Abstract
We conducted a study on shell morphology variation among three populations of Happiella cf. insularis (Boëttger, 1889) inhabiting different areas (Jararaca, Caxadaço, and Parnaioca trails) at Vila Dois Rios, Ilha Grande, Angra dos Reis, state of Rio de Janeiro, Brazil. Linear and angular measurements, shell indices representing shell shape, and whorl counts were obtained from images drawn using a stereomicroscope coupled with a camera lucida. The statistical analysis based on ANOVA (followed by Bonferroni's test), Pearson's correlation matrix, and discriminant analysis enabled discrimination among the populations studied. The variable that most contributed to discriminate among groups was shell height. Mean shell height was greatest for specimens collected from Jararaca, probably reflecting the better conservation status of that area. Good conservation is associated with enhanced shell growth. Mean measurements were smallest for specimens from Parnaioca, the most disturbed area surveyed. Mean aperture height was smallest for specimens from Parnaioca, which may represent a strategy to prevent excessive water loss. Discriminant analysis revealed that the snails from Jararaca differ the most from snails collected in the two other areas, reflecting the different conservation status of these areas: shells reach larger sizes in the localities where the humidity is higher. The similarities in shell morphology were greater between areas that are more similar environmentally (Caxadaço and Parnaioca), suggesting that conchological differences may correspond to adaptations to the environment.
Conchology; discriminant analysis; ecology; morphometry; threatened biome
BIOLOGY
Morphology of the shell of Happiella cf. insularis (Gastropoda: Heterobranchia: Systrophiidae) from three forest areas on Ilha Grande, Southeast Brazil
Amilcar Brum Barbosa; Sonia Barbosa dos Santos* * Corresponding author. E-mail: milkabrum@yahoo.com.br
Laboratório de Malacologia Límnica e Terrestre, Departamento de Zoologia, Instituto de Biologia Roberto Alcantara Gomes, Universidade do Estado do Rio de Janeiro. Rua São Francisco Xavier 524, PHLC sala 525-2, 20550-900 Rio de Janeiro, RJ, Brazil
ABSTRACT
We conducted a study on shell morphology variation among three populations of Happiella cf. insularis (Boëttger, 1889) inhabiting different areas (Jararaca, Caxadaço, and Parnaioca trails) at Vila Dois Rios, Ilha Grande, Angra dos Reis, state of Rio de Janeiro, Brazil. Linear and angular measurements, shell indices representing shell shape, and whorl counts were obtained from images drawn using a stereomicroscope coupled with a camera lucida. The statistical analysis based on ANOVA (followed by Bonferroni's test), Pearson's correlation matrix, and discriminant analysis enabled discrimination among the populations studied. The variable that most contributed to discriminate among groups was shell height. Mean shell height was greatest for specimens collected from Jararaca, probably reflecting the better conservation status of that area. Good conservation is associated with enhanced shell growth. Mean measurements were smallest for specimens from Parnaioca, the most disturbed area surveyed. Mean aperture height was smallest for specimens from Parnaioca, which may represent a strategy to prevent excessive water loss. Discriminant analysis revealed that the snails from Jararaca differ the most from snails collected in the two other areas, reflecting the different conservation status of these areas: shells reach larger sizes in the localities where the humidity is higher. The similarities in shell morphology were greater between areas that are more similar environmentally (Caxadaço and Parnaioca), suggesting that conchological differences may correspond to adaptations to the environment.
Key words: Conchology; discriminant analysis; ecology; morphometry; threatened biome
Land snails are exceptionally diverse in morphology, for instance they display great polymorphism in shell color and variations in shell dimensions. For this reason, they are a good subject for evolutionary biology studies (CLARKE et al. 1978). Differences in size, morphology and growth rates are associated with ecological conditions, natural selection, and phylogenetic history (VERMEIJ 1971, CLARKE et al. 1978, EMBERTON 1994, 1995b, COOK 1997, PARMAKELIS et al. 2003, TESHIMA et al. 2003). According to GOULD (1984), the low mobility of land snails influences character variability. The literature shows that habitat alterations, which result in fragmentation, are an important factor affecting shell morphological differentiation (COOK 1997, GOODFRIEND 1986, EMBERTON 1982, 1994), which can be accelerated in degraded environments (CHIBA 2004, CHIBA & DAVISON 2007).
Ilha Grande, a continental island in the southern portion of the state of Rio de Janeiro, harbors large, continuous and conserved fragments of Atlantic Forest (ROCHA et al. 2006), which is among the most threatened biomes in the world (MYERS et al. 2000). Over 50% of Ilha Grande is covered by ombrophilous dense forest, now at different levels of regeneration (ALHO et al. 2002, OLIVEIRA 2002, ALVES et al. 2005, CALLADO et al. 2009) from disturbances caused by a range of human activities over the past five decades, being now a natural laboratory to study shell morphological differentiation induced by in environment conditions.
The focus of this study was to investigate variations in the morphology of the shell of Happiella cf. insularis in three different environments (Table I). This species was described by BOËTTGER (1889) based on a single shell collected from the type locality, Ilha das Flores, São Gonçalo city, Rio de Janeiro, where additional specimens have not been found (SANTOS et al. 2010). BOËTTGER's (1889) description, which was not accompanied by illustrations, highlighted the following diagnostic features: maximum diameter with 5.25 mm, shell height 2 mm, large umbilicus, one-fourth the size of the shell base; shell pebbleshaped, thin, white, polished, spire apex slightly prominent, with ½ whorls, slightly convex; borders distinct, mildly striated, last border over the third, approximately as wide as shell, less arched at top than bottom, angled below central region; suture deeply impressed. Aperture elliptical-lunular, with small slit, aperture height with 2 mm, aperture width with 2.25 mm, simple peristomatic edge, with curved, spherical, sub-angular syphunculus [sic] protruding to right side of base.
THIELE (1927), in addition to the type locality of H. cf. insularis, also listed it in Piracicaba (state of São Paulo), Blumenau (state of Santa Catarina) and Porto Alegre (state of Rio Grande do Sul); MORRETES (1949) also listed it only in Ilha das Flores and SIMONE (2007) to Xanxerê and São Carlos (state of Santa Catarina).
In the present study, we analyzed the shell morphology of three populations of H. cf. insularis subjected to different environmental conditions, with the goal to assess variability in shell morphology, as detailed morphology and range of variation can prove useful for refining species diagnoses.
MATERIAL AND METHODS
The specimens used in this study were collected from three areas, known as the Jararaca, Caxadaço, and Parnaioca trails, located in Vila Dois Rios, on the ocean side of Ilha Grande, Municipality of Angra dos Reis, southern region of the state of Rio de Janeiro (23º04'25" to 23º13'10"S, 44º05'35" to 44º22'50"W). In each collecting site (Fig. 1), a distinct level of forest regeneration (VERA-Y-CONDE & ROCHA 2006) can be found, making them suitable for investigations on the influence of environmental factors on shell morphology.
Table I contains a summary of the environmental parameters measured at the three areas studied.
We selected intact shells from 102 adults, grown to approximately three whorls and proportionally similar to each other. Thirty-three shells from the Jararaca Trail were selected, in addition to 34 and 35 shells from the Caxadaço and Parnaioca trails, respectively.
Material examined. Happiella cf. insularis. BRAZIL, Rio de Janeiro: Angra dos Reis, Ilha Grande, Vila Dois Rios, Trilha da Jararaca, 14.VI.1998, S.B. Santos leg. (Col. Mol. UERJ 942-1); 27.IX.1996, S.B. Santos leg. (Col. Mol. UERJ 977); 11.I.1996, V.C. Queiroz leg. (Col. Mol. UERJ 980-4 and 5); 12.I.1996, S.B. Santos leg. (Col. Mol. UERJ 990-1 and 2); 21.III.1997, S.B. Santos leg. (Col. Mol. UERJ 1132); 23.III.1997, S.B. Santos leg. (Col. Mol. UERJ 1133-1 and 2); 20.IX.1997, S.B. Santos leg. (Col. Mol. UERJ 1155); 30.XI.1997, D.P. Monteiro leg. (Col. Mol. UERJ 1168-1 and 2); ditto, 26.VI.1999, S.B. Santos leg. (Col. Mol. UERJ 1241-1 and 2); 21.III.1997, S.B. Santos leg. (Col. Mol. UERJ 12522); 14.I.1998, D.P. Monteiro leg. (Col. Mol. UERJ 1617-2); 17.II.1998, A.S. Alencar leg. (Col. Mol. UERJ 1618-2); 17.II.1998, D.P. Monteiro leg. (Col. Mol. UERJ 1646); 15.I.1998, S.B. Santos leg. (Col. Mol. UERJ 1647-2); 17.II.1998, M.A. Fernandez leg. (Col. Mol. UERJ 1650); 14.I.1998, A.S. Alencar leg. (Col. Mol. UERJ 1651); 14.I.1998, D.P. Monteiro leg. (Col. Mol. UERJ 16532); 17.I.1998, S.B. Santos leg. (Col. Mol. UERJ 1656-2 and 3); 17.II.1998, D.P. Monteiro leg. (Col. Mol. UERJ 1658-2, 3, 4 and 6); 17.II.1998, S.B. Santos leg. (Col. Mol. UERJ 1659-1, 2, and 3). Vila Dois Rios, Trilha do Caxadaço, 19.X.1995, V.C. Queiroz leg. (Col. Mol. UERJ 999-2, 3, 4, 5, and 6); 30.V.1997, S.B. Santos leg. (Col. Mol. UERJ 1064-7 and 3); 28.XI.1997, D.P. Monteiro leg. (Col. Mol. UERJ 1110-1, 2, 3, 4, and 5); 15.VIII.1996, S.B. Santos leg. (Col. Mol. UERJ 1114); 19.X.1995, V.C. Queiroz leg. (Col. Mol. UERJ 1144-1, 2, 3, and 4); 08.VIII.1999, M. Sttorti leg. (Col. Mol. UERJ 1310); 21.X.2000, D.P. Monteiro leg. (Col. Mol. UERJ 2061-1, 2, and 3); 15.III.2001, S.B. Santos leg. (Col. Mol. UERJ 2156-2, 3, 4, 5, and 6); 28.X.2001, C.C. Siqueira leg. (Col. Mol. UERJ 2225-2, 3, 4, 5, and 6); 2.VIII.2005, A.B Barbosa, Lacerda, L.E.M., T.A. Viana leg. (Col. Mol. UERJ 7445-1, 2, and 3). Vila Dois Rios, Trilha da Parnaioca), 28.V.1997, N. Salgado A.B. Barbosa & S.B. dos Santos leg. (Col. Mol. UERJ 1129-1); 13.VIII.1996, S.B. Santos leg. (Col. Mol. UERJ 1139); 13.VIII.1996, S.B. Santos leg. (Col. Mol. UERJ 1177-1); 13.VIII.1996, S.B. Santos leg. (Col. Mol. UERJ 1175); 13.VIII.1996, S.B. Santos leg. (Col. Mol. UERJ 1178-1 and 2); 16.VI.2002, S.B. Santos leg. (Col. Mol. UERJ 1827-2); 02.II.2002, D.P. Monteiro leg. (Col. Mol. UERJ 2989-1); 01.II.2000, D.P. Monteiro leg. (Col. Mol. UERJ 3005-1, 2, and 3); 31.I.2000, D.P. Monteiro leg. (Col. Mol. UERJ 3006-1, 2, 3, 4, 5, 6, 7, 8, 10, and 11); 31.I.2000, S.B. Santos leg. (Col. Mol. UERJ 3007-1, 2, 3, 4, 5, 6, 7, and 8); 31.I.2000, D.P. Monteiro leg. (Col. Mol. UERJ 3288-2); 28.I.2000, P. Coelho leg. (Col. Mol. UERJ 3289); 3.VIII.2005, A.B. Barbosa, Lacerda, L.E.M., T.A. Viana leg. (Col. Mol. UERJ 7444-1, 2, and 3).
Drawings of the shells in apical, umbilical, and lateral views were made with the aid of a camera lucida under an Olympus SZH10 stereomicroscope. The drawings were used to obtain angular and linear measurements, establish the number of whorls, and calculate the ratios between measurements, according to the criteria proposed by DIVER (1931), PARODIZ (1951), SOLEM & CLIMO (1985) and FONSECA & THOMÉ (1994). The following angular measurements were considered: maximum angle (MA), columellar angle (CA), sutural angle (SA), lower sutural angle (SS'), and spire angle (SPA) (Figs 2 and 3). The linear measurements taken were: shell height (h), aperture height (ah), aperture width (aw), spire height (sh) (Fig. 2), first whorl diameter (1wd), maximum diameter (D), smaller diameter (d), first whorl width (1ww), and second whorl width (2ww) (Fig. 4), diameter umbilical (ud) (Fig. 5). The following ratios were calculated: shell height/maximum diameter (h/D), maximum diameter/umbilical diameter (D/ud), umbilical diameter/ shell height (ud/h), aperture height/aperture width (ah/aw), aperture height/smaller diameter (ah/d), first whorl diameter/ maximum diameter (1wd/D), maximum diameter/total number of whorls (D/NW), and first whorl width/second whorl width (1ww/2ww) (SOLEM & CLIMO 1985, FONSECA & THOMÉ 1994, EMBERTON 1995a). The method proposed by DIVER (1931) was applied to obtain the number of protoconch whorls (pW), total number of whorls (NW), and total number of teleoconch whorls (TW) (Fig. 4).
The analysis of shell morphological variation followed VALORVITA & VÄISÄNEN (1986), with some modifications. Descriptive statistics were performed for each variable in each group, and normality was tested using the skewness test. In cases when a given variable had asymmetrical distribution, the following transformation procedures were applied to normalize it as appropriate: e-base logarithm of X (Neperian logarithm) [1wd/ D, NW], square root of X [D/ud], sin X [MA, ah], cos X [SA, 1ww/2ww], tan X [ah/aw, ah/d], and reciprocal of X (i.e., 1/X) [1ww, 2ww, h/D, D/NW] (KREBS 1998, ZAR 1999), where X is the variable considered.
After normalization, each variable was standardized by reduction (SPIEGEL 1993) and compared using analysis of variance (ANOVA) followed by the Bonferroni's test. Differences at p < 0.05 were considered statistically significant. The third decimal place was dropped and differences at p 0.05 were considered statistically significant.
After the exclusion of the highly correlated variables (KLECK 1982, ENGELMAN 1997), discriminant analysis was performed on 15 variables: MA, CA, SA, SS, SPA, 1wd,h, ah,aw,sh, 1ww, 2ww, pW, NW, and TW. Preliminary Pearson's correlation matrix revealed a high correlation (r 0.90) between the variables D, d, ud, and ah. These were removed from the analysis, and the variable h, representing all correlated variables excluded, was kept. Upon analysis, the variables MA, SA, sh, and 1ww were also removed, owing to their low contribution to group discrimination, as shown by the discriminant function coefficients. Statistical analyses were performed with the aid of the SYSTAT 7.0 statistical package (ENGELMAN 1997).
RESULTS
Shell morphology
Happiella cf. insularis (Fig. 6) has thin, translucent, depressed, shiny shells; periostracum color varies from yellow amber (when alive) to whitish yellow (when fixed in alcohol or in cases when only the shell is found in situ); spire slightly elevated (BOËTTGER 1889) (Fig. 9); under the optical microscope, the texture is smooth, with mildly marked growth lines (BOËTTGER 1889) (Fig. 7); under scanning microscopy, protoconch, more granular, and teleoconch, of rougher aspect (Fig. 10), umbilicus opened (Fig. 8) (BOËTTGER 1889); aperture wide, crescent-shaped, slightly oblique; peristome simple (BOËTTGER 1889), thin sharp edges, without teeth (Fig. 9) (THIELE 1931, ZILCH 1959, MONTEIRO & SANTOS 2001); suture not impressed (Fig. 7) (R.L. RAMÍREZ unpubl. data); body whorl rounded (Fig. 10) (R.L. RAMÍREZ unpubl. data); ments, except for the mean maximum angle. Specimens from rapid increment's shell growth (Fig. 7) (EMBERTON 1995a).
Shell morphometry
between samples from Jararaca and Caxadaço and
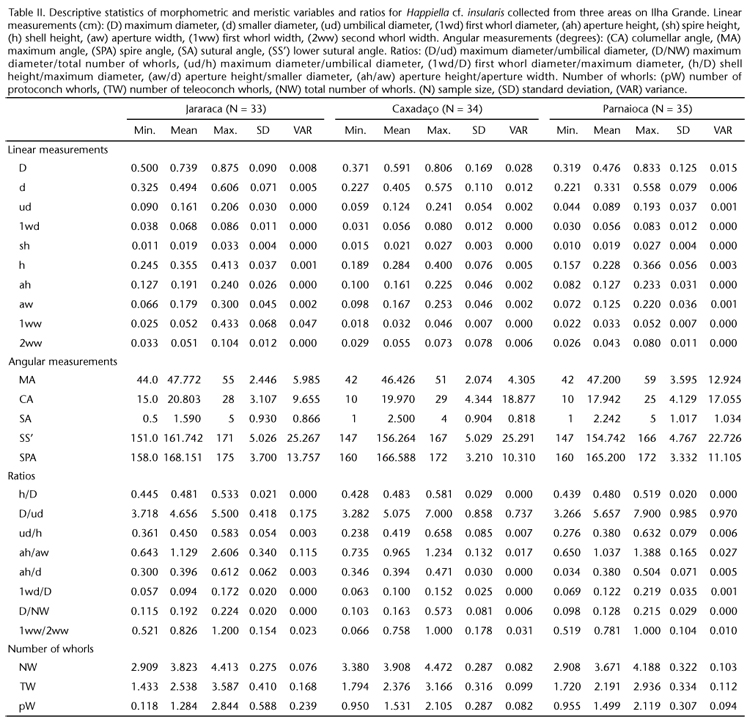
Table II shows the morphometric and meristic data of from Parnaioca and Caxadaço were not statistically significant. the 102 shells examined. The mean values of these features The shells from Jararaca differed from those from the Caxadaço were lowest in specimens from the Parnaioca Trail.
The results of the ANOVA, distinguished among the three shells from Caxadaço and Parnaioca, however, were statistically samples collected from Jararaca, Caxadaço, and Parnaioca, re-similar with regards to these two variables. The Bonferroni's test vealed significant differences in all linear and angular measurements, except for the mean maximum angle. Specimens from the Jararaca and Parnaioca trails differed significantly in mean columellar and mean spire angles, but the differences in these measurements between samples from Jararaca and Caxadaço and from Parnaioca and Caxadaço were not statistically significant. The shells from Jararaca differed from those from the Caxadaço and Parnaioca trails in the mean sutural and lower sutural angles; shells from Caxadaço and Parnaioca, however, were statistically similar with regards to these two variables. The Bonferroni's test revealed differences between samples from Parnaioca and Jararaca in nine morphological features, between Jararaca and Caxadaço samples in eight features, and between samples from Caxadaço and Parnaioca in seven features i.e., samples from Caxadaço and Parnaioca were less dissimilar to each other than to the sample from Jararaca (Table III).
ANOVA revealed significant differences in the D/ud, ud/ h, ah/aw, 1wd/D, and D/NW ratios across samples. Bonferroni's test showed Parnaioca and Jararaca samples to differ in four of these ratios (D/ud, ud/h, 1wd/D, and D/NW), Caxadaço and Parnaioca samples to differ in three (D/ud, 1wd/D, and D/NW), and Jararaca and Caxadaço samples to differ on two of these ratios (ah/aw and D/NW) i.e., differences in measurement ratios were most pronounced between samples collected from the Jararaca and Parnaioca trails, as were differences in linear measurements (Table III).
The mean total number of whorls (NW) differed significantly between the samples from Caxadaço (greater mean) and Parnaioca (Table III). The mean total number of teleoconch whorls (TW) differed significantly between the Jararaca and Parnaioca samples. The mean number of protoconch whorls (pW) differed significantly not only between samples from Jararaca and Caxadaço, but also between samples from Jararaca and Parnaioca (Table III).
Discriminant analysis
The discriminant analysis (Fig. 11) allowed the distinction of all three samples (Wilks's Lambda = 0.300, F = 6.689, df = 22, p = 0.000), particularly with respect to the sample from Jararaca, which differed the most from the others. The samples from Caxadaço and Parnaioca were more similar to each other than each was to the sample from Jararaca (Fig. 11). This analysis correctly classified 67% of the specimens (Fig. 11), with 34 out of 102 being incorrectly classified.
The proportions of explanation were of 84.6% and 15.4% for the first and second discriminant functions, respectively. The coefficients also revealed the following variables to be major contributors to the degree of differentiation achieved with the first function: shell height (h), lower sutural angle (SS'), spire angle (SPA), aperture height (ah), number of protoconch whorls (pW) e number of teleoconch whorls (TW).
Discriminant function 1 = -2.263 h 1.077 SS' + 0,964 SPA + 0.877 ah + 0.736 pW + 0.674 TW 0.411 2ww + 0.296 aw + 0.294 + 0.113 1wd + 0.038 CA.
Discriminant function 2 = -1.046 NW + 0.992 SS' 0.941 SPA + 0.900TW 0.870 ah + 0.799 pW 0.780 aw + 0.465 h + 0.396 CA + 0.249 1wd + 0.035 2ww.
DISCUSSION
The morphology of the shell of H. cf. insularis fits the description for Systrophiidae perfectly: shell thin, translucent, polished, generally smooth, spire apex slightly prominent, discoid, simple peristomatic edge. According to BAKER (1925), Happiella shells are characterized by a very low spire and an umbilicus normally reduced to a small perforation. In his original description of H. cf. insularis, BOËTTGER (1889) reported a maximum diameter of 5.25 mm, very close to the mean values found for the Caxadaço and Parnaioca samples, and shell height of 2 mm, very close to the mean obtained for the sample from Parnaioca. Aperture height and aperture width were originally described as measuring 2 mm and 2.25 mm, respectively, with the latter measurement falling within the confidence interval of the sample from Jararaca. The original description of the number of whorls (3.5) is also within the confidence intervals of both Jararaca and Parnaioca samples. According to the original description, the umbilicus size is one-fourth the maximum diameter of the shell, a similar ratio to that found in our specimens.
Our results revealed significant differences among the three populations of H. cf. insularis examined, which may be explained by differences in the degree of forest conservation in each area surveyed. The original vegetation in the Caxadaço and Parnaioca areas has been disturbed, more dramatically so in the latter, where the vegetation was entirely slashed down in some areas to make way for the now disabled Vila Dois Rios-Jararaca Dam road and for a number of plantations that served the now defunct Cândido Mendes Penal Colony. Along the Caxadaço Trail, inhabited until the construction of the penal colony by local native fishermen, the environmental changes were less pronounced. The Jararaca Trail region, by contrast, is better preserved. It has a relatively undisturbed secondary forest in lower-altitude areas and primary forest in higher areas. This translates into a deeper leaf litter layer, a more closed canopy, and lower temperature (Table I). As in other instances (SHIMEK 1930, BOYCOTT 1934, CAIN 1977, CLARKE et al. 1978, TILLIER 1981, EMBERTON 1995b, COOK 1997, WELTER-SCHULTES 2000, TESHIMA et al. 2003), better conservation certainly influences the environmental conditions overall, including leaf litter quality, structure, humidity, and depth, which in turn influence mollusk morphology (GOULD 1968, PEAKE 1978, CIPRIANI 2007). Investigating Ainohelix editha (Adams, 1868) in Hokkaido, Japan, TESHIMA et al. (2003) demonstrated that shell size and growth rates are adaptations to the environmental conditions; CHIBA (2004), investigating the genus Mandarina Pilsbry, 1894 on the Bonin Islands, found that differentiation in shell shape and dimensions are accelerated in degraded environments.
In the present study, the smallest mean values were obtained for specimens collected from the Parnaioca Trail area (Table II), the most disturbed of the three areas (shallower leaf litter, more open canopy, higher temperature). The lower capacity of the local leaf litter to retain water is likely responsible for the smaller size of the snails. The smaller mean aperture height of the shell may represent a strategy to prevent excessive water loss (GOODFRIEND 1986). MACHIN (1967), PEAKE (1978), EMBERTON (1982), and GOODFRIEND (1986), along with other investigators, have reported that smaller specimens are found in terrestrial gastropod populations living in dry areas with a strong incidence of sunlight.
The mean shell diameter and number of whorls were greatest in snails collected from Jararaca (Table II), consistent with the hypothesis that higher humidity and lower temperatures promote increased rates of shell growth in terrestrial gastropods (GOULD 1984, GOODFRIEND 1986, EMBERTON 1994). BAUR (1988) concluded that the size of the shell of Chondrina clienta (Westerlund, 1883) increases in higher temperatures and lower population densities, as verified by ANDERSON et al. (2007) for Oreohelix cooperi (Binney, 1838). BAUR (1988) commented that the phenotypic plasticity found in C. clienta may be adaptive, as the genetic makeup of snails allows for different shell growth patterns under different environmental conditions.
The populations of the most disturbed areas the Caxadaço and Parnaioca trails are more similar to each other than to the population of the Jararaca Trail, which is the bestpreserved area (Fig. 11). The greater similarity observed between the Parnaioca and Caxadaço samples can be explained by the intermediate degree of conservation of the Caxadaço region, which is closer to the degree of conservation of the Parnaioca area than to that of the Jararaca region.
The findings of this study corroborate investigations conducted in other countries showing that morphological differentiation is a result of the isolation of populations in areas that are distinct in vegetation cover, dominant plant species, maximum altitude, and soil type i.e., areas that offer different microhabitats. As CHIBA (2004) pointed out, degraded environments accelerate differentiation by eliciting new ecological interactions and new habitat conditions, thus subjecting species to a number of selective pressures.
We believe that a similar process has occurred in the areas investigated in the present study, where the different degrees of forest degradation, added to different degrees of moisture, contributed to the morphological differentiation of the three studied populations of H. cf. insularis. However, as Brazilian species of Systrophiidae are not yet well defined, we have decided not to treat the three populations as separate species, and we recommend that they continue to be identified as H. cf. insularis until ongoing anatomical studies are concluded, and a decision on their taxonimical status is reached.
ACKNOWLEDGEMENTS
This study is one of the results of the Project "Fauna malacológica aquática e terrestre da Ilha Grande", supported by research grants from the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) to the second author (APQ1 E-26/110.402/2010 and E-26/110.362/2012). We would like to express our gratitude to PEIG/INEA (Parque Estadual da Ilha Grande/Instituto Estadual do Ambiente) for license 18/ 2007; to IBAMA/Sisbio (Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis/Sistema de Autorização e Informação em Biodiversidade) for license 19836-1 to ABB and 10812-1 to SBS; to Capes (Coordenação de Aperfeiçoamento do Pessoal de Ensino Superior for a PhD scholarship to ABB; to the reviewers who gave valuable suggestions to improve the manuscript; to Cientifica Consultoria for review of English and to CEADS (Centro de Estudos Ambientais e Desenvolvimento Sustentável da UERJ) for logistic support.
LITERATURE CITED
Submitted: 13.IV.2013;
Accepted: 06.IV.2014
Editorial responsibility: Marcos D.S. Tavares
All content of the journal, except where identified, is licensed under a Creative Commons attribution-type BY-NC.
- ALHO, C.J.R.; M. SCHNEIDER & L.A. VASCONCELLOS. 2002. Degree of threat to the biological diversity in the Ilha Grande State Park (RJ) and guidelines for conservation. Brazilian Journal of Biology 62 (3): 375-385. doi: 10.1590/S1519-69842002000300001.
- ALVES, S.L.; A.S. ZAÚ; R.R. OLIVEIRA; D.F. LIMA & C.J.R. MOURA. 2005. Sucessão florestal e grupos ecológicos em Floresta Atlântica de encosta, Ilha Grande, Angra dos Reis/RJ. Revista Universidade Rural: Série Ciências da Vida 25 (1): 26-32.
- ANDERSON, T.K.; K.F. WEAVER & R.P. GURALNICK. 2007. Variation in adult shell morphology and life-history traits in the land snail Oreohelix cooperi in relation to biotic and abiotic factors. Journal of Molluscan Studies 73: 129-137. doi: 10.1093/ mollus/eym006.
- BAKER, H.B. 1925. The Mollusca collected by the University of Michigan-Williamson expedition in Venezuela. Part III. Occasional Papers of the Museum of Zoology 156: 1-44.
- BAUR, B. 1988. Microgeographical variation in shell size of the land snail Chondrina clienta Biological Journal ofthe Linnean Society 35: 247-259. doi: 10.1111/j.1095-8312.1988.tb00469.x.
- BOËTTGER, O. 1889. Bemerkung uber ein paar brasilianische Landschneken, nebst Beschreibung drein neuer Hyalinien von dort. Nachrichtsblatt der deutschen Malakozoologischen 20 (1-2): 27-30.
- BOYCOTT, A.E. 1934. The habitats of land mollusca in Britain. Journal of Ecology 22: 1-38.
- CAIN, A.J. 1977. Variation in the spire index of some coiled gastropods shells, and its evolutionary significance. Philosophical Transactions of the Royal Society B277: 377-428.
- CALLADO, C.H.; A.A.M. BARROS; L.A. RIBAS; N. ALBARELLO; R. GAGLIARDI & C.E.S. JASCONE. 2009. Flora e cobertura vegetal, p. 91-162. In: M. BASTOS & C.H. CALLADO (Eds). O Ambiente da Ilha Grande Rio de Janeiro, UERJ/CEADS, 562p.
- CHIBA, S. 2004. Ecological and morphological patterns in communities of land snails of the genus Mandarina from the Bonin Islands. Journal of Evolutionary Biology 17: 131-143. doi: 10.1046/j.1420-9101.2004.00639.x.
- CHIBA, S. & A. DAVISON. 2007. Shell shape and habitat use in the North-west Pacific land snail Mandarina polita from Hahajima, Ogasawara: current adaptation or ghost of species past? Biological Journal of the Linnean Society 91: 149-159. doi: 10.1111/j.1095-8312.2007.00790.x.
- CIPRIANI, R. 2007. Modelando las conchas de los moluscos, o la búsqueda de la espiral perfecta, p. 3-11. In: S.B. SANTOS; A.D. PIMENTA; S.C. THIENGO; M.A. FERNANDEZ & R.S. ABSALÃO (Eds). Tópicos em Malacologia Ecos do XVIII Encontro Brasileiro de Malacologia Rio de Janeiro, Sociedade Brasileira de Malacologia, XIV+365p.
- CLARKE, B.; W. ARTHUR; D.T. HORSLEY & D.T. PARKIN. 1978. Genetic variation and natural selection in pulmonate molluscs. 219-270. In: V. FRETTER & J. PEAKE (Eds). Pulmonates: Systematics, Evolution and Ecology Londres, Academy Press, 540p.
- COOK, L.M. 1997. Geographic and ecological patterns in Turkish land snails. Journal of Biogeography 24: 409-418. doi: 10.1111/j.1365-2699.1997.00139.x.
- DIVER, C. 1931. A method to determining the number of whorls of a shell and its application to Cepaea hortensis Müll. Proceedings of the Malacological Society of London 19: 1931.
- EMBERTON, K.C. 1982. Environment and shell shape in the Tahitian land snail Partula otaheitana Malacologia 23 (1): 23-35.
- EMBERTON, K.C. 1994. Partitioning a morphology among its controlling factors. Biological Journal of the Linnean Society 53: 353-369.
- EMBERTON, K.C. 1995a. Land-snail community morphologies of the highest-diversity sites of Madagascar, North America and New Zealand, with recommended alternatives to heightdiameter plots. Malacologia 36 (1-2): 43-66.
- EMBERTON, K.C. 1995b. Sympatric convergence and environmental correlation between two land-snail species. Evolution 3: 469-475.
- ENGELMAN, K. 1997. SYSTAT 7.0 Chicago, SPSS Inc press, 421p.
- FONSECA, A.L.M. & J.W. THOMÉ. 1994. Conquiliomorfologia e anatomia dos sistemas excretor e reprodutor de Radiodiscus thomei Weirauch, 1965 (Gastropoda, Stylommatophora, Charopidae). Biociências 2 (1): 163-188.
- GOODFRIEND, G.A. 1986. Variation in land-snail shell form and size and its causes: a review. Systematic Zoology 2: 204-223.
- GOULD, S.J. 1968. Ontogeny and the explanation of form: an allometric analysis. Paleontological Society Memoirs 2: 81-98.
- GOULD, S.J. 1984. Covariance sets and ordered geographic variation in Cerion from from Aruba, Bonaire and Curaçao: a way of studying nonadaptation. Systematic Zoology 33 (2): 217-237.
- KLECK, W. 1982. Discriminant analysis Sage University Paper Series on Quantitative Applications in the Social Sciences, 07-0119. Beverly Hills, Sage Publications, 71p.
- KREBS, J.C. 1998. Ecological Methodology New York, Benjamin Cummings, XII + 620p.
- LEVINE, D. M.; M.L. BERENSON & D. STEPHAN. 2000. Estatística: teoria e aplicações Rio de Janeiro, LTC, 812 p.
- MACHIN, J. 1967. Strutural adaptation for reducing water-loss in three species of terrestrial snail. Journal of Zoology 152: 55-65. doi: 10.1111/j.1469-7998.1967.tb01638.x.
- MONTEIRO, D.P. & S.B. SANTOS. 2001. Conquiliomorfologia de Tamayoa (Tamayops) banghaasi (Thiele) (Gastropoda, Systrophiidae). Revista Brasileira de Zoologia 18 (4): 10491055. doi: 10.1590/S0101-81752001000400002.
- MORRETES, F.L. 1949. Ensaio de catálogo dos moluscos do Brasil. Arquivos do Museu Paranaense 7: 1-216.
- MYERS, N.; R.A. MITTERMEIER; C.G. MITTERMEIER; G.A.B. FONSECA & J. KENT. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853-858. doi: 10.1126/science.1067728.
- OLIVEIRA, R.R. 2002. Ação antrópica e resultantes sobre a estrutura e composição da Mata Atlântica na Ilha Grande, RJ. Rodriguésia 53 (82): 33-58. doi: 10.1590/S1414-753X2007000200002.
- PARMAKELIS, A.; E. SPANOS; G. PAPAGIANNAKIS; C. LOUIS & M. MYLONAS. 2003. Mitochondrial DNA phylogeny and morphological diversity in the genus Mastus (Beck, 1837): a study in recent (Holocene) island group (Koufonisi, south-east Crete). Biological Journal of the Linnean Society 78: 383-399. doi: 10.1046/j.1095-8312.2003.00152.x.
- PARODIZ, J.J. 1951. Métodos de Conquiliometria. Physis 20 (38): 241-248.
- PEAKE, J.F. 1978. Distribution and ecology of Stylommatophora, p. 429-526. In: V. FRETTER & J. PEAKE (Eds). Pulmonates. Systematics, Evolution and Ecology New York, Academic Press, vol. 2A, 540p.
- ROCHA, C.F.D.; H.G. BERGALLO; M.A.S. ALVES & M.V. SLUYS. 2003. A biodiversidade nos grandes remanescentes florestais do Estado do Rio de Janeiro e nas restingas da Mata Atlântica São Carlos, Editora Rima, 160p.
- SANTOS, S.B.; A.B. BARBOSA; R.M.R.B. BRAGA; J.L. OLIVEIRA & R.F. XIMENES. 2010. Moluscos da Ilha das Flores, São Gonçalo, Rio de Janeiro. Informativo SBMa 173: 10-14.
- SHIMEK, B. 1930. Land snails as indicators of ecological conditions. Ecology 11 (4): 673-686. doi: 10.2307/1932328.
- SIMONE, L.R.L. 2007. Land and freshwater molluscs of Brazil São Paulo, EGB, Fapesp, 390p.
- SLUYS, M.V.; R.V. MARRA; L. BOQUIMPANI-FREITAS; & C.F.D. ROCHA. 2012. Environmental factors affecting calling behavior of sympatric frog species at an Atlantic Rain Forest area, Southeastern Brazil. Journal of Herpetology 46 (1): 41-46.
- SOLEM, A. & F.M. CLIMO. 1985. Structure and habitat correlations of sympatric New Zealand land snail species. Malacologia 26: 1-30.
- SPIEGEL, M.R. 1993. Estatística São Paulo, Makron Books, Coleção Schaum, 643p.
- TESHIMA, H.; A. DA VISON; Y. KUWAHARA; J. YOKOHAMA; S. CHIBA; T. FUKUDA; H. OGIMURA & M. KAWATA. 2003. The evolution of extreme shell shape variation in the land snail Ainohelix editha: a phylogeny and hybrid zone analysis. Molecular Ecology 12: 1869-1878. doi: 10.1046/j.1365-294X.2003.01862.x.
- THIELE, J. 1927. Über einige brasilianische Landschnecken. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft 40 (3): 307-329.
- THIELE, J. 1931. Handbuch der Systematischen Weichtierkunde Jena, Gustav Fischer, vol. 1, 778p.
- TILLIER, S. 1981. Clines, convergence and character displacement in new Caledonian diplommatinids (land prosobranchs). Malacologia 21 (1-2): 177-208.
- VALORVITA, I. & VÄISÄNEN, R. A. 1986. Multivariate morphological discrimination between Vitrea contracta (Westerlund) and V. crystallina (Müller)(Gastropoda, Zonitidae). Journal Molluscan Studies 52: 62-67. doi: 10.1093/mollus/52.1.62
- VERMEIJ, G.J. 1971. Gastropod evolution and morphological diversity in relation to shell geometry. Journal of Zoology 163: 15-23. doi: 10.1111/j.1469-7998.1971.tb04522.x.
- VERA-Y-CONDE, C.F. & C.F.D. ROCHA. 2006. Habitat disturbance and small mammal richness and diversity in an atlantic rainforest area in southeastern Brazil. Brazilian Journal of Biology 66 (4): 983-990. doi: 10.1590/S1519-69842006000600005.
- WELTER-SCHULTES, F.W. 2000. Human-dispersed land snails in Crete, with special reference to Albinaria (Gastropoda: Clausiliidae). Biologia Gallo-hellenica 24: 83-106.
- ZAR, J. H. 1999. Biostatistical Analysis New Jersey, Prentice-Hall, 663p.
- ZILCH, A. 1959. Gastropoda: Euthyneura Berlim, Borträger, vol. 2, 834p.
Publication Dates
-
Publication in this collection
04 July 2014 -
Date of issue
June 2014
History
-
Received
13 Apr 2013 -
Accepted
06 Apr 2014