ABSTRACT
We described immature stages of Nilio (Nilio ) brunneus Thomson, 1860 and provide a supplementary description for adults, including new data on the anatomy of the female and male terminalia. We observed N. brunneus feeding on the lichen Parmotrema sp., and that immature and adult are gregarious, with sessile pupae and generations overlapping. In laboratory, eggs hatched in 14 days and adults emerged after seven days in the pupal stage, the adults survived only a few days.
KEY WORDS:
Host fungus; larva; lichen; morphology; pupa
Nilioninae is a subfamily of Tenebrionidae comprising 42 extant Neotropical species and one fossil species from the Dominican amber (Poinar & Brown 2011Poinar Jr G, Brown AE (2011) Descriptions of a broad-nosed weevil (Eudiagogini: Curculionidae) and false ladybird beetle (Nilionini: Nilionidae) in Dominican amber. Historical Biology 23(2-3): 231-235.), all in the single genus Nilio Latreille, 1802. Nilio is divided into three subgenera, defined mostly by the number or lack of elytral striae: Nilio Latreille, 1802 and Linio Mader, 1936, separation proposed by Mader (1936Mader L (1936) Bestimmungstabelle der Coleopterenfamilie Nilionidae. Entomologisches Nachrichtenblatt 10(2): 73-102.); and Micronilio Pic, 1936. Eight species of Nilio occur in the Brazilian Atlantic Forest: Nilio (Nilio ) lutzi Ihering, 1914, Nilio (Nilio ) marginellus Erichson, 1847, Nilio (Nilio ) brunneus Thomson, 1860, Nilio (Linio ) lanatus Germar, 1824, Nilio (Linio ) maculatus Germar, 1824, Nilio (Micronilio ) pusillus Ihering, 1914, Nilio (Micronilio ) gounellei Ihering, 1914 and Nilio (Micronilio ) barthi Costa Lima & Seabra, 1954.
Little is known about the biology of Nilio species, and immature stages have been seldom studied. Larvae and adults of a few species have been observed in dead or live trunks, probably feeding on fungi, usually on lichens, and there are records of gregarious behavior. Immature stages of only four species are described: N. (N .) brunneus described by Ihering (1914Ihering R von (1914) As espécies brazileiras de Nilionidas (Coleopteros) e a posição systematica da família, pelo estudo das larvas. Revista do Museu Paulista 9: 281-306., as N. bouvieri ), N. (L. ) varius Ihering, 1914 by Jorge (1974Jorge ME (1974) Immature stages of Nilionidae: a contribution toward the taxonomic position of the family (Coleoptera). Revista Brasileira de Entomologia 18(4): 123-128.) and redescribed by Costa et al. (1988Costa C, Vanin SA, Casari-Chen SA (1988) Larvas de Coleoptera do Brasil. São Paulo, Universidade de São Paulo, 282p.), N. (L .) lanatus by Simões et al. (2009Simões MVP, Quintino HYS, Monné ML (2009) Larva and pupa of Nilio (Linio) lanatus Germar, 1824 (Coleoptera: Tenebrionidae). Zootaxa 2175: 51-56.), and N. (M.) barthi by Gil-Santana & Marques (2008Gil-Santana HR, Marques OM (2008) Contribuição ao conhecimento de Nilio barthi Costa Lima & Seabra (Coleoptera: Tenebrionidae: Nilioninae). Boletín de la Sociedad Entomológica Aragonesa 42: 271-278.).
Our objectives here are to describe the egg, last instar larva and pupa of N. brunneus , and to provide a supplementary description of adults, including the description of the terminalia of male and female.
MATERIAL AND METHODS
The specimens of N. brunneus were found in living trunks of ipê rosa (Handroanthus impetiginosus (Mart. ex DC.) Mattos, Lamiales: Bignoniaceae) covered by lichens, in the campus of the Universidade Federal de Viçosa, state of Minas Gerais, Brazil. The locality belongs to the Brazilian Atlantic Forest biome, but the forest itself is very fragmented. Specimens of N. brunneus were collected only in the urban area of the campus, far from the surrounding forest remnants.
Specimens were examined and measured, and adult male and female terminalia extracted under a Zeiss Stemi 2000-C stereomicroscope. Female terminalia, including spermatheca, were stained with a solution of 0.5% Chlorazol Black E in 85% alcohol to enhance contrast. Whole mount preparations of dissected sclerites were made using a water-soluble mounting media based on polyvinyl alcohol and lactic acid. We photographed slides under a Zeiss AxioLab compound microscope equipped with an AxioCam MRc digital camera, and adult specimens under a Zeiss Discovery V8 stereomicroscope with an AxioCam MRc. Final images were the result of montaging 25 to 125 image slices at different focal lengths using the extended focus module of Zeiss AxioVision 4.8 software. Slide preparations of gut contents of one adult and lichens found on the tree host were made to confirm the feeding habits of the species. The key provided by Fleig et al. (2008Fleig M, Grüniger W, Mayer WE, Hampp R (2008) Liquens da Floresta com Araucária no Rio Grande do Sul/Lichens of the Araucaria Forest of Rio Grande do Sul/Flechten des Araukarienwaldes von Rio Grande do Sul. Pró-Mata: Guia de Campo nº 3/Field Guide No. 3/Naturführer Nr. 3. Tübingen, University of Tübingen, 217p.) was used for identifying the lichen.
We based the redescription of N. brunneus on a plesiotype (a specimen used for a redescription, supplementary description, or illustration published subsequent to the original description; sensu Evenhuis 2008Evenhuis NL (2008) A compendium of zoological type nomenclature: A reference source. Honolulu, Bishop Museum Technical Report 41, 23p.). Terms for external morphology, including sclerites of terminalia, follow Lawrence et al. (2011Lawrence JF, Slipinski A, Seago AE, Thayer MK, Newton AF, Marvaldi AE (2011) Phylogeny of the Coleoptera based on morphological characters of adults and larvae. Annales Zoologici 61(1): 1-217.). The term basale refers to the phallobase, and apicale to the fused parameres (Lawrence et al. 2011Lawrence JF, Slipinski A, Seago AE, Thayer MK, Newton AF, Marvaldi AE (2011) Phylogeny of the Coleoptera based on morphological characters of adults and larvae. Annales Zoologici 61(1): 1-217.). The following symbols are used for measurements (in mm) and ratios, for adults: EL, elytral length (at midline, from base of scutellum to elytral apex); EW, greatest elytral width; GD, greatest depth of the body (from elytra to metaventrite); PL, pronotal length along midline; PW, greatest pronotal width; TL, total length (= EL+PL; head not included); for larvae: TL, total length (including head); GW, greatest width. The ratio GD/EW (adult) was recorded as an indication of degree of convexity; TL/EW (adult) and TL/GW (larva) indicate degree of body elongation.
Label data are cited verbatim inside quotation marks; a backslash separates different labels. The number of specimens bearing these labels are stated immediately before the label data.
All specimens were deposited in the Coleção Entomológica do Laboratório de Sistemática e Biologia de Coleoptera (CELC), Universidade Federal de Viçosa, Viçosa, Minas Gerais.
TAXONOMY
Tenebrionidae Latreille, 1802
Nilioninae Oken, 1843
Nilio Latreille, 1802
Nilio (Nilio ) brunneus Thomson, 1860
urn:lsid:zoobank.org:pub:26DB11F0-3DA6-4BF8-AA6A-3879F4E62253
Diagnosis. Adults of Nilio brunneus differ from all other Nilio by having 11 longitudinal striae on each elytron. Larvae of N. brunneus are elongate, have four stemmata on each side of head, with dark head and pronotum, differing from larvae of N. varius , N. lanatus and N. barthi , respectively. Pupae of N. brunneus are bigger and darker than pupae of N. barthi , N. brunneus has a dense pilosity while N. barthi is glabrous. Pupae of N. brunneus are dark colored and have the halteriform projections lighter than body, differing from pupae of N. lanatus , which are light colored and have the halteriform projections darker than body. Pupae of N. brunneus and N. varius differ from each other by the coloration of pilosity, white in N. brunneus and darkish brown in N. varius .
Redescription. Immature stages. Egg (Fig. 1). Total length: 1 mm. Glossy, shiny, ovoid and darkish brown. Mature larvae (Figs. 3-4, 8-14). Total length: 5.40-6.50 mm, width: 3.50-4.60 mm. Oblong, convex, highly pigmented. Dorsally darkish brown with head, lateral and posterior edges of pronotum, lateral of abdominal segments I and II, and last two abdominal segments reddish brown. Ventral surface light yellow. Dorsal surface with vestiture of two types of setae, one white, long, with about half the width of the segment, and another darkish brown, smaller, reaching a quarter the size of the major setae. Head hypognathous, articulating ventrally with prothorax. Epicranial suture present and wide. Coronal suture long. Frontal arms U-shaped, slightly widest distally. Four stemmata on each side, three dorsally arranged in a semicircle right behind antennal insertion, and one isolated ventrally, slightly bigger. Antennae (Fig. 8) inserted laterally, equidistant from base of mandibles, posteriorly in the head with the insertions almost reaching the pronotum. Antennae with three antennomeres, the first and third annular, the second subcylindrical and longer than the other two together. Frontoclypeal suture visible. Clypeus transverse, setose, membranous distally. Labrum (Fig. 9) transverse, setose, anterior angles rounded. Epipharynx heavily setose, setae longer laterally. Hypopharyngeal sclerome (Fig. 13) symmetrical, with three teeth at apex. Gula transverse with short sutures. Buccal pieces protracted. Mandibles mobile, asymmetrical, heavily sclerotized, apex with three teeth, lateral margin setose at base, mola highly developed, rounded, prominent in the right mandible. Maxillae elongate; mala long, apex slightly rounded, presenting thick setae distally and laterally. Maxillary palpi with three palpomeres, subglabrous, third segment with a tuft of thick bristles distally. Stipe elongate, subglabrous. Cardo subtriangular. Labium with prementum transverse, mentum and submentum elongate, heavily setose. Ligula elongate, apex rounded, setose distally. Palpiger membranous with one seta. Labial palpi with two palpomeres. Prothorax wider and shorter than mesothorax. Legs long, subequal in length, presenting many short and narrow setae, coxa elongate, trochanter subtriangular, femur thicker than tibia, tarsungulus subglabrous. Abdomen with nine segments visible dorsally, segments I to VIII transverse, about the same length, gradually narrowing to apex, with a pair of dorsolateral annular spiracles each, segment IX smaller, without urogomphi, segment X below the IX, very small, apex bilobate. Anal aperture between apical lobes of segment X.
Nilio brunneus immature stages. Egg cluster (1); first instar larvae (2); mature larva, dorsal (3) and ventral (4) views; pupa, dorsal (5), lateral (arrow - halteriform projection) (6) and ventral (7) views. Scale bars: 1 = 2 mm, 2-6 = 1 mm.
Nilio brunneus larval parts. Antenna (8); labrum (9); left (10) and right (11) mandibles; maxila (12); labium (13); leg (14). hs - hypopharyngeal sclerome. Scale bars: 8-13 = 0.1 mm, 14 = 0.5 mm.
Pupa (Figs. 5-7). Adecticous and exarate. Ventral surface darkish brown with the first abdominal segment goldish yellow, the middle portion of the second to the last abdominal segment reddish brown. Ventral surface light yellow. Dorsal surface covered with sparse small white setae, ventral surface glabrous. Head not visible from above. Pronotum transverse, slightly projected laterally. Abdominal segments I-V each presenting a pair of light colored, almost transparent, halteriform projections (Fig. 6, arrowed).
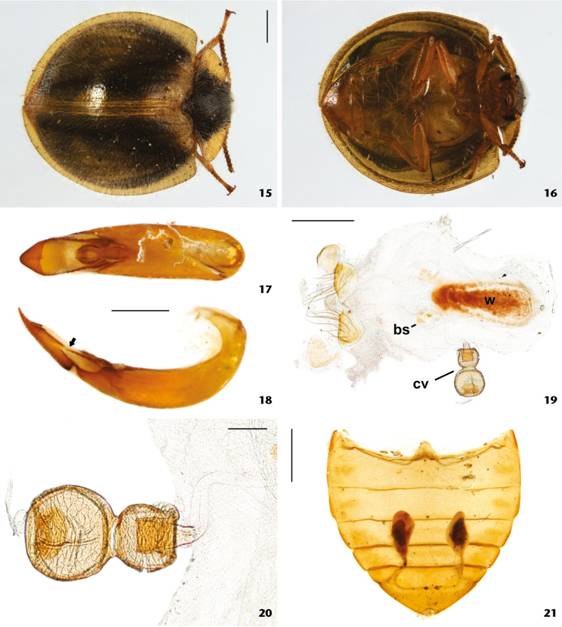
Adults (Figs. 15-21). Male. Body convex, opaque, with a dense vestiture; length 5.40-6.00 mm; head, central disc of pronotum, scutellum and middle portion of elytra darkish brown; edges of pronotum, edges and surface around suture of elytra and scutellum yellow to light brown; ventral surface yellow to light brown; antennae yellow to light brown, darkening from the fourth antennomere to apex. Head not visible dorsally. Eyes with anterior portion emarginated by antennal insertion, forming a lower lobe about three times larger than upper lobe. Antennae with antennomeres 4-11 slightly expanded forming a light club, bearing multi-pronged sensilla (sensillifers) at the upper portion. Pronotum transverse, twice as wide as long, widest and longest at middle; lateral edges explanate, visible for their entire lengths from above; anterior edge curved outward, posterior edge strongly convex. Elytra approximately four times as long as pronotum; sides rounded, posterior edges straight in dorsal view, epipleura extending to apex. Hind wings developed, apparently functional. Prosternum short; prosternal process subparallel, apex hidden by the mesosternal projection. Mesosternum short with a spear-like projection towards the prosternum. Tibiae simple, covered with setae, inner angle bearing a row of setae. Aedeagus (Figs. 17-18) with basale about three times as long as apicale; basale most expanded near its middle, strongly curved ventrally; basale completely closed ventrally, forming a tube that bears a Y-shaped projection at middle, this projection directed towards apicale; apicale with sides subparallel, tapering at apical 1/3 and bearing two lateral projections (ala) that are articulated (Fig. 18, arrowed), directed anteriorly and fitting the basale, each ala bearing four teeth in outer edge and expanding near basale; penis about as long as basale, cylindrical, with an expanded sclerotized apex.
Nilio brunneus adults. Dorsal (15) and ventral (16) views; aedeagus ventral (17) and lateral (arrow - ala) (18) views; female terminalia (19); spermatheca (20); abdominal tergites with defensive glands (21). (bs) Sclerites of bursa, (cv) check valve, (w) window of bursa. Sclae bars: 15, 16, 21 = 1 mm, 17-19 = 0.5 mm, 20 = 0.1 mm.
Female. Not distinguishable from males externally. Bursa copulatrix (Fig. 19) with small sclerites; window of bursa about three times the length of gonocoxites and two times the length of spermatheca. Spermatheca (Fig. 20) with a check valve one and a half times the length of gonocoxites; check valve constricted around middle, forming two lobes, the lobe closer to bursa slightly smaller than the other. First lobe of check valve with an invagination almost as long as the lobe; second lobe with an invagination reaching the middle of the lobe. Paraprocts subequal in length with gonocoxites. Gonocoxites bearing long setae, about twice the length of gonocoxites. Gonostyli inserted laterally on gonocoxites.
Variation. Mature larvae (n = 6): TL = 5.40-6.00 (5.78 ± 0.37); GW = 3.50-4.50 (3.88 ± 0.42); TL/GW = 1.40-1.60 (1.50 ± 0.07). Adults (n = 21): TL = 5.1-6.7 (5.89 ± 0.48); EL = 4.00-5.80 (4.95 ± 0.46); EW = 4.50-6.20 (5.49 ± 0.43); PL = 0.70-1.10 (0.89 ± 0.10); PW = 3.00-4.00 (3.55 ± 0.25); GD = 2.20-3.50 (2.73 ± 0.41); GD/EW = 0.43-0.60 (0.50 ± 0.05); TL/EW = 1.02-1.13 (1.07 ± 0.03).
Material examined. 3 adults (CELC), labeled "BRASIL: MG, Viçosa/Campus UFV/2.viii.2013/leg. S. Aloquio & C. Lopes-Andrade". 10 eggs, 6 larvae, 3 pupae and 18 adults (CELC), labeled "BRASIL: MG, Viçosa/Campus UFV/15-16.vii.2013/leg. S. Aloquio".
Host fungus. We observed immature and adult N. brunneus on and around lichens of an unidentified Parmotrema A. Massal. (Ascomycota: Lecanoromycetes: Parmeliaceae; Figs. 25-28). We observed the intestinal content in a single adult and found only spores, hyphae, green algae and other lichen remnants. These structures found in the intestinal content matched those observed in slide preparations of Parmotrema collected in the same trees inhabited by N. brunneus .
Nilio brunneus . Larva and adult on lichen (22); group of adults (23); pupal cluster (24).
Parmotrema sp. Thallus (25); apotecia and soredia (26); lower cortex (27); edges of lower cortex (28). Scale bars: 25 = 10 mm, 26-28 = 1 mm.
Biological remarks. We observed that larvae and adults of N. brunneus move slowly and pupae are sessile. Individuals are gregarious and usually found in great number. Larvae seem to choose specific spots on the tree where mature larvae tightly attach to the substrate using their tarsungulus (Fig. 24), and after ecdysis pupae remain inside the last larval exuvium (Figs. 5-6). We observed generations overlapping, with larvae, pupae and adults staying close to each other (Figs. 22, 24). Females lay eggs in clusters (Fig. 1), but we have not observed eggs close to immature or adult forms. In laboratory we have observed one cluster of eggs, and the first instar larvae hatched from the eggs in about 14 days (Fig. 2). Some of the last instar larvae we collected have pupated in laboratory and adults emerged after seven days. We were not successful in breeding larvae from other instars and the emerged adults survived for only a few days.
DISCUSSION
Nilio species are supposed to use lichens as food. There are works reporting these beetles on branches covered with lichens (Gil-Santana & Marques 2008Gil-Santana HR, Marques OM (2008) Contribuição ao conhecimento de Nilio barthi Costa Lima & Seabra (Coleoptera: Tenebrionidae: Nilioninae). Boletín de la Sociedad Entomológica Aragonesa 42: 271-278., Ihering 1914Ihering R von (1914) As espécies brazileiras de Nilionidas (Coleopteros) e a posição systematica da família, pelo estudo das larvas. Revista do Museu Paulista 9: 281-306., Simões et al. 2009Simões MVP, Quintino HYS, Monné ML (2009) Larva and pupa of Nilio (Linio) lanatus Germar, 1824 (Coleoptera: Tenebrionidae). Zootaxa 2175: 51-56.), but these provided no evidence on their feeding habit. And the lichens were not identified in these works. Here we confirm that N. brunneus eats lichens of the genus Parmotrema , both by observing them on these fungi and by examining the gut content of an adult. It would be important to examine the gut content of immatures and adults of more specimens of N. brunneus , and also of other Nilio species, to elucidate the extension of this biological interaction.
Many arthropods are known to have some kind of association with lichens. The lichen huntsman spider Pandercetes gracilis Koch, 1875 (Araneae: Sparassidae), from Australia and New Guinea, camouflages on lichen-covered tree trunks. The body and legs are very hairy, breaking up its outline, and it presses itself very close to the trunk (Beccaloni 2009Beccaloni J (2009) Arachnids. Collinwood, CSIRO Publishing, 320p.). The debris-carrying lacewing larva Leocochyrsa pavida (Hagen, 1861) (Neuroptera: Chrysopidae) constructs a debris packet using minute lichen thallus fragments and attaches it to its dorsal surface to use as camouflage (Brodo et al. 2001Brodo IM, Sharnoff SD, Sharnoff S (2001) Lichens of North America. New Haven, Yale University Press, 795p.). Nilio species do not seem to camouflage on lichens, but more field observations are necessary to evaluate this (pers. obs.). Many tiny terrestrial arthropods such as mites, springtails, bark lice and silverfish may eat lichens (Brodo et al. 2001Brodo IM, Sharnoff SD, Sharnoff S (2001) Lichens of North America. New Haven, Yale University Press, 795p.). But larger arthropods feeding on lichens are uncommon. Among the latters is the nymph of Lichenodraculus matti Braun, 2011 (Orthoptera: Tettigoniidae), which feeds exclusively on lichens, but adults seem to have a broader diet (Braun 2011Braun H (2011) The Little Lichen Dragon - an extraordinary katydid from the Ecuadorian Andes (Orthoptera, Tettigoniidae, Phaneropterinae, Dysoniini). Zootaxa 3032: 33-39.). The available information on feeding habits of Nilio is that adults and larvae are usually found on trunks covered with lichens and other fungi and probably eat them (Costa et al. 1988Costa C, Vanin SA, Casari-Chen SA (1988) Larvas de Coleoptera do Brasil. São Paulo, Universidade de São Paulo, 282p.). However, no attempt of identification of those fungi was made and authors do not mention gut content. Here we confirm that at least adult N. brunneus feeds on lichens and we believe it is a common habit in Nilio species (pers. obs.). Most lichens are high in carbohydrates but low in proteins (Brodo et al. 2001Brodo IM, Sharnoff SD, Sharnoff S (2001) Lichens of North America. New Haven, Yale University Press, 795p.), and are also good sources of vitamins. Those are the primary metabolites (intracellular) and are synthetized by both fungus and alga (Elix & Stocker-Wörgötter 2008Elix JA, Stocker-Wörgötter E (2008) Biochemistry and secondary metabolites, p. 106-135. In: Nash III TH (Ed.) Lichen Biology. Cambridge, Cambridge University Press, 2nd ed., 489p.). The secondary metabolites (extracellular) are synthetized only by the fungus, and can include mycotoxines that are known to be harmful to insects, as vulpinic acid produced by the lichen Letharia vulpina (L.) Hue (1899) (Lecanorales: Parmeliaceae) (Elix & Stocker-Wörgötter 2008Elix JA, Stocker-Wörgötter E (2008) Biochemistry and secondary metabolites, p. 106-135. In: Nash III TH (Ed.) Lichen Biology. Cambridge, Cambridge University Press, 2nd ed., 489p.). In addition to their secondary metabolites, lichens are capable of accumulating many harmful elements such as mercury, cadmium, copper and magnesium, which can make them unpalatable and toxic (Nash 2008Nash III TH (2008) Nutrients, elemental accumulation, and mineral cycling. p. 236-253. In: Nash III TH (Ed.) Lichen Biology. Cambridge, Cambridge University Press, 2nd ed., 489p.). Thus lichens are good sources of nutrients, but bear secondary components acting as deterrent to lichenophagy. Therefore, it is important to study further the feeding habits of Nilio species, as they possibly represent an uncommon example of lichenophagous insects.
The larvae of N. bouvieri , a species described by Ihering (1914Ihering R von (1914) As espécies brazileiras de Nilionidas (Coleopteros) e a posição systematica da família, pelo estudo das larvas. Revista do Museu Paulista 9: 281-306.) that is currently considered a junior synonym of N. brunneus , are completely different from those we describe here. The most noticeable differences are that larvae of N. bouvieri are comparatively more elongate and bigger, and have the last two thoracic terga darker than the first thoracic tergum and the abdominal terga. In the larvae of N. brunneus we describe here, the color of all thoracic and abdominal terga is similar, being mostly dark brown. We observed larvae pupating in the laboratory and adults emerging from them, therefore we can assure that the larvae we observed and collected are conspecific to pupae and adults. The same cannot be said about larvae and adults of N. bouvieri and N. lutzi described by Ihering (1914Ihering R von (1914) As espécies brazileiras de Nilionidas (Coleopteros) e a posição systematica da família, pelo estudo das larvas. Revista do Museu Paulista 9: 281-306.). In his work, Ihering (1914Ihering R von (1914) As espécies brazileiras de Nilionidas (Coleopteros) e a posição systematica da família, pelo estudo das larvas. Revista do Museu Paulista 9: 281-306.) described larvae and adults of N. bouvieri in detail, the larvae being about 9 mm long and 4 mm wide, and adults only 6.5 mm long and 5.7 mm wide, which is much unexpected in Nilio , in which adults are usually similar in size to mature larvae (see Gil-Santana & Marques 2008Gil-Santana HR, Marques OM (2008) Contribuição ao conhecimento de Nilio barthi Costa Lima & Seabra (Coleoptera: Tenebrionidae: Nilioninae). Boletín de la Sociedad Entomológica Aragonesa 42: 271-278. and Simões et al. 2009Simões MVP, Quintino HYS, Monné ML (2009) Larva and pupa of Nilio (Linio) lanatus Germar, 1824 (Coleoptera: Tenebrionidae). Zootaxa 2175: 51-56.). It is important to note that for N. lutzi he provided a complete description only for adults, which were 8 mm long and 7 mm wide, but mentioned that larvae of N. lutzi were darker than the ones of N. bouvieri . The author did not mention whether ecdyses were observed, in order to make sure that collected larvae and adults were conspecific. Another interesting fact is that the type locality of N. bouvieri is "Ypiranga" and that of N. lutzi is "Cantareira", which are separated by less than 15Km. So close that we shall consider the possibility that both species are sympatric. In our opinion, the larvae of N. bouvieri described by Ihering (1914Ihering R von (1914) As espécies brazileiras de Nilionidas (Coleopteros) e a posição systematica da família, pelo estudo das larvas. Revista do Museu Paulista 9: 281-306.) are probably of N. lutzi , and vice versa. Moreover, the dark larvae Ihering (1914Ihering R von (1914) As espécies brazileiras de Nilionidas (Coleopteros) e a posição systematica da família, pelo estudo das larvas. Revista do Museu Paulista 9: 281-306.) assumed to be of N. lutzi match the coloration of the larvae of N. brunneus we describe here. Although we have not examined the type series of N. bouvieri , we agree that it is possibly a true synonym of N. brunneus , as proposed by Mader (1936Mader L (1936) Bestimmungstabelle der Coleopterenfamilie Nilionidae. Entomologisches Nachrichtenblatt 10(2): 73-102.) and not questioned up to date. The diagnostic features of adult N. bouvieri that would separate it from N. brunneus (antennae light colored at base and gradually darkening to apex, presence of fine punctation between elytral rows of coarse punctures and elytral suture conspicuous) are intraspecific variations found in adult N. brunneus .
Nilioninae is classified within the lagrioid branch, together with Lagriinae and Phrenapatinae, mostly by its larval characters. However, some of those characters may be the results of adaptive convergence, such as the overall body shape of larvae. Other character that places Nilioninae close to Lagriinae is the halteriform process found in pupae of Nilio , being similar to the "processii motorii" found in pupae of Lagria hirta (Linnaeus, 1758), but both probably have different functions and origins. Given the highly specialized female terminalia, bearing window of bursa, check valve, laterally inserted gonostyli, which are considered advanced character states (Tschinkel & Doyen 1980Tschinkel WR, Doyen JT (1980) Comparative anatomy of the defensive glands, ovipositor and female genital tubes of tenebrionid beetles (Coleoptera). International Journal of Insect Morphology and Embryology 9: 321-368.), the "eleodine type" of defensive glands (Fig. 21) and ovipositor, as well as the symmetrical hypopharyngeal sclerome, it is possible that Nilioninae is, in fact, close to Diaperinae as a highly specialized tenebrionoid. But as discussed by Tchisnkel & Doyen (1980Tschinkel WR, Doyen JT (1980) Comparative anatomy of the defensive glands, ovipositor and female genital tubes of tenebrionid beetles (Coleoptera). International Journal of Insect Morphology and Embryology 9: 321-368.), those characters may have appeared independently several times.
ACKNOWLEDGMENTS
We wish to express our thanks to Samuel Hosken for confirming the identification of the host tree. Financial support was provided by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG: doctor degree grant to the senior author, Universal APQ-00653-12, PPM-00026-14), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq: Universal 479737/2012-6, research grant to CLA 307116/2015-8) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES: PVE 88881.030447/2013-01).
LITERATURE CITED
- Beccaloni J (2009) Arachnids. Collinwood, CSIRO Publishing, 320p.
- Braun H (2011) The Little Lichen Dragon - an extraordinary katydid from the Ecuadorian Andes (Orthoptera, Tettigoniidae, Phaneropterinae, Dysoniini). Zootaxa 3032: 33-39.
- Brodo IM, Sharnoff SD, Sharnoff S (2001) Lichens of North America. New Haven, Yale University Press, 795p.
- Costa C, Vanin SA, Casari-Chen SA (1988) Larvas de Coleoptera do Brasil. São Paulo, Universidade de São Paulo, 282p.
- Elix JA, Stocker-Wörgötter E (2008) Biochemistry and secondary metabolites, p. 106-135. In: Nash III TH (Ed.) Lichen Biology. Cambridge, Cambridge University Press, 2nd ed., 489p.
- Evenhuis NL (2008) A compendium of zoological type nomenclature: A reference source. Honolulu, Bishop Museum Technical Report 41, 23p.
- Fleig M, Grüniger W, Mayer WE, Hampp R (2008) Liquens da Floresta com Araucária no Rio Grande do Sul/Lichens of the Araucaria Forest of Rio Grande do Sul/Flechten des Araukarienwaldes von Rio Grande do Sul. Pró-Mata: Guia de Campo nº 3/Field Guide No. 3/Naturführer Nr. 3. Tübingen, University of Tübingen, 217p.
- Gil-Santana HR, Marques OM (2008) Contribuição ao conhecimento de Nilio barthi Costa Lima & Seabra (Coleoptera: Tenebrionidae: Nilioninae). Boletín de la Sociedad Entomológica Aragonesa 42: 271-278.
- Ihering R von (1914) As espécies brazileiras de Nilionidas (Coleopteros) e a posição systematica da família, pelo estudo das larvas. Revista do Museu Paulista 9: 281-306.
- Jorge ME (1974) Immature stages of Nilionidae: a contribution toward the taxonomic position of the family (Coleoptera). Revista Brasileira de Entomologia 18(4): 123-128.
- Lawrence JF, Slipinski A, Seago AE, Thayer MK, Newton AF, Marvaldi AE (2011) Phylogeny of the Coleoptera based on morphological characters of adults and larvae. Annales Zoologici 61(1): 1-217.
- Mader L (1936) Bestimmungstabelle der Coleopterenfamilie Nilionidae. Entomologisches Nachrichtenblatt 10(2): 73-102.
- Nash III TH (2008) Nutrients, elemental accumulation, and mineral cycling. p. 236-253. In: Nash III TH (Ed.) Lichen Biology. Cambridge, Cambridge University Press, 2nd ed., 489p.
- Poinar Jr G, Brown AE (2011) Descriptions of a broad-nosed weevil (Eudiagogini: Curculionidae) and false ladybird beetle (Nilionini: Nilionidae) in Dominican amber. Historical Biology 23(2-3): 231-235.
- Simões MVP, Quintino HYS, Monné ML (2009) Larva and pupa of Nilio (Linio) lanatus Germar, 1824 (Coleoptera: Tenebrionidae). Zootaxa 2175: 51-56.
- Tschinkel WR, Doyen JT (1980) Comparative anatomy of the defensive glands, ovipositor and female genital tubes of tenebrionid beetles (Coleoptera). International Journal of Insect Morphology and Embryology 9: 321-368.
Publication Dates
-
Publication in this collection
2016
History
-
Received
19 Nov 2015 -
Reviewed
19 Jan 2016 -
Accepted
24 Jan 2016