Abstract
Evidence that the bill of the Toco Toucan, Ramphastos toco Statius Muller, 1776, has a specialized role in heat dissipation suggests a new function for the large and light-weight bill of the toucan family (Piciformes: Ramphastidae). A prediction of this hypothesis is that bill length in toucans will increase with body mass at a rate greater than the isometric expectation. This hypothesis was tested in a phylogenetic context with measurements of skeletal elements in adult males of 21 toucan species. In these species, 64.3% of variance in relative skeletal measurements was accounted for by the contrast between bill and body size. Maxilla length and depth increased with body mass at a greater than isometric rate relative to both body mass and other linear skeletal measures. By contrast, no such trend was seen in a parallel analysis of 24 hornbill species (Bucerotiformes), sometimes considered ecological equivalents of toucans. The unique relationship between bill size and body mass in toucans supports the hypothesis that the evolution of a heat dissipation function has been a persistent theme of bill evolution in toucans.
Allometry; heat dissipation; Ramphastidae
SYSTEMATICS AND EVOLUTION
Evolution of bill size in relation to body size in toucans and hornbills (Aves: Piciformes and Bucerotiformes)
Austin L. Hughes
Department of Biological Sciences, University of South Carolina, Columbia SC 29205 USA. E-mail: austin@biol.sc.edu
ABSTRACT
Evidence that the bill of the Toco Toucan, Ramphastos toco Statius Muller, 1776, has a specialized role in heat dissipation suggests a new function for the large and light-weight bill of the toucan family (Piciformes: Ramphastidae). A prediction of this hypothesis is that bill length in toucans will increase with body mass at a rate greater than the isometric expectation. This hypothesis was tested in a phylogenetic context with measurements of skeletal elements in adult males of 21 toucan species. In these species, 64.3% of variance in relative skeletal measurements was accounted for by the contrast between bill and body size. Maxilla length and depth increased with body mass at a greater than isometric rate relative to both body mass and other linear skeletal measures. By contrast, no such trend was seen in a parallel analysis of 24 hornbill species (Bucerotiformes), sometimes considered ecological equivalents of toucans. The unique relationship between bill size and body mass in toucans supports the hypothesis that the evolution of a heat dissipation function has been a persistent theme of bill evolution in toucans.
Key words: Allometry; heat dissipation; Ramphastidae
The adaptive significance of the large and remarkably light-weight bill of members of the Neotropical toucan family (Piciformes: Ramphastidae) has been the subject of much speculation (SHORT & HORNE 2001, TATTERSALL et al. 2009). Although VAN TYNE (1929: 39) suggested that the toucan's bill has no "especial adaptive function", a number of adaptive hypotheses have been proposed. BÜHLER (1995) proposed that the bill's large size and serrated edges originally evolved primarily as an adaptation for reaching and grasping fruit; later "tooth-like" markings on the bill may have evolved as adaptations to minimize mobbing by other birds when toucans prey on their nests (SICK 1993, BÜHLER 1995). SHORT & HORNE (2001) suggested a similar evolutionary sequence, while emphasizing the likely importance of species-specific bill markings in species recognition and courtship. Toucan bills are often brightly colored, and a few species show sexual dimorphism in bill coloration (SHORT & HORNE 2001). When bill color dimorphism occurs it is usually not very marked (SHORT & HORNE 2001), but its presence suggests that sexual selection may be another evolutionary force acting on toucan bills, at least in some species.
A further contribution to understanding the function of the toucan's bill was provided by evidence that the bill of the Toco Toucan, Ramphastos toco Statius Muller, 1776, serves as a key surface area for heat dissipation (TATTERSALL et al. 2009), which the bird can use to regulate body temperature by controlling blood flow. There is evidence that bills of a variety of avian taxa can function in heat dissipation (HAGAN & HEATH 1980, SCOTT et al. 2008, GREENBERG et al. 2012a, b, GREENBERG & DANNER 2013), suggesting that heat dissipation may be a plesiomorphic function of the avian bill. In the Ramphastidae, it might be hypothesized that the ancestral heat-dissipation function has become elaborated by the evolution of a highly modifiable vascular radiator (TATTERSALL et al. 2009). On this hypothesis, the emergence of this vascular adaptation has been an additional factor favoring the evolution of large bill size in toucans, in conjunction with other selective pressures such as frugivory and signaling. Relatively little is known of toucans' thermal biology in nature, but the family is entirely Neotropical in distribution, and most species inhabit tropical lowland forests (SHORT & HORNE 2001), where high daily maximum temperatures occur year-round (GRUBB & WHITMORE 1996).
Consistent with a role for the toucan bill in heat-dissipation, TATTERSALL et al. (2009) presented evidence that bill length in juvenile and adult Toco Toucan increases as a function of body mass at a rate greater than the isometric expectation; i.e., greater than an exponent of 1/3 expected for a linear dimension (ALEXANDER 1971). Likewise, SYMONS & TATTERSALL (2010) provided evidence that across toucan species bill length increases as a function of body mass at a rate greater than linear expectation, using published data on 34 species of Ramphastidae. Such a relationship is expected if the bill plays a role in dissipating body heat, since metabolic rate increases with body mass with an exponent between 2/3 and 1.0, depending on activity level (GLAZIER 2008).
Here I analyze the evolution of bill size in relation both to the size of other major skeletal elements and to body mass across the family Ramphastidae in order to test the hypothesis of isometry against an alternative consistent with the bill's proposed role in heat dissipation, using statistical methods that control for phylogenetic relationships. A phylogenetic approach makes it possible to test the hypothesis that there has been a trend toward bill sizes greater than the isometric expectation throughout the evolution of this family. The hornbills (Bucerotiformes) are considered Old World ecological equivalents of the toucans, filling similar ecological niches in their respective ecosystems; most members of both families are cavity-nesting frugivores of tropical forests, and the two families have convergently evolved large slightly downcurved bills (KEMP 1995, KINNAIRD & O'BRIEN 2007). Because of these ecological parallels, I conduct a similar analysis with hornbills to compare the patterns of bill evolution relative to body size in the two groups of large-billed tropical birds. By comparing pattern of bill allometry in these two families, I test for a distinctive pattern of bill evolution in toucans, which would be consistent with the hypothesis that the toucan's bill plays an exceptionally highly developed role in heat dissipation.
MATERIAL AND METHODS
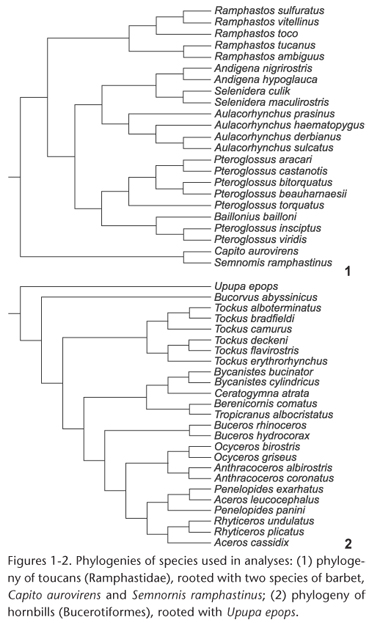
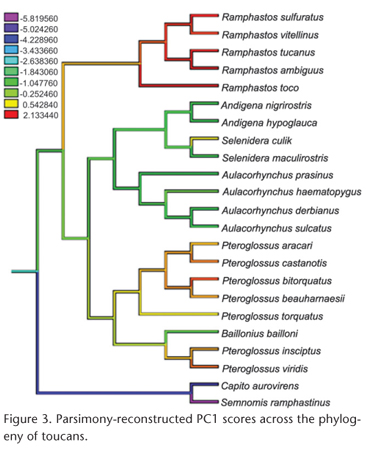
Measurements were made on complete skeletal specimens of adult males belonging to 21 species of toucans (Piciformes: Ramphastidae) and on complete skeletons of adult males of 24 species of hornbills (Bucerotiformes: Bucorvidae and Bucerotidae) from the U.S. National Museum of Natural History. The same measurements were also made on adult females of 16 of the toucan species and 14 of the hornbill species; since the patterns were similar for males and females, only the results for males are reported here. As an outgroup to root the phylogenetic tree of toucans, two species of New World barbets (Piciformes: Capitonidae) were used, Capito aurovirens (Cuvier, 1829) and Semnornis ramphastinus (Jardine, 1855) (Fig. 1). As an outgroup to root the phylogenetic tree of hornbills, the Eurasian Hoopoe, Upupa epops Linnaeus, 1758, (Upupiformes: Upupidae) was used (Fig. 2). Species were included based on available specimens but sampled all major lineages of both toucans and hornbills (Figs 1 and 2).
Because no comprehensive molecular phylogeny of toucans has been published, the phylogeny of toucans (Fig. 1) was derived from a combination of published DNA sequencebased phylogenies. The relationships among the ramphastid genera were based on NAHUMet al. (2003); see also PATANÉet al. (2009). Relationships within the genus Ramphastos were based on PATANÉet al. (2009); see also WECKSTEIN (2005). Relationships within Pteroglossus and Baillonius were based on EBERHARD & BERMINGHAM (2005) and PATELet al. (2011). Relationships within Aulacorhynchus were based on BONACCORSO et al. (2011); and those within Andigena and Selenidera were based on LUTZet al. (2013). The phylogeny of hornbills (Fig. 2) was based on the DNA sequence phylogeny of GONZALEZ et al. (2013). Most branching patterns indicated in Figs 1 and 2 were strongly supported in the original phylogenetic analyses by bootstrap probabilities, Bayesian posterior probabilities, or both. Preliminary analyses using the phylogeny of Ramphastos from HAFFER (1974, 1997) showed essentially identical results to those based on the phylogeny of PATANÉet al. (2009); only the latter results are reported here.
In the case of toucans and barbets, the following nine skeletal measurements were made by digital caliper (BAUMEL 1993): 1) maxilla length, measured from the dorsal junction of the maxilla with the cranium to the tip of the bill (Rostrum maxillare); 2) maxilla depth, measured at the point of widest dorsal to ventral depth; 3) maxilla width, measured at the point of greatest lateral width; 4) cranium length, measured from the dorsal junction of the maxilla with the cranium to the posterior end (Proeminentia cerebellaris) of the cranium; 5) cranium width, measured at the point of greatest lateral width; 6) sternum, measured from Apex carinae to Margo caudalis; 7) synsacrum, measured from the anterior edge of Ala preacetabularis to the posterior edge of Ala ischii; 8) femur, measured from the proximal point of Crista trochanteris to the distal point of Condylus lateralis; and 9) tibiotarsus, measured from the proximal point of Facies gastrocnemialis to Incisura intercondylaris. In the case of the hornbill sample, Maxilla depth and cranium length were not included in analyses because the presence of the casque prevented comparable measurements in most species. Mean body mass values (in grams) for each species were obtained from DUNNING (2008). In most species, values for males and females were given separately (DUNNING 2008); and in those cases values for males were used. Data for male and female toucans are available in Appendix S1*, while male and female hornbills are available in Appendix S2*.
To test the sensitivity of these measurements to withinspecies variation, the same nine measurements were made on 10 adult males of Ramphastos sulfuratus Lesson, 1830 and 11 adult males of R. toco. Analysis of variance applied to log-transformed measurements was used to test for the relative magnitude of within-species and between-species components of variance in each of the nine measurements. In the case of all measurements, between species variance was significantly greater than within-species variance (p < 0.001 in every case except for cranium length, where p = 0.017, F-tests). The less pronounced between-species difference in cranium length than in the other measures was consistent with previous reports of low variance in similar measures (HÖFLING 1991).
Size-corrected transformations ("Mosimann transformations" MOSIMANN 1970) were computed for the 9 skeletal measurements on toucans. Where the xi are the individual measurements, let zi = ln [xi/G(x)], where G(x) is the geometric mean of the nine measurements within each species (MOSIMANN 1970). Principal components (PCs) were extracted from the correlation matrix of the zis; the PC scores were used to provide size-independent indices of body shape for each toucan species (DARROCH & MOSIMANN 1985, JUNGERS et al. 1995, HUGHES 2013). The values used in these computations are shown in Appendix S1*. Principal components extracted Mosimanntransformed variables are preferable to principal components extracted from raw data, because the former are more effective in correctly identifying similarities in shape independent of body size (JUNGERS et al. 1995). Because maxilla depth and cranium length could not be accurately measured in the case of hornbills, in order to compare the two families, Mosimann transformations were computed for the remaining seven variables separately for each family; and principal components were extracted from these transformed variables.
To test hypotheses regarding isometric relationships among skeletal measures and between skeletal measures and body mass, all measurements were first log-transformed. The isometric expectation for the slope (b) of a log-log regression (i.e., the allometric exponent) of any linear skeletal measure on any other linear skeletal measure is 1.0. The isometric expectation for b in a log-log regression of a linear measure on body mass (predicted to be proportional to body volume) is 1/ 3 (ALEXANDER 1971). Because the toucan's maxilla is approximately triangular in cross-section (SHORT & HORNE 2001), the external surface area of the bill consists largely of the area on the two lateral bill surfaces. Assuming that each of these surfaces has the approximate shape of an elongated triangle, the surface area of the maxilla can be roughly approximated by the product maxilla length times maxilla depth. The isometric expectation for b in a log-log regression of the product of two linear measures on body mass is 2/3 (ALEXANDER 1971).
Isometric expectations were tested in two ways: 1) traditional analyses, in which phylogeny was not taken into account but rather each species was treated an independent unit of analysis; and 2) phylogenetically independent contrasts. In traditional analyses, the outgroup species were not included in the regressions. On the basis of the phylogenetic trees (Figs 1 and 2), phylogenetically independent contrasts were constructed using the PDAP (GARLAND et al. 1993) contrasts plug-in within Mesquite version 2.75 (MADDISON & MADDISON 2011). Regressions between phylogenetically independent contrasts were conducted without fitting an intercept (GARLAND et al. 1992). PCs extracted from the correlation matrix of the zis were mapped on the toucan phylogeny by maximum parsimony using the "Map Continuous" function in Mesquite with default settings.
Following the recommendation of SMITH (2009) for testing the null hypothesis of isometry, reduced major axis (RMA) was used rather than ordinary least squares (OLS) to estimate regression coefficients (SOKAL & ROHLF 1995). The results with OLS (not shown) were very similar to those of RMA in the present case because correlations between variables were high. For all allometric regressions reported here (N = 58), the linear correlation coefficient ranged from 0.735 to 0.988 (mean = 0.887 ± 0.008 S.E., median = 0.893). OLS was used to estimate regression lines (SMITH 2009). All reported significance levels are corrected for multiple testing by the Bonferroni method (SOKAL & ROHLF 1995). Statistical analyses were conducted in Minitab (http://www.minitab.com).
RESULTS
Relative length of skeletal elements
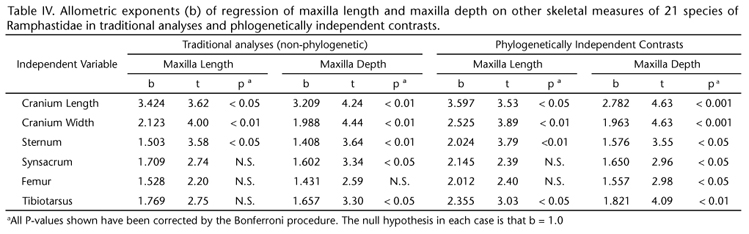
The first principle component (PC1) extracted from the correlation matrix of size-corrected transformations of nine linear skeletal measures of toucans accounted for 64.3% of the variance and represented a contrast between two sets of variables: 1) maxilla length and maxilla depth; and 2) the other variables except for sternum (Table I). Thus PC1 could be interpreted as a size-corrected measure of the contrast between bill size and body size. PC2, accounting for 17.7% of the variance, seemed to mainly consist of a contrast between sternum and maxilla width (Table I). In order to provide a visual image of how the contrast between bill and body size has evolved across the Ramphastidae, PC1 values were mapped across the phylogeny of toucans. The highest values (indicating greatest bill size relative to body size) were seen in Ramphastos (Fig. 3). The phylogeny also supported the hypothesis of a parallel increase in bill size relative to body size in the Pteroglossus/Baillonius lineage (Fig. 3).
Because maxilla depth and cranium length could not be accurately measured in the case of hornbills, principal were extracted from the correlation matrix of size-corrected transformations of remaining seven linear skeletal measures of in each family (Table II). Even excluding maxilla depth and cranium length, PC1 (accounting for 60.8% of the variance) in the toucan data again appeared mainly to represent a contrast between bill size and body size (Table II). By contrast, in hornbills, PC1 accounted for only 30.8% of the variance and appeared to reflect mainly a contrast between body size and the width of both bill and cranium (Table II). The loading of maxilla length on PC1 in hornbills (-0.063) differed strikingly from that in toucans (0.481, Table II). Thus hornbills appeared to differ from toucans in that bill length relative to body size was not a major factor in cross-species comparisons of major skeletal elements.
Allometric relationships
In traditional analyses, not accounting for the phylogeny, the 9 log-transformed skeletal measures were regressed against log body mass (Table III). Likewise, phylogenetically independent contrasts in the same 9 log-transformed skeletal measures were regressed against phylogenetically independent contrasts in log body mass (Table III). The results were broadly similar in the two types of analysis (Table III). In both cases, the allometric exponent (b) for maxilla length and maxilla depth were significantly greater than the isometric expectation (1/3; Table III). In the case of phylogenetically independent contrasts, b for maxilla width was also significantly greater than the isometric expectation (Table III). In traditional analyses, but not in phylogenetically independent contrasts, b for femur was significantly greater than the isometric expectation (Table III). No other linear measure showed b significantly greater than the linear expectation in either type of analysis, but in the traditional analyses b for cranium length was significantly less than the isometric expectation (Table III). When the log of the product of maxilla length and maxilla depth, was regressed against log body mass, in both types of analyses, b was significantly greater than the isometric expectation (2/3; Table III). These results imply that in the Ramphastidae both bill size and bill surface area increase with body mass at a greater rate than expected under isometry.
The hypothesis that maxilla length and maxilla depth show a distinctive pattern of evolution in the Ramphastidae was further tested by regressing logarithms of these measures on those of linear measures of non-maxillary structures (Table IV). In both traditional and phylogenetically based analyses, b exceeded the isometric expectation (1.0) in every case (Table IV). In both kinds of analyses, the regressions with maxilla length as the dependent variable, the b was significantly greater than the isometric expectation with cranium length, cranium width, and sternum as dependent variables (Table III). In both kinds of analyses, in regressions with maxilla depth as the dependent variable, b was significantly greater than the isometric expectation with cranium length, cranium width, sternum, synsacrum and tibiotarsus as dependent variables (Table IV). In the phylogenetically based analysis, maxilla length showed b greater than the isometric expectation when regressed on tibiotarsus, and maxilla depth also showed b greater than the isometric expectation when regressed on femur (Table IV).
When log-transformed skeletal measures of hornbills were regressed against log body mass, a very different pattern was seen from that seen in toucans (Table V). In hornbills, b for maxilla length did not differ significantly from the isometric expectation (Table V), resulting in distinct patterns in toucans and hornbills (Fig. 4). In traditional analyses, the only measure for which the slope significantly exceeded the isometric expectation was synsacrum, while the slope for cranium width was significantly less than the isometric expectation (Fig. 4). Likewise, in phylogenetically based analyses, the slope of the relationship for contrasts in log maxilla length did not differ significantly from the isometric expectation, and the only measure for which the slope exceeded the isometric expectation was synsacrum (Table V).
The Northern Ground Hornbill Bucorvus abyssinicus (Boddaert, 1783) (Fig. 2) had a relatively large synsacrum (129.9 mm) in comparison to the 23 other hornbill species of (mean = 62.6 ± 5.0 mm, range = 30.5 to 105.mm, Appendix S2*). A relatively large is consistent with the terrestrial habits and relatively large legs of the Northern Ground Hornbill (KEMP 1995). However, even when the Northern Ground Hornbill was excluded from the data set, a similar relationship was seen in the traditional analysis of the relationship between log synsacrum and log body mass (b = 0.409; test of equality to isometric expectation, p < 0.001). Likewise, in phylogenetically independent contrasts, when both the ancestral node and the node linking the Northern Ground Hornbill to the other hornbills (Fig. 2) were excluded, there was a similar relationship between contrasts in log synsacrum and contrasts in log body mass (b = 0.429, test of equality to isometric expectation, p < 0.01).
DISCUSSION
An examination of the relationship among linear measures of major skeletal measures and between those measures and body mass supported an unusual pattern of bill size evolution in the toucan family. Throughout the toucan family, the length and depth of the maxilla increased as a function of body mass at a rate greater than expected under isometry, implying disproportionately large bills per unit body mass in large-bodied toucan species, consistent with the hypothesis that heat dissipation has been an important factor in the evolution of the large bills of toucans (TATTERSALL et al. 2009). Since the capacity for radiation of heat from the bill is a function of surface area, it is further expected that bill surface area will increase with body mass at a rate greater than expected under isometry. The present analyses supported this prediction, since the results showed that product of toucan bill length and depth increases with body mass at a rate greater than the isometric expectation.
In spite of the ecological parallels between toucans and hornbills (KEMP 1995, KINNAIRD & O'BRIEN 2007), the present analyses provided no evidence of a greater than isometric increase in hornbill maxilla length as a function of body mass. These results are consistent with the hypothesis that the bill of hornbills does not play a role in heat dissipation analogous to that of toucans. This hypothesis will require further testing through physiological study of hornbills. It is of interest, however, that hornbills appear to make use of alternative heatdissipation mechanisms from those seen in toucans; for instance, evaporative water loss from the bare skin under the wings, which is exposed by the hornbills' unique lack of underwing-coverts (KEMP 1995).
In contrast to the maxilla, in hornbills synsacrum length increased with body mass at a greater rate than expected under isometry. This pattern was seen even when the terrestrial Northern Ground Hornbill, in which the synsacrum was unusually large, was excluded from the analysis. The increase in the length of the synsacrum with body mass may reflect an enhanced need for weight support in the larger hornbills. That no similar trend is seen in toucans may reflect their substantially smaller body masses, as well as the fact that even arboreal hornbills spend more time on the ground than toucans (KEMP 1995), with a consequent requirement to support the body weight on the pelvic girdle.
All phylogenies represent hypotheses, which are subject to revision in the light of additional data (GARLAND et al. 2005). In the present case, the fact that traditional and phylogenetic analyses yielded very similar results suggests that the conclusions are likely to be robust to phylogenetic revision. In addition, the phylogenetic perspective provided evidence that bill size increased relative to body size independently in different toucan lineages. In the toucans, 64.3% of variance in size-adjusted skeletal measures was accounted for by a composite variable (PC1) that could be interpreted as reflecting the contrast between bill and body size. PC1 increased markedly the genus Ramphastos and the Pteroglossus/Baillonius lineage (Fig. 3). Thus, the relationship between bill dimensions and body mass was a recurring feature of evolution across the phylogeny of toucans.
A fuller understanding of the evolution of the bill in toucans and hornbills will require investigation of the thermal biology of these species in a natural setting. At present little is known about the temperature regimes encountered by these birds in nature and the variety of behavioral and physiological strategies which they employ to cope with temperature extremes. Additional studies of morphological evolution, combining data on both within-species and between-species variation, can provide further insights into the selective forces acting on bill morphology. In particular, comparative study of the evolution of bill morphology in males and females will help to elucidate the potential role of sexual selection as a factor in shaping the evolution of the bill in these families.
Typically biological structures are multi-functional; thus, support for the heat-dissipation hypothesis precludes neither the hypothesis that reaching for and grasping fruit played a key role in the origin of the toucan's large bill, nor the hypothesis that the bill has secondarily evolved roles in aposematic and intraspecific signaling, including a role in sexual selection (BÜHLER 1995, SHORT & HORNE 2001). The apparent convergence between the bills of toucans and hornbills lends plausibility to the hypothesis that the original selective pressure favoring large bills in the toucan lineage arose from frugivory (BÜHLER 1995). At the same time, some role in heat dissipation is likely to be a plesiomorphic character of the bills of birds (HAGAN & HEATH 1980, SCOTT et al. 2008; GREENBERG et al. al. 2012a, b, GREENBERG & DANNER 2013). Thus, the relatively elaborate mechanisms of heat-dissipation seen in toucans may have arisen as an exaptation (GOULD & VRBA 1982); that is, the co-option of an existing structure for a new function. The present results, because they reveal that the relationship between bill dimensions and body mass has persisted across the toucan phylogeny, suggest that the co-option of the toucan bill for heat dissipation may represent an ancient feature within this family, which has acted in concert with other selective factors favoring large bill size.
ACKNOWLEDGMENTS
I am grateful to the staff of the U.S. National Museum, Bird Division, especially Chris Milensky, for access to specimens. Comments by Glenn J. Tattersall and an anonymous reviewer greatly improved the manuscript.
LITERATURE CITED
Submitted: 26.XII.2013;
Accepted: 10.V.2014
Editorial responsibility: Mauricio O. Moura
All content of the journal, except where identified, is licensed under a Creative Commons attribution-type BY-NC.
Supplementary Information
Appendix_S1
The supplementary material is available in pdf: [Supplementary material]
Appendix_S2
The supplementary material is available in pdf: [Supplementary material]
- ALEXANDER, R.M. 1971. Size and Shape London, Edward Arnold.
- BAUMEL, J.J. 1993. Handbook of Avian Anatomy: Nomina Anatomica Avium Cambridge, Nuttall Ornithological Club, 2nd ed.
- BONACCORSO, E.; J.M. GUAYASAMIN; A.T. PETERSON & A.G. NAVARROSIGÜENZA. 2011. Molecular phylogeny and systematics of Neotropical toucanets in the genus Aulacorhynchus (Aves, Ramphastidae). Zoologica Scripta 40: 336-349. doi: 10.1111/ j.1463-6409.2011.00475.x
- BÜHLER, P. 1995. Grösse, Form und Färbung des Tukanschnabels Grundlage für den evolutiven Erflog der Ramphastiden? Journal für Ornithologie 136: 187-193.
- DARROCH, J.N.& J.E. MOSIMANN. 1985. Canonical and principal components of shape. Biometrika 72: 241-252.
- DUNNING, J.C. 2008. CRC handbook of Avian Body Masses Boca Raton, CRC Press, 2nd ed.
- EBERHARD, J.R. & E. BERMONGHAM. 2005. Phylogeny and comparative biogeography of Pionopsitta parrots and Pteroglossus toucans. Molecular Phylogenetics and Evolution 36: 288-304. doi: 10.1016/j.ympev.2005.01.022
- GARLAND JR, T.; P.H. HARVEY & A.R. IVES. 1992. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Systematic Biology 41: 18-32. doi: 10.1093/sysbio/42.3.265
- GARLAND JR, T.; A.W. DICKERMAN; C.M. JANIS & J.A. JONES. 1993. Phylogenetic analysis of covariance by computer simulation. Systematic Biology 42: 265-292.
- GARLAND JR, T.; A.F. BENNET & E.L. REZENDE. 2005. Phylogenetic approaches in comparative physiology. Journal of Experimental Biology 208: 3015-3035. doi: 10.1242/jeb.01745
- GLAZIER, D.S. 2008. Effects of metabolic level on the body size scaling of metabolic rate in birds and mammals. Proceedings of the Royal Society B 275: 1405-1410. doi:10.1098/ rspb.2008.0118
- GONZALEZ, J.-C.; B.C. SHELDON; N.J. COLLAR & J.A. TOBIAS. 2013. A comprehensive molecular phylogeny for the hornbills (Aves: Bucerotidae). Molecular Phylogenetics and Evolution 67: 468-483. doi: 10.1016/j.ympev.2013.02.012
- GOULD, S.J. & E.S. VRBA. 1982. Exaptation a missing term in the science of form. Paleobiology 8: 4-15.
- GREENBERG, R. & R.M. DANNER. 2013. Climate, ecological release and bill dimorphism in an island songbird. Biology Letters 9: 20130118. doi:10.1098/rsbl.2013.0118
- GREENBERG, R.; V. CADENA; R.M. DANNER & G. TATTERSALL. 2012a. Heat loss may explain bill size differences between birds occupying different habitats. Plos One 7 (7): e40933. doi: 10.1371/journal.pone.0040933
- GREENBERG, R.; R. DANNER; B. OLSEN & D. LUTER. 2012b. High summer temperature explains bill size variation in salt marsh sparrows. Ecography 35: 146-152. doi: 10.1111/j.16000587.2011.07002.x
- GRUBB, P.J. & T.C. WHITMORE. 1966. A comparison of montane and lowland rain forest in Ecuador: II. The climate and its effects on the distribution and physiognomy of the forests. Journal of Ecology 54: 303-333.
- HAFFER, J. 1974. Avian Speciation in Tropical South America Cambridge, Nuttal Ornithological Club.
- HAFFER, J. 1997. Foreword: species concepts and species limits in ornithology, p. 11-24. In: J. DEL HOYO; A. ELLIOTT & J. SARGATAL (Eds). Handbook of the Birds of the World Barcelona, Lynx Ediciones, vol. 4.
- HAGAN, A.A. & J.E. HEATH. 1980. Regulation of heat loss in the duck by vasomotion in the bill. Journal of Thermal Biology 5: 95-101. doi: 10.1016/0306-4565(80)90006-6
- HÖFLING, E. 1991. Étude comparative du crâne chez les Ramphastidae (Aves, Piciformes). Bonner Zoologische Beiträger 42: 55-65.
- HUGHES, A.L. 2013. Indices of Anseriform body shape based on relative size of major skeletal elements and the relationship to reproductive effort. Ibis 155: 835-846. doi: 10.1111/ibi.12087
- JUNGERS, W.L.; A.B. FALSETTI & C.E. WALL. 1995. Shape, relative size, and size-adjustments in morphometrics. American Journal of Physical Anthropology 38: 137-161. doi: 10.1002/ajpa.1330380608
- KEMP, A. 1995. The Hornbills Oxford, Oxford University Press.
- KINNAIRD, M.F. & T.G. O'BRIEN. 2007. The Ecology and Conservation of Asian Hornbills: Farmers of the Forest Chicago, University of Chicago Press.
- LUTZ, H.L.; J.D. WECKSTEIN; J.S. PATANÉ; J.M. BATES & A. ALEIXO. 2013. Biogeography and spatio-temporal diversification of Selenidera and Andigena toucans (Aves: Ramphastidae). Molecular Phylogenetics and Evolution 69: 873-883. doi: 10.1016/j.ympev.2013.06.017
- MADDISON, W.P. & D.R. MADDISON. 2011. Mesquite: a modular system for evolutionary analysis Version 2.75, available online at: http://mesquiteproject.org [Accessed: 30/IX/2011]
- MOSIMANN, J. 1970. Size allometry: size and shape variables with characterizations of the lognormal and generalized gamma distributions. Journal of the American Statistical Association 65: 930-945.
- NAHUM, L.A.; S.L. PEREIRA; F.M. FERNANDES; S.R. MATIOLI & A.WAJNTAL. 2003. Diversification of Ramphastinae (Aves, Ramphastidae) prior to the Cretaceous/Tertiary boundary as shown by molecular clock of mtDNA sequences. Genetics and Molecular Biology 26: 411-418. doi: 10.1590/S141547572003000400003.
- PATANÉ, J.S.; J.D. WECKSTEIN; A. ALEIXO & J.M. BATES. 2009. Evolutionary history of Ramphastos toucans: molecular phylogenetics, temporal diversification, and biogeography. Molecular Phylogenetics and Evolution 53: 923-934. doi: 10.1016/j.ympev.2009.08.017
- PATEL, S.; J.D. WECKSTEIN; J.S. PATANÉ; J.M. BATES & A. ALEIXO. 2011. Temporal and spatial diversification of Pteroglossus araçaris (Aves: Ramphastidae) in the neotropics: constant rate of diversification does not support an increase in radiation during the Pleistocene. Molecular Phylogenetics and Evolution 58: 105-115. doi: 10.1016/j.ympev.2010.10.016
- SCOTT, G.R.; V. CADENA; G.R. TATTERSALL & W.K. MILSOM. 2008. Body temperature depression and peripheral heat loss accompany the metabolic and ventilatory responses to hypoxia in low and high altitude birds. Journal of Experimental Biology 211: 1326-1335. doi: 10.1242/ jeb.015958
- SHORT, L.L. & J.F. HORNE. 2001. Toucans, Barbets and Honeyguides Oxford, Oxford University Press.
- SICK, H. 1993. Birds in Brazil Princeton, Princeton University Press.
- SMITH, R.J. 2009. Use and misuse of the reduced major axis for line-fitting. American Journal of Physical Anthropology 140: 476-486. doi: 10.1002/ajpa.21090
- SOKAL, R.R. & F.J. ROHLF. 1995. Biometry San Francisco, W.H. Freeman, 3rd ed.
- SYMONS, R.E. & G.J. TATTERSALL. 2010. Geographical variation in bill size across bird species provides evidence for Allen's Rule. The American Naturalist 176: 188-197. doi: 10.1086/ 653666
- TATTERSALL, G.J.; D.V. ANDRADE & A.S. ABE. 2009. Heat exchange from the toucan bill reveals a controllable vascular thermal radiator. Science 325: 468-470. doi: 10.1126/science.1175553
- VAN TYNE, J. 1929. The life history of the toucan Ramphastos brevicarinatus University of Michigan Museum of Zoology, Miscellaneous Publications 19: 1-43.
- WECKSTEIN, J.D. 2005. Molecular phylogenetics of the Ramphastos toucans: implications for the evolution of morphology, vocalizations, and coloration. Auk 122: 1191-1209. doi: doi: 10.1642/0004-8038(2005)122[1191:MPOTRT]2.0.CO;2
Publication Dates
-
Publication in this collection
04 July 2014 -
Date of issue
June 2014
History
-
Received
26 Dec 2013 -
Accepted
10 May 2014