1907). Venezuela, 1 male, SMF 39012. Citharacanthus livingstoni Schmidt & Weinmann, 1996. Guatemala, 1 male, SMF 38559. Cyriocosmus chicoi Pérez-Miles, 1998. Brazil, State of Rondonia, Porto Velho, U.H.E. Samuel, 1 male, 1 female, IBSP 4947. Cyriocosmus ritae Pérez-Miles, 1998. Brazil, State of Acre, Rio Branco, Humaitá, 1 male, IBSP 4951. Cyrtopholis portoricae Chamberlin, 1917. Puerto Rico, Guayama, 1 male, RCW. Euathlus truculentus L. Koch, 1875. Chile, Valparaíso, 1 male, IBSP 3744; 1 female, IBSP 3720. Euathus vulpinus (Karsch, 1880). Chile, Osorno, 3 males, IBSP 3817-A; 1 female, 3817-B. Eupalaestrus campestratus (Simon, 1891). Brazil, State of Mato Grosso do Sul, Nova Andradina, 1 male, IBSP 4302; Coxim, 1 male IBSP 4149; Brilhante, 1 female, IBSP 2830; Palmeiras, 1female, IBSP 2346. Eupalaestrus weijenberghi (Thorell, 1894). Uruguay, Montevideo, 1 male, IBSP 7979; 1 female, IBSP 7980. Grammostola sp. Brazil, State of Minas Gerais, Cunha, 1 male, IBSP 4390; Itajubá, 1 female, IBSP 3595. Grammostola rosea (Walckenaer, 1837). Chile, 1 male, IBSP 9672. Hapalopus sp. Brazil, State of Tocantins, Lajeado, 1 male, IBSP 9211; 1 female, IBSP 9217. Homoeomma montanum (Mello-Leitão, 1923). Brazil, State of São Paulo, Santo André, 2 males, IBSP 4649; Tapiraí, 1 female, IBSP 9666. Homoeomma brasilianum (Chamberlin, 1917). Brazil, State of São Paulo, Capão Bonito, 1 male, IBSP 4389. Homoeomma stradlingi O. P.-Cambridge, 1881. Brazil, State of Rio de Janeiro, Teresópolis, 1 male, 1 female, IBSP 4242; Rio de Janeiro, 1 male, IBSP 4147. Lasiodora sp. Brazil, State of Pernambuco, Caruaru, 1 male, IBSP 6416; State of Bahia, Morro do Chapéu, Gruta dos Brejões, 1 female, IBSP 2828. Lasiodorides polycuspulatus Schmidt & Bischoff, 1997. Peru, 1 male, SMF 39007. Maraca horrida (Schmidt, 1994). Brazil, State of Amazonas, Miratucu, Parque Nacional do Jaú, 1 male, 1 female, IBSP 9377. Megaphobema robustum (Ausserer, 1875). Colombia, 1 male, IBSP 8529. Megaphobema mesomelas (O. P.-Cambridge, 1892). Costa Rica, 1 female, IBSP 9640. Nhandu carapoensis Lucas, 1983. Brazil, State of Mato Grosso do Sul, Piraputanga, 1 male, IBSP 6558; Dourados, 1 male, IBSP 6559; Caarapó, 1 female, IBSP 4553; State of São Paulo, Araras, 1 male, IBSP 6559. Nhandu cerradensis Bertani, 2001. Brazil, State of Goiás, Porangatú, 1 male, IBSP 3809; State of Bahia, 1 female, IBSP 2726. Nhandu coloratovillosus (Schmidt, 1998). Brazil, State of Mato Grosso, Barra do Garças, 1 male, IBSP 4078; Between Rivers Coluene and Sete de Setembro, 1 male, IBSP 2748; State of Mato Grosso do Sul, 1 female, IBSP 2959. Pamphobeteus crassifemur Bertani, Fukushima & Silva, 2008. Brazil, State of Rondonia, Porto Velho, U.H.E. Samuel, 1 male, IBSP 4944; 1 female, IBSP 7025. Paraphysa sp. Perú, Fundo Ingahuasi, 3 females, IBSP 3791-B. Phormictopus sp. Dominican Republic, Barahona, 1 male, RCW; Haiti, 1 female, IBSP 7021. Phormictopus atrichomatus Schmidt, 1991. Honduras, 1 female, SMF 37587. Plesiopelma insulare (Mello-Leitão, 1923). Brazil, State of São Paulo, Ilha da Queimada Grande, 1 male, IBSP 8922; 1 female, IBSP 8363. Proshapalopus anomalus Mello-Leitão, 1923. Brazil, State of Espírito Santo, Domingos Martins, 1 male, IBSP 6857; 1 female, IBSP 6858. Proshapalopus multicuspidatus (Mello-Leitão, 1929). Brazil, State of Bahia, Porto Seguro, 1 male, IBSP 6848; 1 female, IBSP 6846. Proshapalopus amazonicus Bertani, 2001. Brazil, State of Mato Grosso, Alta Floresta, 1 male, IBSP 4747; 1 female, IBSP 6915. Pseudhapalopus spinulopalpus Schmidt & Weinmann, 1997. Colombia, 1 male, 1 female, SMF 39018. Stichoplastoris longistylus (Kraus, 1955). El Salvador, 1 male, SMF 8575/1; 1 female SMF 8576/1. Theraphosa sp. Brazil, State of Amazonas, Presidente Figueiredo, U.H.E. Balbina, 1 male, IBSP 7834; 1 female, IBSP 7830. Theraphosa apophysis (Tinter, 1991). Brazil, Roraima, Alto Mazaruni, 1 male, IBSP 2277. Thrixopelma pruriens Schmidt, 1998. Chile, 1 male, SMF 39212. Tmesiphantes nubilus Simon, 1892. Brazil, State of Bahia, Rio de Contas, 1 male, IBSP 7068; Arraial D'Ajuda, 1 female, IBSP 9665. Vitalius buecherli Bertani, 2001. Brazil, State of São Paulo, Juquitiba, 1 male, IBSP 6586; 1 male, IBSP 6594; 1 male, IBSP 6600; 1 female, IBSP 6630; 1 female, IBSP 6630; 1 female, IBSP 6646. Vitalius dubius (Mello-Leitão, 1923). Brazil, State of São Paulo, Mogi Mirim, 1 male, IBSP 5920; Pirassununga, 1 male, IBS P 5907; São Roque, 1 male, IBSP 5898; Jundiai, 1 female, IBSP 5544; Aguaí, 1 female, IBSP 5590. Vitalius longisternalis Bertani, 2001. Brazil, State of Paraná, Guarapuava, 1 male, IBSP 3939; Candói, U.H.E. Segredo, 1 male, IBSP 6671; 1 male, IBSP 6775; 1 female, IBSP 6754; 1 female, IBSP 6764. Vitalius lucasae Bertani, 2001. Brazil, State of Paraná, Irati, 1 male, IBSP 6829; Curitiba, 1 female, IBSP 4392. Vitalius sorocabae (Mello-Leitão, 1923). Brazil, State of São Paulo, Tietê, 1 male, IBSP 5215; Ibiúna, 1 female, IBSP 5442. Vitalius vellutinus (Mello-Leitão, 1923). Brazil, State of São Paulo, Palmital, 1 male, IBSP 6342; 1 male, IBSP 6346; 1 female, IBSP 5678; Assis, 1 female, IBSP 6984. Vitalius wacketi (Mello-Leitão, 1923). Brazil, State of Rio de Janeiro, Angra dos Reis, 1 male, IBSP 6833; State of São Paulo, Itanhaém, 1 female, IBSP 6955; Serra de Santos, 1 female, IBSP 6957; Praia Grande, 1 female, IBSP 6958. Xenesthis immanis (Ausserer, 1875). Venezuela, 1 male, IBSP 7026; 1 female, IBSP 4267.
For comparison, representatives of the following species lacking urticating setae were analyzed: Ischnocolinae - Oligoxystre caatinga Guadanucci, 2007. Brazil, State of Bahia, Central, 1 male, IBSP 8554; 1 female, IBSP 9470. Aviculariinae - Psalmopoeus sp. Trinidad-Tobago, 1 male, IBSP 9653; Venezuela, 1 female IBSP 9655. Stromatopelma sp. Sierra Leone, 1 male, IBSP 9662; 1 female, IBSP 9661. Heteroscodra maculata Pocock, 1899. Africa, 1 male, IBSP 9642; Guinea-Bissau, 1 female, IBSP 9644. Tapinauchenius sp. Brazil, State of Pará, Belém, 1 male, 2 females, 7 immatures, IBSP 9639. Harpactirinae - Pterinochilus sp. Angola, Buila, Lunda Sul, 2 males, IBSP 9647; Kenya, 1 female, IBSP 8765. Ceratogyrus sp. Zimbabwe, 1 female, IBSP 9638. Eumenophorinae - Hysterocrates sp. Nigeria, 1 female, IBSP 9641. Pelinobius muticus Karsch, 1885. Kenya, 1 male, IBSP 8530; 1 female, IBSP 9643. Ornithoctoninae - Haplopelma minax (Thorell, 1897). Thailand, Bangkok, 2 females, IBSP 9645. Selenocosmiinae - Selenocosmia sp. Myanmar, 1 female, IBSP 9648. Poecilotheria sp. India, 1 male, IBSP 9660; Sri Lanka, 1 female, IBSP 8788.
SEM photographs of setae were taken from preserved and living specimens, as well as from exuviae. Electron scanning microscopes LEO 440 from Museu de Zoologia and Zeiss DSM 940 from Instituto de Biociências, both from Universidade de São Paulo, were used.
In order to test the effects of molting webs on ants - Camponotus rufipes (Fabricius, 1775) - we proceeded as follows: two plastic vials 70 mm long and 105 mm wide were connected by a cardboard cylinder (100 mm long and 45 mm wide). Inside the cylinder we attached a piece of paper where a female Lasiodora sp. had molted. The paper was fully covered with silk strands incorporated with type I and III urticating setae. A second similar device was built in which the piece of paper was covered only with silk strands produced by a female of Avicularia juruensis Mello-Leitão, 1923, used as the control experiment. We placed 15 ants in one of the vials of each of the two devices. The experiment was observed during the first hour and after 24 hours. The number of ants that succeeded in passing through the cylinder was counted and their behavior was observed.
Ten larvae of Megaselia scalaris (Loew, 1866) (Diptera: Phoridae), a cosmopolitan and synanthropic scuttle fly (Disney 1994), were obtained from spider carcasses and tested on molting webs incorporated with urticating setae types I and III of a Lasiodora sp. specimen. The larvae were placed at the center of the molting web with a forceps. The larval behavior in contact with urticating setae was observed under a stereoscopic microscope.
RESULTS
Distribution of types of urticating setae on the abdominal regions
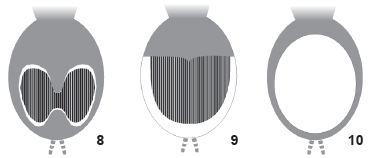
Setae type patterns found in the species of Theraphosinae studied are shown in Table II and figures 5-13. Most of the patterns include type III, which were found in the median region (Figs 6-9 and 11-13 ). Whenever occurring along with type III, type I (Fig. 7) and IV (Figs 8 and 9) were found in regions surrounding the patch of type III setae. In species having type IV hair, there is normally a conspicuous median patch formed by the long type III urticating setae, which is surrounded by the short type IV urticating setae. The type IV setae area can be very narrow, sometimes making it difficult to find them (Fig. 8).
Besides the presence of a "median patch of type III urticating setae" in most specimens examined, we also found some variation of this pattern as follows: nearly totally lacking urticating setae on the MA region in Grammostola sp. (Fig. 9); division of the original patch into two, so that each type III patch is surrounded laterally by type IV setae in E. vulpinus (Fig. 12 ); extreme reduction of the urticating setae patch, found in an unidentified Paraphysa species from Peru (Fig. 13 ).
Morphological variation of urticating setae types
Although types I, III and IV seem very distinct at first glance (Fig. 1), we found intermediates between types I and III (in agreement with Bertani 2001), as well as between III and IV (Fig. 14). Intermediates between types I and III share characteristics with both type I (presence of basal reversed barb region and large barbs helicoidally arranged at the distal region) and type III (small barbs distributed at least along the basal half, so that the "b" region is absent) (Figs 14 and 15). The presence of both the "b" region and the reversed barbed region was considered by Cooke et al. (1972) as a combination of features characterizing type I setae.
Types III and IV differ chiefly in size, with type III ranging from 0.3 to 1.8 mm and type IV from 0.06 to 0.2 mm (Cooke et al. 1972). Other differences are related to barbs being restricted to the posterior end in type IV (Cooke et al. 1972). However, in the same Grammostola sp. individuals studied, a variation from the medially positioned type III setae to laterally positioned type IV setae was found. Intermediate sized setae were found between these two regions, including nearly all intermediate ranges from 1.3 to 0.1 mm (Fig. 14). Thus, type IV can be better characterized by the barbs pointing towards the convex side of the seta (Fig. 16).
No morphological intermediates between types I and IV were found, nor any intermediates between type II of aviculariine with types I, III, or IV of theraphosines.
We found that the remaining part of abdomen of species having urticating setae is covered by two main types of non urticating setae. These are very long (4 mm) tactile setae ("tactile hairs" of Foelix 2010) or "guard-setae ("guard-hairs" of Cooke et al. 1972), which can be recognized by their insertion point into a socket, which allows them to move (Foelix 2010) (Fig. 17); and a "basic pelage" (Cooke et al. 1972) "constituted by strong pilose, 0.5 mm long setae, at a density of 300-400/mm2", that seems to be the "short body hairs" of Foelix (2010). They cover the abdomen, as well as the remaining body parts completely. These short body setae are highly plumose and frequently strongly curved, narrow at their most basal portion, and inserted into small sockets (Figs 17 and 18).
In species with urticating setae types III and IV (some theraphosine), and II (some aviculariine), body setae were found on the areas where urticating setae were lacking, on the boundary of the patch of urticating setae, or intermixed. In species having type I, I and III (some theraphosine) or only III urticating setae - Theraphosa blondi (Latreille, 1804), body setae were found mainly on the ventral region.
We found another non urticating seta type on the dorsal abdominal region in species having type I urticating setae and in T. blondi. They are highly plumose, longer than the typical body setae (Fig. 19), being probably the "intermediate, 1 mm long hair" found on the abdomen of Aphonopelma chalcodes Chamberlin, 1940 by Cooke et al. (1972). This seta bears a close morphological resemblance with body setae because of the strong plumosity and the similar insertion into the abdomen. However, it is long (0.7-2.0 mm), and normally weakly curved at the basal region and many of them have a helicoidal basal region (Figs 20 and 21). These long body setae are found intermixed with types I and III urticating setae in a proportion of 1 intermediate seta per 20 urticating setae (Cooke et al. 1972), and are released along with urticating setae (pers. obs.) (Fig. 22). Because they are longer than urticating setae, they give the abdomen a homogeneous aspect, in contrast with the conspicuous median setae patch in species bearing setae types III and IV (pers. obs.). These long body setae are the first ones to be touched when one tries to remove urticating setae from the abdomen. Also, attempts to pull them out often result in removing urticating setae as well. It is possible that the helicoidal basal part holds some urticating setae, aiding the release of the latter (pers. obs.).
When comparing urticating setae with body setae, from which they probably derived (Battisti et al. 2011), we found that their morphological similarity is based mainly on the mode of attachment to the spider's cuticle. They both have an internal chitinized structure (Figs 23 and 24) which appears on the outside as a small socket where either a body seta (Fig. 23) or an urticating seta (Fig. 24) attaches. The only difference detected between them is that dislodged body setae leave an empty socket, whereas urticating setae leave their basal part, the stalk (Figs 20-21 and 24). Thus, contrary to Townsend & Felgenhauer (1998), there is no "dramatic" difference between the proximal end of cuticular scales and urticating setae. Other similarities were found between body setae variants with either type II or III urticating setae, but not with types I and IV. Figure 25 shows body setae variants with the typical plumose aspect on its distal half, whereas the basal half bears barbs basally oriented and similar to type III hair. One of them has the basal portion truncated, resembling a stalk. In the same way, in A. avicularia we found morphological intermediates between body setae and type II setae in the same individual, such as pilose setae having a stalk; or, scale-like barbed setae lacking a perforating tip and break-off region. As previously stressed, we found no intermediates between body setae and types I and IV setae; on the other hand, intermediates between types I and III and III and IV were quite common (Fig. 14).
Length of the urticating setae
We found that the longest setae are concentrated at the median and median posterior regions of the abdomen of V. sorocabae and Grammostola sp. Urticating setae from those regions can be three times longer than those found in the MA region in V. sorocabae (Fig. 26) and more than ten times longer at the median region than at the lateral and far posterior region in Grammostola sp. (Fig. 27). For A. avicularia the setae length distribution is rather more homogeneous (Fig. 28).
Two-Way ANOVA for V. sorocabae showed dimorphism (p = 0.001), and no significance was detected for the interaction between sex and position (p = 0.079). As shown above, setae length was found to be affected by position. Tukey's HSD test detected high significance (p < 0.001) for all differences among positions, except between MA and LA (p = 0.460).
The first set (type III hair, comprising regions 4, 5, and 6) of Grammostola sp. showed dimorphism (p = 0.002), and no significance was detected for the interaction between sex and position (p = 0.643). The non-significance for the positions (p = 0.916) indicates high homogeneity for these regions. The second set (type IV hair, regions 1, 2 and 3) showed neither dimorphism (p = 0.453), nor significance due to position (p = 0.473), nor interaction between sex and position (p = 0.465). Only a few urticating setae were found in region 7 in some individuals, and their presence seems to be due to dislodgement from other regions.
For A. avicularia sexual dimorphism was detected related to setae length (p < 0.001), in agreement with Stradling (1978); whereas interaction between sex and position (p = 0.997), as well as differences among positions, were not significant (p = 0.561).
Effect of urticating setae on ants
Of the 15 ants tested, only two succeeded passing through the cylinder covered with silk and urticating setae. One of them succeeded after getting entangled and struggling for 60 minutes to break free. Four other ants returned to the vial immediately after having contact with the molting web, spending at least an hour afterwards grooming themselves. Other ants got entangled with the contaminated individuals, mainly after contacting their distal leg region. SEM micrographs showed that their mandibles and distal leg segments were particularly covered with type I urticating setae (Figs 29 and 30). Ants in the control experiment device moved freely between the vials without any detectable disturbance. After 24 hours, they used the cylinder as shelter, since it offered some protection against light. On the contrary, no ants in the device used for the test were found inside the cylinder.
Effects of urticating setae on phorid larvae
We observed that phorid larvae put in contact with a molt web were perforated by type I setae up to the main barbed region of the setae (Figs 31 and 32). The reversed barbed area remained on the outside, preventing the larva from moving freely by getting entangled with other setae and silk threads, thus anchoring the larva. Figures 31 and 32 show a dead phorid larva perforated by many type I setae. During preparation of the material for the photograph, we lifted the larva slightly to show its ventral region. The reversed barbs are missing in nearly all of these setae (Fig. 32), because they were entangled with silk threads and broke when we lifted the larva.
DISCUSSION
Length of the urticating setae
Consistent with the results of Marshall & Uetz's (1990b) for T. blondi, the longest setae are concentrated on the MM and MP regions of the abdomen of V. sorocabae and Grammostola sp. (Figs 26 and 27). Such differences in the length of the urticating setae could be related to their types, because different urticating setae types are segregated into distinct parts of the abdomen, as previously shown above. However, the difference in the length of the setae was also seen in females of V. sorocabae, which have only type I setae (Fig. 5), and in males and females of T. blondi (Fig. 6), which have only type III setae [Marshall & Uetz (1990b), pers. obs.]. These results show that the variation in the length of the setae depends on their position on the abdomen and not only on seta type. The presence of the longest setae on the MM and MP positions can be seen as a general pattern for Theraphosinae which was present in almost all other species studied, listed in Table II. Exceptions of the "longest setae located on the MM and MP positions" pattern were found in males of Homoeomma spp., which have type III setae that are almost the same size; in Paraphysa sp. from Peru (Fig. 13 ), which have two very small patches with type III setae of similar length; and in E. vulpinus, which has two symmetrical patches of the longest type III setae positioned at the centre of each patch. Each patch is surrounded laterally by short type IV setae (Fig. 12 ). In contrast to Theraphosinae, the aviculariine A. avicularia does not show the same variation concerning the length of the urticating setae (Fig. 28).
The longest setae occur on the abdomen, which the spider usually flicks first when disturbed, i.e., MM and MP regions. Pérez-Miles & Prandi (1991) observed that Grammostola mollicoma (Ausserer, 1875) uses setae from the median region whereas Megaphobema sp. uses setae from the median posterior (Marshall & Uetz 1990b). The results of the latter are in agreement with ours: spiders with setae types IV and III, such as Grammostola spp., have the longest setae positioned centrally (type III, Fig. 9), whereas in species bearing types I and III (such as Megaphobema spp.), the longest setae are positioned slightly more posteriorly (type III, Fig. 7). We propose that the length of setae are directly proportional to their efficacy towards a predator, i.e., long setae cause greater reactions than short ones. This is the reason why setae from the MM and MP regions are the first to be used by the spiders. The differential urticaria felt by people who handle these spiders (Cooke et al. 1972) could be, in part, due to the contact with setae of different lengths. Thus, species with long setae, such as B. smithi (1.8 mm), Grammostola sp. (1.3 mm), Megaphobema sp. (1.0 mm), Acanthoscurria sp. (0.9 mm), Lasiodora sp. (0.7 mm), and T. blondi (0.6 mm) would cause strong irritation. Besides setae length, the type of seta seems also to play a role in the differential urticaria, as proposed by Cooke et al. (1972). According to them, genera mentioned in literature as being particularly urticarious, such as Lasiodora C.L. Koch, 1850, Grammostola, and Acanthoscurria Ausserer, 1871 (Vellard 1936, Bücherl 1951), and adding to the list B. smithi, all have type III urticating setae, which should be considered more urticarious than type I. Thus, the urticarious effects of such setae may be the combination of morphology and length. Moreover, the number of setae present on the spiders' abdomen is also subject to variation. Large specimens such as T. blondi can surely release a greater number of setae than smaller specimens, thus causing more severe urticaria.
Cooke et al. (1972) attributed the drastic effects on the respiratory mucosa of rats and mice while in contact with Grammostola setae (Bücherl 1951) to the short type IV hair. However, it seems more plausible that these symptoms are an effect of the contact with the long type III setae present in species of Grammostola. Additional studies are necessary to establish whether type IV setae have any specialized function.
Urticating setae morphology and evolution
It has been proposed that urticating setae evolved from typical setae that cover the spider's body (Battisti et al. 2011). Thus, both the primitive and the derived character states are present in the same individual. We consider it plausible, since setae covering the spider's body are repetitions of the same structure, i.e., "anatomical plurals" (Patterson 1982), morphological "multiples" (Coddington 1989), or "homonoms" (Riedl 1979 apud Patterson 1982). Homonomy is "mass homology", "when there are several or many copies of the homologue in one individual" (Patterson 1982). Homonoms are expected to pass the similarity and congruence tests of homology, but not of conjunction, since the two supposed homologues are found in the same organism (Patterson 1982). Even though one can make exactly specifiable homology statements about a single structure, or "morphological singulars", only groups or sets of homonoms, or "morphological multiples", can be homologized (Coddington 1989).
Therefore, we propose that primitive regions having short body setae underwent modifications and gave origin to regions bearing urticating setae. Morphological similarities between body setae and urticating setae, mainly in the manner they are inserted into the spider's tegument, support for this hypothesis (Figs 23 and 24). The existence of intermediates between short body setae and type III urticating seta (Fig. 25) strengthens this idea.
When it comes to the evolution of different types of setae in the same individual, the question is whether the three types evolved independently from the ancestor's body setae or whether one urticating seta gave origin to another urticating seta. The morphological similarity suggests that both type I and IV evolved from an ancestor type III urticating setae, as we found intermediates between types I and III and III and IV but no intermediates between types I and IV were found.
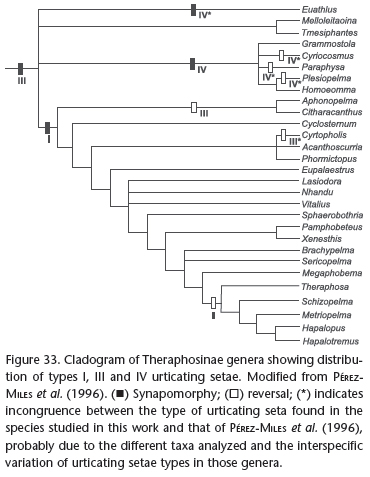
The cladogram of Pérez-Miles et al. (1996) for Theraphosinae shows type III urticating setae as a synapomorphy for the subfamily, and types I and IV as synapomorphies, with some reversions, for two distinct groups within Theraphosinae (Fig. 33). This is congruent with our discussion on similarity above, and suggests that an ancestor body setae underwent modifications on a restricted abdominal region, resulting in the type III urticating setae patch. A further step would be the modification of the marginal region of the primitive type III patch into either a type I or IV seta. This hypothesis passes both the similarity (topographic, compositional) and congruence (with other homologies) tests of homology. This can be seen as the putative ground plan in the evolution of urticating setae, from which the different patterns shown on Figs 5-13 probably evolved. Knowing more about the diversity patters of urticating setae may provide characters for theraphosid systematics. This is particularly true for species bearing type III and IV setae, which have more variable patterns (Figs 8-13 ), in contrast with species having type I setae, which exhibited a very limited number of patterns (Figs 5-7).
A distinct scenario was presented by Pérez-Miles (2002) based on ontogenetic evidence. He demonstrated that types I and IV appear earlier on the development of some theraphosines than urticating setae type III, suggesting a polarity for this particular character transformation. He made the following statement: "(...) if polarization is inverted another conflict remains: type III setae could not be derived from two different states (type IV and type I)". Thus, this series of transformation would not pass the congruence test of homology, the most valuable, "for it is the only test that discriminates useful comparisons from homoplasy" (Patterson 1982). An alternative explanation for the unexpected ontogeny of urticating setae regards the adaptive value of their different uses: we propose that type I setae are used against small invertebrates (see discussion below), as even a few urticating setae would be useful when incorporated into molting webs. The long type III setae are used against large vertebrate predators, and they would be useless for very small spiders.
Concerning type II setae, there is no evidence of homology with other urticating setae types, apart from the position criterion. Type II seta is morphologically very different from type III seta in many aspects, for instance in the basal perforating tip, and the small scale-like barbs (Cooke et al. 1972, Bertani & Marques 1996) (Fig. 1). The well developed barbs of A. versicolor were considered a convergence with type III setae (Bertani et al. 2003). The similarity of the body setae strengthens the idea of parallelism, as already proposed by Bertani & Marques (1996). Furthermore, the presence of urticating setae type II does not represent a synapomorphy of Aviculariinae, and type V is found only in Ephebopus (West et al. 2008). The way type V urticating seta is inserted in the palpal tegument of Ephebopus species (Foelix et al. 2009) is also distinct from the insertion found in Grammostola sp. (Theraphosinae) (Fig. 24). Therefore, it is possible to affirm that urticating setae appeared convergently at least three times in theraphosid evolution.
Types of urticating setae and differential usage
The diversity of urticating setae types in New World Theraphosidae is remarkable, contrasting with the Old World species which seem to lack them (Marshall & Uetz 1990a). "The occurrence of all these morphological types indicates that a variety of selective advantages may account for possession of such defenses" (Marshall & Uetz 1990a). Yet more remarkable is the presence of two setae types in the same theraphosine individual, a fact that suggests that they are used differently (pers. obs.). Two uses of urticating setae have been proposed: active defense against potential predators such as vertebrates (Torres 1921, Bristowe 1941, Gertsch 1949, Bücherl 1951, Cooke et al. 1972, Pérez-Miles & Prandi 1991), and passive defense against fly larvae (Diptera: Phoridae) (Marshall & Uetz 1990b). Even though the active defensive function against vertebrate predators seems to be reasonably defined, the same is not true for adult invertebrates. There is no record of urticating setae used against them, though many wasps and dipterans are amongst spiders' main predators (Bristowe 1941, Gertsch 1949, Cooke et al. 1972). Some theraphosines incorporate urticating setae into the silk mesh that covers the substrate where they molt (Marshall & Uetz 1990b, Pérez-Miles & Costa 1994) or use them in eggsac construction (Marshall & Uetz 1990b). The function seems to be protecting either the spider or the eggsac against phorid fly larvae (Marshall & Uetz 1990b). Eggsacs usually contain a few to several hundred eggs, which, in conjunction with the relatively large size of their eggs when compared with those of insects, renders them a potentially valuable food resource for any predator (Austin 1985). While predation on araneomorph eggsacs is well recorded, there are only a few records of it for theraphosids. These involve mainly phorid flies (Diptera: Phoridae) whose puparia have been found attached to the outer surface of an eggsac of M. robustum (Weinmann & Disney 1997). Theraphosine species known to incorporate urticating setae in the molting web are Acanthoscurria atrox Vellard, 1924 (Pérez-Miles & Costa 1994), T. blondi (Marshall & Uetz 1990b), B. smithi and B. emilia (White, 1856) Breene in Pérez-Miles & Costa 1994; additionally, we observed a great amount of urticating setae in the molting web of several specimens of Lasiodora spp., Acanthoscurria spp., Vitalius spp., Proshapalopus spp., and Nhandu spp. Similarly to eggsacs, it has been suggested that this provides protection against phorids. Phorid larvae were seen crawling over the body of the theraphosids T. blondi (Marshall & Uetz 1990b), M. robustum, and Pamphobeteus sp. (Weinmann & Disney 1997); and in one case a larva was seen entering the book lung opening of a M. robustum (Weinmann & Disney 1997). One phorid larva (Megaselia dimorphica Weinmann & Disney, 1997) is specific to T. blondi, and lives on the spiders' body (Weinmann & Disney 1997). Phorids are amongst the most biologically diverse insect families in the world (Disney 1990 apud Disney 1994) and the diversity of larval lifestyles is apparently without rival among insect families (Disney 1994). Some species are known to associate with living spiders, so that adults can feed from the prey of spiders; furthermore, several species have larvae that prey upon spider eggs (Weinmann & Disney 1997). Therefore, we consider plausible to assume that these flies are a potential source of risk to the spiders and their eggsacs. The use of urticating setae by incorporation on the eggsac wall and on the silk mat where they molt is a defensive strategy.
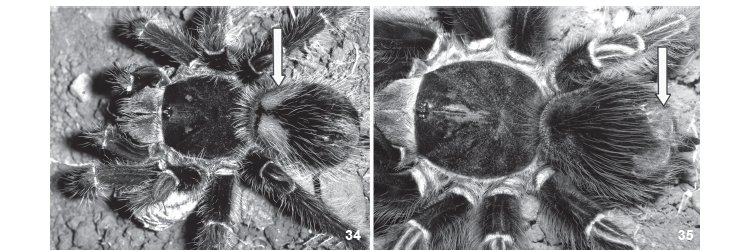
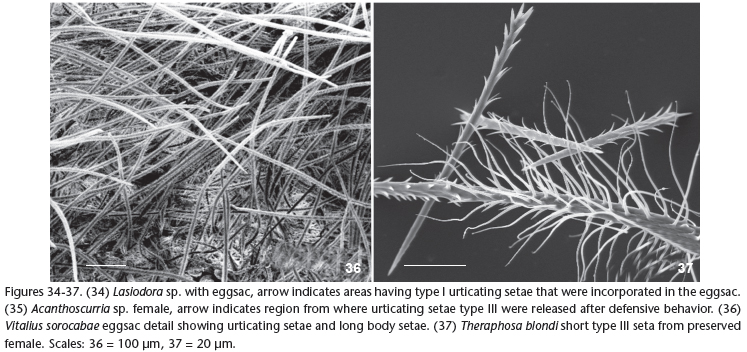
When incorporating urticating setae into the molting web, the spider dislodges them by distal metatarsal friction, produced by downward movements of the fourth leg against the abdomen, which is distinct from the upward movements used to throw urticating setae (Pérez-Miles & Costa 1994). Setae from all parts of the abdomen are used (Marshall & Uetz 1990b, pers. obs.). However, in eggsac construction the setae are scraped from specific regions of the abdomen: the MA, LA and LP regions (Fig. 34) as seen in T. blondi and Megaphobema sp. (Marshall & Uetz 1990b) which also contrasts with the shedding of setae from regions MM and MP during defensive displays (Fig. 35) (Marshall & Uetz 1990b). We also observed the use of setae from these regions in eggsacs (Fig. 34) by V. sorocabae (4 specimens), V. longisternalis (5 specimens), V. wacketi (2 specimens), Lasiodora sp. (2 specimens) and P. multicuspidatus (1 specimen). In Vitalius spp. the setae are deposited in each silk layer, together with long body setae in such quantity that the eggsac becomes hairy (Fig. 36).
Interestingly, apart from T. blondi, all species known to readily incorporate urticating setae in the web mat or eggsac bear type I urticating setae, which are very effective against phorid larvae, stopping the larval movements (Figs 31 and 32). Web mats and eggsacs examined for species having types III and IV showed the presence of few urticating setae when compared with species having type I setae. However, because we never witnessed the process of eggsac or shedding mat construction by those species, it was not possible to determine whether the spiders deliberately incorporate the setae into the silk or if their presence is fortuitous, as it seems likely.
While setae from the MM and MP regions, the longest type III setae, are used for defense, (Fig. 33), the shortest type I setae or the very short (0.1 mm) type III setae of T. blondi are used in the eggsac (Marshall & Uetz 1990b) (Fig. 37). Type I urticating setae are only weakly irritant for man (Cooke et al. 1972) when compared with type III. In addition, their morphology is intriguing because of the presence of reversed barbs that prevent them from going deep into the skin (Cooke et al. 1972) (Figs 1 and 14). Moreover, when we pulled type I urticating setae from the spider's abdomen with a sharp forceps, we observed strings of these setae getting entangled with each other upon contact with the barbed areas, as well as the contact with the long body setae that have a helicoidal basal region (Figs 20-22). It is possible that the helicoidal basal part of the long body setae holds some urticating setae and aids in their release. This could be advantageous for the spider in the process of removing setae from the anterior abdominal regions during eggsac construction.
Besides guarding against phorid flies, other possibilities of specialized uses of type I urticating setae exist. Bertkau (1880) was the first author to publish drawings of theraphosid urticating setae. Astonishingly, based on the reversed barbed area, he drew attention to the similarity between the abdominal setae of his new species, Crypsidromus fallax Bertkau, 1880 (actually a type I seta of Cooke et al. 1972), and the setae tuft present at the posterior region of Polyxenus Latreille, 1802/1803 (Diplopoda: Polyxenidae) millipedes. Eisner et al. (1996) showed that in Polyxenus fasciculatus Say, 1821 these setae are actively used against ants. When put in contact with ants, the polyxenid raises its rear and rubs the tufts against the ants, causing them to become covered with setae detached from the tufts. When trying to groom itself, the ant movements cause the setae to cross-link, entrapping and immobilizing the ant, which may even die. The experiments conducted with C. rufipes showed that type I urticating setae act in a similar way (Figs 29 and 30), being effective against at least this ant species.
We conclude that, though both types I and III setae can be actively used against vertebrate predators, as well as used passively in molting webs and eggsacs against some species of fly larvae and ants, each type seems to be more effective for one particular situation.
The presence of only type I setae in female representatives of Vitalius spp., N. carapoensis, and P. amazonicus would indicate that, to these species, they are more important for defense against invertebrates rather than vertebrates. Males do not molt and wander after females when the reach adulthood (Janowski-Bell & Horner 1999), exposing themselves to vertebrate predators. Then, it would be more advantageous for males to have type III urticating setae. The sexual dimorphism detected here, in which males always have longer urticating setae than females, strengthens this hypothesis. On the other hand, sedentary females incorporate type I setae from the LA and MA regions of the abdomen in the eggsac, while they should save some type I setae from the MM and MP regions to be used in the next molting web, since adult females readily molt yearly.
Phorid and ant attacks may be the reason for some behaviors exhibited by theraphosids in captivity. For instance, their behavior towards food remains, which can attract parasites. Food remains are usually put in the corners of the cage, i.e. as far as possible from the spider; dropped into the water dish; or covered with silk strands (pers. obs.). Additionally, Yanez & Floater (2000) observed in the field that B. klaasi individuals remove prey remains from their burrows and deposits them near the entrance. Exuvial fluids found in recently shed exuvia can also be consumed by phorid larvae (Weinmann & Disney 1997) and sometimes these exuviae are covered with silk. Silk strands help stop larval movements, but are not as effective as silk with type III urticating setae (Marshall & Uetz 1990b). We consider the strategy of using silk strands against phorid larvae a primitive condition, whereas the incorporation of type III urticating setae into silk is a derived condition. Morphological specialization of urticating seta (type I) leading it to be used mainly against fly larvae and ants would be regarded as a further evolutionary step.
ACKNOWLEDGMENTS
Thanks are due to Sérgio A. Vanin (advisor of RB), Pedro Ismael da Silva Júnior, Fernando Pérez-Miles, Martha Yanez, Otávio A.V. Marques, Pedro Gnaspini, Ricardo Pinto-da-Rocha, and Samuel Marshall for their valuable comments on early drafts of the manuscript. Kátia de Mendonça Faria kindly drew the figures. The curators A.B. Kury (MNRJ), N. Platnick (AMNH), P. Jäger (SMF) and R.C. West (Victoria) for the loan of specimens. Sérgio Rosso kindly helped with the statistics. Alberto de Freitas Ribeiro, Miriam David Marques, Ênio Mattos and Lara Maria Guimarães are thanked for SEM facilities. Ana Eugênia de Carvalho Campos identified the ant species. Emma Shaw for the English revision. Peter Jordan called attention to important references. This paper is part of the PhD of RB at the Departamento de Zoologia, Universidade de São Paulo.
LITERATURE CITED
Submitted: 19.XI.2012; Accepted: 29.I.2013.
Editorial responsibility: Mauricio O. Moura
- Austin, A.D. 1985. The function of spider egg sacs in relation to parasitoids and predators, with special reference to the Australian fauna. Journal of Natural History19:359-376.
- Battisti, R.; G. Holm; B. Fagrell & S. Larsson. 2011. Urticating Hairs in Arthropods: Their Nature and Medical Significance. Annual Review of Entomology 56:203-220.
- Bertani, R. 2001. Revision, cladistic analysis, and zoogeography of Vitalius, Nhandu, and Proshapalopus, with notes on other theraphosine genera (Araneae, Theraphosidae). Arquivos de Zoologia 36(3):265-356.
- Bertani, R. & O.A.V. Marques. 1996. Defensive behaviors in Mygalomorph spiders: Release of urticating hairs by some Aviculariinae (Araneae, Theraphosidae). Zoologischer Anzeiger234:161-165.
- Bertani, R.; T. Boston; Y. Evenou & J.P.L. Guadanucci. 2003. Release of urticating hairs by Avicularia versicolor (Walckenaer, 1837) (Araneae, Theraphosidae). Bulletin of the British Arachnological Society 12(9):395-398.
- Bertkau, P. 1880. Verzeichnis der von Prof. Ed. von Beneden auf seiner im Auftrage der Belgischen Regierung unternommenen wissenschaftlichen Reise nach Brasilien und La Plata I. J. 1879-1875 gesammelten Arachniden. Mémoires Couronnes de la Academie Royale des Sciences, Lettres et Beaux-Arts de Belgique 43:1-120.
- Bristowe, W.S. 1941. The comity of spiders. London, Ray Society, 560p.
- Bücherl, W. 1951. Estudo sobre a biologia e a sistemática do gênero Grammostola Simon, 1892. Monografias do Instituto Butantan 1:1-126.
- Coddington, J.A. 1989. Spinneret silk spigot morphology: evidence for the monophyly of orbweaving spiders, Cyrtophorinae (Araneae), and the group Theridiidae plus Nesticidae. The Journal of Arachnology 17:71-95.
- Cooke, J.A.L.; V.A. Roth & F.H. Miller. 1972. The urticating hairs of theraphosid spiders. American Museum Novitates 2498:1-43.
- Disney, R.H.L. 1994. Scuttle flies: The Phoridae. London, Chapman & Hall, 467p.
- Eisner, T.; M. Eisner & M. Deyrup. 1996. Millipede defense: Use of deta chablebristles to entangle ants. Proceedings of the National Academy of Sciences of the United States of America 93:10848-10851.
- Foelix, R.F. 2010. Biology of Spiders. Oxford, University Press, 3rd ed., 432p.
- Foelix, R.F.; R. Bastian & B. Erb. 2009. Palpal urticating hairs in the tarantula Ephebopus: fine structure and mechanism of release. The Journal of Arachnology 37:292-298.
- Gertsch, W.J. 1949. American Spiders. New York, Van Nostrand, 285p.
- Janowski-Bell, M.E. & N.V. Horner. 1999. Movement of the male brown tarantula, Aphonopelma hentzi (Araneae, Theraphosidae), using radio telemetry. The Journal of Arachnology 27:503-512.
- Langsdorff, G.H. von. 1812. Bemerkungen auf eine Reise um die Welt in den Jahren 1803 bis 1807. Frankfurt am Main, In Verlag bei Friedrich Wilmans, 303p.
- Marshall, S.D. & G.W. Uetz. 1990a. The pedipalpal brush of Ephebopus sp. (Araneae, Theraphosidae): Evidence of a new site for urticating hairs. Bulletin of the British Arachnological Society 8:122-124.
- Marshall, S.D. & G.W. Uetz. 1990b. Incorporation of urticating hairs into silk: A novel defense mechanism in two Neotropical tarantulas (Araneae, Theraphosidae). The Journal of Arachnology 18:143-149.
- Melchers, M. 1964. Zur Biologie Der Vogelpinnen (Fam. Aviculariidae). Zeitschrift für Morphologie und Ökologie der Tiere 53:517-536.
- Patterson, C. 1982. Morphological characters and homology, p. 21-74. In: K.A. Joysey & A.E. Friday (Eds). Problems of Phylogenetic Reconstruction. London, Academic Press, Systematics Association Special Volume.
- Pérez-Miles, F. 1998. Notes on the systematics of the little known theraphosid spider Hemirrhagus cervinus, with a description of a new type of urticating hair. The Journal of Arachnology 26(1):120-123.
- Pérez-Miles, F. 2002. The occurrence of abdominal urticating hairs during development in Theraphosinae (Araneae, Theraphosidae): phylogenetic implications. The Journal of Arachnology 30(2):316-320.
- Pérez-Miles, F. & F.G. Costa. 1994. Acanthoscurria atrox incorporates urticating hairs into its shedding mat. The Forum of the American Tarantula Society 3(3):63-64.
- Pérez-Miles, F. & L. Prandi. 1991. El comportamiento de emision de pelos urticantes en Grammostola mollicoma (Araneae, Theraphosidae): un analisis experimental. Boletin de la Sociedad Zoológica del Uruguay, 2Ş época 6:47-53.
- Pérez-Miles, F.; S.M. Lucas; P.I. Silva Jr & R. Bertani. 1996. Systematic revision and cladistic analysis of Theraphosinae (Araneae: Theraphosidae). Mygalomorph 1:33-68.
- Schmidt, G.E.W. 2003. Reizhaare bei Ischnocolinae? (Araneae: Theraphosidae). Tarantulas of the World 78:16-18.
- Stradling, D.J. 1978. The growth and maturation of the "tarantula" Avicularia avicularia Zoological Journal of the Linnean Society 62:291-303.
- Torres, O. 1921. Algumas observações sobre a biologia das aranhas do genero Theraphosa Revista de Sciencias, Sociedade Brasileira de Sciencias 5:181-185.
- Towsend, V.R. & B.E. Felgenhauer. 1998. Cuticular scales of spiders. Invertebrate Biology 117(4):318-330.
- Vellard, J. 1936. Le Venin des Araignées. Paris, Masson, 311p.
- Weinmann, D. & R.H.L. Disney. 1997. Two new species of Phoridae (Diptera) whose larvae associate with large spiders (Araneae: Theraphosidae). Journal of Zoology 243:319-328.
- West, R.C.; S.D. Marshall; C.S. Fukushima & R. Bertani. 2008. Review and cladistics analysis of the Neotropical tarantula genus Ephebopus Simon 1892 (Araneae: Theraphosidae) with notes on the Aviculariinae. Zootaxa 1849:35-58.
- Yanez, M. & G. Floater. 2000. Spatial distribution and habitat preference of the endangered tarantula, Brachypelma klaasi (Araneae: Theraphosidae) in Mexico. Biodiversity and Conservation 9:795-810.
Publication Dates
-
Publication in this collection
06 Sept 2013 -
Date of issue
Aug 2013
History
-
Received
19 Nov 2012 -
Accepted
29 Jan 2013