Abstract
Purpose:
To investigate the impact of Ramipril (RAM) on the expressions of insulin-like growth factor-1 (IGF-1) and renal mesangial matrix (RMM) in rats with diabetic nephropathy (DN).
Methods:
The Sprague Dawley rats were divided into normal control (NC) group (n = 12), DN group (n = 11), and DN+RAM group (n = 12). The ratio of renal weight to body weight (RBT), fasting blood glucose (FBG), HbA1c, 24-h urine protein (TPU), blood urea nitrogen (BUN), creatinine (Cr), renal pathological changes, the levels of IGF-1, fibronectin (FN), type IV collagen (Col-IV), and matrix metalloproteinases (MMP)-2 were compared among the groups.
Results:
Compared with NC group, the RBT, FBG, HbA1c, TPU, BUN, Cr, and RMM in DN group were significantly increased (P < 0.05), the IGF-1, FN, and Col-IV were significantly upregulated (P < 0.05), while MMP was significantly downregulated (P < 0.05). Compared with DN group, the indexes except for the FBG and HbA1c in DN+RAM group were significantly improved (P < 0.05), among which IGF-1 exhibited significant positive correlation with TPU(r=0.937), FN(r=0.896) and Col-IV(r=0.871), while significant negative correlation with MMP-2 (r=-0.826) (P<0.05).
Conclusion:
RAM may protect the kidneys by suppressing IGF-1 and mitigating the accumulation of RMM.
Key words:
Ramipril; Insulin-Like Growth Factor I; Diabetic Nephropathies; Rats
Introduction
Diabetes mellitus (DM) is a chronic metabolic disease severely threatening the public’s health. It is involved in many organs of the body with high mortality and morbidity. One epidemiological investigation has shown that the prevalence of DM in Chinese adults in 2013 was increased to 11.6%, and the total number had reached 114 million11 Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D, Zhou M, Tang X, Hu Y, Wang L. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515-23 doi: 10.1001/jama.2017.7596.
https://doi.org/10.1001/jama.2017.7596...
,22 Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J, Xu M, Li Y, Hu N, Li J, Mi S, Chen CS, Li G, Mu Y, Zhao J, Kong L, Chen J, Lai S, Wang W, Zhao W, Ning G; 2010 China Noncommunicable Disease Surveillance Group. Prevalence and control of diabetes in chinese adults. JAMA. 2013;310(9):948-59. doi: 10.1001/jama.2013.168118.
https://doi.org/10.1001/jama.2013.168118...
. Diabetic nephropathy (DN) is a common chronic complication of DM as well as the leading cause of end stage renal failure (ESRD) and death33 Zhang L, Long J, Jiang W, Shi Y, He X, Zhou Z, Li Y, Yeung RO, Wang J, Matsushita K, Coresh J, Zhao MH, Wang H. Trends in chronic kidney disease in China. N Engl J Med. 2016;375(9):905-6. doi: 10.1056/NEJMc1602469.
https://doi.org/10.1056/NEJMc1602469...
,44 Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, Bragg-Gresham J, Balkrishnan R, Chen JL, Cope E, Eggers PW, Gillen D, Gipson D, Hailpern SM, Hall YN, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Kalantar-Zadeh K, Kovesdy CP, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, Nguyen DV, O'Hare AM, Plattner B, Pisoni R, Port FK, Rao P, Rhee CM, Sakhuja A, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, White S, Woodside K, Hirth RA. US Renal Data System 2015 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2016;67(3 Suppl 1):Svii, S1-305. doi: 10.1053/j.ajkd.2015.12.014.
https://doi.org/10.1053/j.ajkd.2015.12.0...
. The characteristic pathological changes of DN are glomerular mesangial cell hypertrophy, basement membrane thickening, and excessive accumulation of extracellular matrix, which thus cause progressive glomerular sclerosis. The pathogenesis is complex and has not been fully elucidated yet55 Pofi R, Di Mario F, Gigante A, Rosato E, Isidori AM, Amoroso A, Cianci R, Barbano B. Diabetic nephropathy: focus on current and future therapeutic strategies. Curr Drug Metab. 2016;17(5):497-502. doi: 10.2174/138920021705160324165553.
https://doi.org/10.2174/1389200217051603...
,66 Macisaac RJ, Ekinci EI, Jerums G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis. 2014;63(2 Suppl 2):S39-62. doi: 10.1053/j.ajkd.2013.10.048.
https://doi.org/10.1053/j.ajkd.2013.10.0...
. Existing studies have shown that the accumulation of mesangial extracellular matrix is one of the most prominent pathological changes in DN, and it’s also the important pathological basis for nodular or diffuse glomerulosclerosis and the occurrence and development of DN77 Ponchiardi C, Mauer M, Najafian B. Temporal profile of diabetic nephropathy pathologic changes. Curr Diab Rep. 2013;13(4):592-9. doi: 10.1007/s11892-013-0395-7.
https://doi.org/10.1007/s11892-013-0395-...
,88 Kolset SO, Reinholt FP, Jenssen T. Diabetic nephropathy and extracellular matrix. J Histochem Cytochem. 2012;60(12):976-86. doi: 10.1369/0022155412465073.
https://doi.org/10.1369/0022155412465073...
. Therefore, exploring the mechanism of mesangial extracellular matrix accumulation and new therapeutic targets have become hot research spots.
Insulin-like growth factor-1 (IGF-1) is a single-chain growth factor composed of 70 amino acids, and has similar structure and functions to insulin such as promoting cell differentiation and proliferation, and exhibiting insulin-like metabolic roles99 Ding J, Sullivan DA. The effects of insulin-like growth factor 1 and growth hormone on human meibomian gland epithelial cells. JAMA Ophthalmol. 2014;132(5):593-9. doi: 10.1001/jamaophthalmol.2013.8295.
https://doi.org/10.1001/jamaophthalmol.2...
. Levin-Iaina et al.1010 Levin-Iaina N, Iaina A, Raz I. The emerging role of NO and IGF-1 in early renal hypertrophy in STZ-induced diabetic rats. Diabetes Metab Res Rev. 2011;27(3):235-43. doi: 10.1002/dmrr.1172.
https://doi.org/10.1002/dmrr.1172...
reported the renal IGF-1 protein is significantly upregulated in rats with Streptozotocin (STZ)-induced early DM, which is also related to renal compensatory hypertrophy and high filtration; Li et al.1111 Li X, Wu TT, Chen J, Qiu W. Elevated expression levels of serum insulin-like growth factor-1, tumor necrosis factor-a and vascular endothelial growth factor 165 might exacerbate type 2 diabetic nephropathy. J Diabetes Investig. 2017;8(1):108-14. doi: 10.1111/jdi.12542.
https://doi.org/10.1111/jdi.12542...
found the serum IGF-1 level is significantly increased in patients with type 2 diabetic nephropathy, which also increases the progress of DN, thus suggesting that IGF-1 may be closely related to the occurrence and development of DN1212 Bach LA, Hale LJ. Insulin-like growth factors and kidney disease. Am J Kidney Dis. 2015;65(2):327-36. doi: 10.1053/j.ajkd.2014.05.024.
https://doi.org/10.1053/j.ajkd.2014.05.0...
. Previous studies have shown that angiotensin converting-enzyme inhibitors (ACEI) can improve the synthesis of mesangial matrix, reduce proteinuria in DN patients, and delay disease progression, so it has become a guide drug for the treatment of DN1313 Strippoli GF, Bonifati C, Craig M, Navaneethan SD, Craig JC. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst Rev. 2006;(4):CD006257. doi: 10.1002/14651858.CD006257.
https://doi.org/10.1002/14651858.CD00625...
. However, studies about the roles of IGF-1are rare in China and abroad. This study observed the effects of ramipril (RAM) on the expression changes of IGF-1, fibronectin (FN), type IV collagen (Col-IV), and matrix metalloproteinases (MMP)-2 in STZ-induced DN rats, hoping to explore the possible mechanism of ACEI in treating DN.
Methods
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Anhui Medical University.
A total of 36 healthy SPF-grade 8-week-old male Sprague Dawley rats, body weight 190 ± 10g, were provided by the Experimental Center of Anhui Medical University (Certificate No. SCXK (Wan) 2005-001) and bred with free diet and water for one-week adaption. This study was conducted in the central laboratory of Anhui Provincial Hospital Affiliated to Anhui Medical University.
The rats were then divided into group normal control (NC, n = 12), group DN (n = 12), and group DN+RAM (n = 12) using the random number method. The rats in group DN and DN+RAM were intraperitoneally injected 60 mg/kg STZ (Sigma-Aldrich, St. Louis, USA) after 14-h fasting, and sampled the venous blood from the tail vein 48 h, 72 h, 1 w, 2 w, and 4 w later, together with the urine samples. The signs of blood glucose >16.7 mmol/L and urinary protein excretion rate > 30 mg/24 h can be seen as the successful establishment of the DN rat model1414 Song Z, Guo Y, Zhou M, Zhang X. The PI3K/p-Akt signaling pathway participates in calcitriol ameliorating podocyte injury in DN rats. Metabolism. 2014;63(10):1324-33. doi: 10.1016/j.metabol.2014.06.013.
https://doi.org/10.1016/j.metabol.2014.0...
. In group DN, one rat was excluded due to not achieving the blood glucose standard. Group NC was intraperitoneally injected the equal volume of citrate buffer. After succeeding in model preparation, group DN+RAM was orally administrated 3 mg/kg/d RAM (Sanofi-Aventis, Paris, France, 2.5 mg/tablet, approval number: H20040517) for 8 weeks , while group NC and DN were given the equal volume of distilled water. During the experiment, standard diet and free water were supplied while no insulin was applied.
Specimen collection
Each rat was collected 24-h urine at the end of the 8th week, after which the rat was killed by intraperitoneally injecting 10% chloral hydrate, sampled t 4-5 mL of cardiac blood for detecting the biochemical indicators in the serum. The left kidney was weighed so as to calculate the ratio of renal weight/body weight (mg/g); partial renal tissue was then fixed in 10% neutral formalin and embedded by paraffin; the rest was cut on ice and stored at -80 °C.
Determination of biochemical indicators
The fasting blood glucose (FBG), blood urea nitrogen (BUN) and serum creatinine (Cr) were determined using one Hitachi 7600-020 automatic biochemical analyzer (Hitachi, Ltd., Tokyo, Japan). The HbA1c values were detected by the HPLC method (VARIANT II Hemoglobin A1c Testing System, BIO-RAD Corp., Hercules, CA, USA) and the 24-hours proteinuria (TPU) was measured by immunoturbidimetry. All biochemical indicators were assayed in the biochemical laboratory of Anhui Provincial Hospital Affiliated to Anhui Medical University.
Renal pathology
The paraffin sections were prepared consecutive slices with 2-3 mm in thickness, followed by conventional dewaxing, HE staining, and observing the histopathological changes under one light microscope (400 times). Each image was taken randomly 10 different visual fields. Partial renal cortex was cut into l mm3 cubes, fixed with 2.5% glutaraldehyde, prepared the ultra-thin slices, preformed Pb-U staining, and observed the renal pathological changes using one JEM-1230 transmission electron microscope (TEM, JEOL Ltd., Tokyo, Japan).
Immunohistochemistry
The expression of IGF-1 in the renal tissue was detected by the Elivision method: The paraffin sections were prepared consecutive slices with 4 mm in thickness, followed by dewaxing, hydration, 3% H2O2 blocking (to eliminate the activity of endogenous peroxidase), PBS washing, and overnight incubation with 50 μl of rabbit anti-rat IGF-1 antibody (Boster Ltd., Wuhan, China) at 4°C (PBS was used to replace the primary antibody as the negative control). The slices were then added the polymer enhancer and the enzyme-labeled goat anti-rabbit polymer (Maxim Bioengineering Ltd. Fuzhou, China) drop wisely for 20-min culture at 37°C, followed by drop wisely adding DAB reagent and microscopically controlled coloration, hematoxylin re-staining, dehydrating, hyalinization, and gum mounting. 20 visual fields in the cortex region were randomly selected under light microscope (×200) and analyzed the accumulative optical density and total area of IGF-1 positive signal by Image Pro Plus 6.0; the average optical density (AOD) of the positive signal was then calculated.
Western blot
100 mg of cryopreserved renal tissue was added 1 ml of total protein extract solution for 30-min ice lysis, followed by 15-min centrifugation at 4°C and 12000 r/min, and the supernatant was then taken to determine the total protein concentration by BCA. 60 μg of the protein was electrophoresed by SDS-PAGE and then transferred onto one PVDF membrane at 260 mA for 90 min; the membrane was then blocked with 5% nonfat dry milk for 1 h, added rabbit anti-rat IGF-1, FN, Col-IV, and MMP-2 monoclonal antibodies (diluted 1: 1000, Santa Cruz, CA, USA), respectively, and incubated overnight at 4°C. After washed with TBST, the membrane was added the horseradish peroxidase (HRP) labeled secondary antibody, incubated at room temperature after 1 h, and performed enhanced chemiluminecence (ECL) coloration. The bands were analyzed by Quantity One software, and the relative expressions of the target proteins were expressed referring to β-actin in the same specimen.
Statistical analysis
SPSS 19.0 was used for the statistical analysis; the measurement data were expressed as ±s; the multi-group comparison used the single factor analysis of variance, and comparison between two groups used the SNK test; the correlation analysis used the Pearson linear correlation analysis, with P <0.05 considered as statistically significance.
Results
General conditions and biochemical indexes
Compared with group NC, the rats in group DN appeared such obvious symptoms as polydipsia, polyphagia, and polyuria, as well as poor spirit and activity, after 8-week medication. The symptoms in group DN+RAM were improved than group DN. Compared with group NC, the RBT (6.31±0.85 vs. 2.76±0.24 mg/g), FBG (24.53±1.21 vs. 5.07±0.38 mmol/L), HbA1c (11.46±0.67 vs. 4.72±0.55%), TPU (120.52±24.39 vs. 3.49±1.16 mg), BUN (19.39±2.07 vs. 7.84±0.61), Cr (97.82±8.65 vs. 34.96±5.17 μmol/L) in group DN were significantly increased (P <0.05). Compared with group DN, there was no significant difference in other indexes except for FBG (23.96±2.03 vs. 24.53±1.21 mmol/L) and HbA1c (11.28±0.81 vs. 11.46±0.67%) in group DN+RAM (P> 0.05) (Table 1).
The RBT (6.31±0.85 vs. 2.76±0.24 mg/g), FBG (24.53±1.21 vs. 5.07±0.38 mmol/L), HbA1c (11.46±0.67 vs. 4.72±0.55%), TPU (120.52±24.39 vs. 3.49±1.16 mg), BUN (19.39±2.07 vs. 7.84±0.61), Cr (97.82±8.65 vs. 34.96±5.17 μmol/L), and RMM in group DN were significantly increased, the IGF-1 (4.53±0.16 vs. 2.17±0.10), FN (3.87±0.19 vs. 2.56±0.15), and Col-IV (3.46±0.20 vs. 2.23±0.25) were significantly upregulated, while MMP (2.20±0.19 vs. 3.41±0.18) was significantly downregulated. Compared with group DN, the indexes except for the FBG (23.96±2.03 vs. 24.53±1.21 mmol/L) and HbA1c (11.28±0.81 vs. 11.46±0.67%) in group DN+RAM were significantly improved, among which IGF-1 and TPU (r=0.937), as well as FN (r=0.896) and Col-IV (r=0.871), exhibited significant positive correlation, while significant negative correlation with MMP-2 (r=-0.826, P<0.05).
Renal histopathological changes
Light microscopy (×400)
The glomerular appearance and structure in group NC showed no significant change, while compares with group NC, group DN exhibited glomerular hypertrophy, mesangial cell proliferation, significantly increased extracellular matrix, and significantly enlarged mesangial region. Compared with group DN, group DN+RAM exhibited significant improvement of the above pathological changes, the renal glomerulus reduce, the glomerular mesangial cells reduced, and the extracellular matrix only exhibited mild hyperplasia (Fig. 1).
Pathological changes of renal tissue at the end of the 8th week (HE staining, ×400). A: NC; B: DN; C: DN+RAM.
TEM
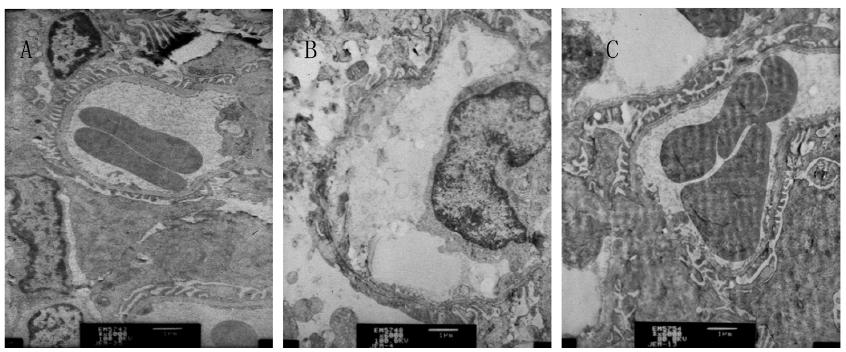
Compared with group NC, the glomerular basement membrane in group DN was thickened, the mesangial matrix was proliferated and swollen, together with the deposition of a little electron dense material. Compared with group DN, the glomerular basement membrane in group DN+RAM showed no obvious thickening, and the mesangial matrix appeared mild hyperplasia (Fig. 2).
Changes of renal tissue of the three groups by TEM (Pb-U staining, ×6000). A: NC; B: DN; C: DN+RAM.
Expressions of IGF-1 by immunohistochemistry
Immunohistochemisty
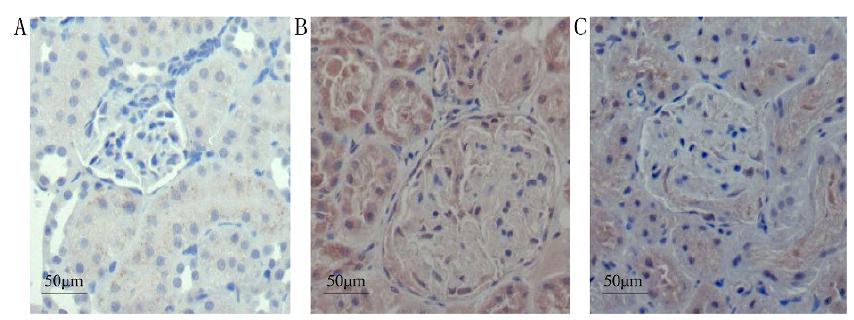
The expression of IGF-1 in group NC was minor, but that in group DN was significantly increased, exhibiting staining in the cytoplasm and nuclei, mainly in the glomerular mesangial region, as well as in the renal tubules, with the AOD value as 0.37 ± 0.02. Group DN+RAM exhibited reduced staining in the glomerular mesangial region, with the AOD value as 0.24 ± 0.03, significantly lower than group DN (P <0.05), but still significantly higher than group NC (P <0.05) (Fig. 3).
Immunohistochemisty of IGF-1in renal tissue of the three groups (Elivision, ×400). A: NC; B: DN; C: DN+RAM.
Expressions of IGF-1, FN, Col-IV, and MMP-2 by western blot
Western blot
Compared with group NC, the proteins of IGF-1 (4.53±0.16 vs. 2.17±0.10), FN (3.87±0.19 vs. 2.56±0.15), and Col-IV (3.46±0.20 vs. 2.23±0.25) were significantly increased in group DN, while the MMP-2 protein (2.20±0.19 vs. 3.41±0.18) was significantly decreased (P <0.05). Compared with group DN, the IGF-1 (3.24±0.15 vs. 4.53±0.16), FN (3.11±0.12 vs. 3.87±0.19) and Col-IV (2.98±0.19 vs. 3.46±0.20) proteins in group DN+RAM were significantly downregulated, while the MMP-2 protein (2.83±0.14 vs. 2.20±0.19) was significantly upregulated (P <0.05) (Table 2).
Correlation analysis
The Pearson correlation analysis showed that the expression of IGF-1 protein was significantly positively correlated with TPU (r = 0.937), FN (r = 0.896), and Col-IV (r = 0.871), while significantly negatively correlated with MMP-2 (r = -0.826) (P <0.05).
Discussion
The pathogenesis of DN is complex and may be related to gene, glucose metabolic disorder, hemodynamic disturbance, insulin resistance, oxidative stress, or immunoinflammatory reaction, and it has been a hot spot all over the world. The main pathological features of DN are glomerular mesangial cell hypertrophy, basement membrane thickening, mesangial cell proliferation, and excessive accumulation of extracellular matrix, as well as glomerular and renal tubulointerstitial fibrosis in late stages, which eventually leads to renal failure. The excessive accumulation of extracellular matrix in the mesangial area is the most important pathologic feature of DN, and it is the common result of the increase of FN, Col-IV, and laminin (LN) and the decrease of MMP-266 Macisaac RJ, Ekinci EI, Jerums G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis. 2014;63(2 Suppl 2):S39-62. doi: 10.1053/j.ajkd.2013.10.048.
https://doi.org/10.1053/j.ajkd.2013.10.0...
. MMP-2 is a zinc-dependent matrix metalloproteinase, as the main gelatinase that degrades FN, Col-IV, and LN in the mesangium, it can reduce the accumulation of extracellular matrix and plays an important role in the occurrence and development of DN1515 Takamiya Y, Fukami K, Yamagishi S, Kaida Y, Nakayama Y, Obara N, Iwatani R, Ando R, Koike K, Matsui T, Nishino Y, Ueda S, Cooper ME, Okuda S. Experimental diabetic nephropathy is accelerated in matrix metalloproteinase-2 knockout mice. Nephrol Dial Transplant. 2013;28(1):55-62. doi: 10.1093/ndt/gfs387.
https://doi.org/10.1093/ndt/gfs387...
,1616 Xu X, Xiao L, Xiao P, Yang S, Chen G, Liu F, Kanwar YS, Sun L. A glimpse of matrix metalloproteinases in diabetic nephropathy. Curr Med Chem. 2014;21(28):3244-60. doi: 10.2174/0929867321666140716092052.
https://doi.org/10.2174/0929867321666140...
.
The Western blot results in this study showed that the MMP-2 protein in group DN was significantly reduced, while the FN and Col-IV proteins were significantly increased. The immunohistochemical and Western blot results showed that the IGF-1 and IGF-1 proteins in group DN were significantly increased, negatively correlated with MMP-2, while positively correlated with TPU, FN, and Col-IV, suggesting that IGF-1 may downregulate MMP-2, which reduces its roles of degrading the FN and Col-IV proteins, thus increasing the deposition of mesangial matrix and resulting in the increase of TPU. After 8 weeks of treatment with RAM, the RBT, TPU, BUN, Cr, and the protein expressions of FN, Col-IV, and IGF-1 were significantly reduced, while MMP-2 was significantly increased. The pathological results also showed that group DN exhibited more serious renal hypertrophy and the accumulation of a large number of mesangial matrix, which were improved after applied RAM, suggesting that RAM may improve the accumulation of mesangial matrix and play a renal protective role by inhibiting the expression of IGF-1 protein.
IGF-1 is a single-chain protein encoded by chromosome 12, consisting of 70 amino acids and with a molecular weight of about 7500; it has nearly 50% of structural homology with insulin99 Ding J, Sullivan DA. The effects of insulin-like growth factor 1 and growth hormone on human meibomian gland epithelial cells. JAMA Ophthalmol. 2014;132(5):593-9. doi: 10.1001/jamaophthalmol.2013.8295.
https://doi.org/10.1001/jamaophthalmol.2...
. IGF-1 is synthesized and secreted by the liver, kidneys, bones, and fat, and distributed widely in various tissues. It binds to specific IGF-1 receptors by autocrine or paracrine, thus promoting the cell proliferation and differentiation, inhibiting the apoptosis, promoting the protein synthesis, etc.; in addition, it has a certain cross-role with insulin receptors99 Ding J, Sullivan DA. The effects of insulin-like growth factor 1 and growth hormone on human meibomian gland epithelial cells. JAMA Ophthalmol. 2014;132(5):593-9. doi: 10.1001/jamaophthalmol.2013.8295.
https://doi.org/10.1001/jamaophthalmol.2...
. Kong et al.1717 Kong YL, Shen Y, Ni J, Shao DC, Miao NJ, Xu JL, Zhou L, Xue H, Zhang W, Wang XX, Lu LM. Insulin deficiency induces rat renal mesangial cell dysfunction via activation of IGF-1/IGF-1R pathway. Acta Pharmacol Sin. 2016;37(2):217-27. doi: 10.1038/aps.2015.128.
https://doi.org/10.1038/aps.2015.128...
found that insulin deficiency can upregulate the expressions of IGF-1 and its receptor IGF-1R in the renal mesangial cells and DN rats’ kidneys, thus causing mesangial cell proliferation as well as the increase of serum creatinine and urinary proteins; Singh et al.1818 Singh LP, Jiang Y, Cheng DW. Proteomic identification of 14-3-3zeta as an adapter for IGF-1 and Akt/GSK-3beta signaling and survival of renal mesangial cells. Int J Biol Sci. 2006;3(1):27-39. doi:10.7150/ijbs.3.27.
https://doi.org/10.7150/ijbs.3.27...
found through proteomics that the overexpression of IGF-1 can activate the Akt/GSK-3β signal pathway, thus promoting the growth of mesangial cells and protein synthesis in DN. Many studies suggest that the overexpression of IGF-1 is an important risk factor for DN progression. This study further confirmed the role of IGF-1 in mesangial matrix aggregation in DN and suggest that it may be related to the expression inhibition of MMP-2.
A large number of studies have confirmed that ACEI drugs can reduce the activity of the renin-angiotensin-aldosterone system, thereby improving the renal hemodynamics and playing a protective effect1313 Strippoli GF, Bonifati C, Craig M, Navaneethan SD, Craig JC. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst Rev. 2006;(4):CD006257. doi: 10.1002/14651858.CD006257.
https://doi.org/10.1002/14651858.CD00625...
. Recent studies have shown that ACEIs can play roles through non-hemodynamic factors, such as reducing inflammatory responses, reducing oxidative stress, inhibiting matrix protein synthesis, etc1919 Ertürküner SP, Basar M, Tunçdemir M, Seçkin I. The comparative effects of perindopril and catechin on mesangial matrix and podocytes in the streptozotocin induced diabetic rats. Pharmacol Rep. 2014;66(2):279-87. doi: 10.1016/j.pharep.2013.09.010.
https://doi.org/10.1016/j.pharep.2013.09...
,2020 Zhang MZ, Wang S, Yang S, Yang H, Fan X, Takahashi T, Harris RC. Role of blood pressure and the renin-angiotensin system in development of diabetic nephropathy (DN) in eNOS-/- db/db mice. Am J Physiol Renal Physiol. 2012;302(4):F433-8. doi: 10.1152/ajprenal.00292.2011.
https://doi.org/10.1152/ajprenal.00292.2...
. McLennan found that perindopril can inhibit the degradation of mesangial matrix in DN rats by upregulating MMP-22121 McLennan SV, Kelly DJ, Cox AJ, Cao Z, Lyons JG, Yue DK, Gilbert RE. Decreased matrix degradation in diabetic nephropathy: effects of ACE inhibition on the expression and activities of matrix metalloproteinases. Diabetologia. 2002;45(2):268-75. doi: 10.1007/s00125-001-0730-4.
https://doi.org/10.1007/s00125-001-0730-...
. The results of this study were consistent with previous studies, and further suggest that the specific mechanism may be through inhibiting the expression of IGF-1. It is noticeable that this study didn’t find RAM can significantly improve the FBG and HbA1c in DN rats, suggesting that the expression of IGF-1 is independent from the changes of blood glucose.
In short, RAM may inhibit the expression of IGF-1 in the renal tissue of DN rats, and upregulate the expression of MMP-2, thus reducing the accumulation of mesangial matrix and delaying the progression of DN, which may exhibit significance for clinical treatments.
References
-
1Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D, Zhou M, Tang X, Hu Y, Wang L. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515-23 doi: 10.1001/jama.2017.7596.
» https://doi.org/10.1001/jama.2017.7596 -
2Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J, Xu M, Li Y, Hu N, Li J, Mi S, Chen CS, Li G, Mu Y, Zhao J, Kong L, Chen J, Lai S, Wang W, Zhao W, Ning G; 2010 China Noncommunicable Disease Surveillance Group. Prevalence and control of diabetes in chinese adults. JAMA. 2013;310(9):948-59. doi: 10.1001/jama.2013.168118.
» https://doi.org/10.1001/jama.2013.168118 -
3Zhang L, Long J, Jiang W, Shi Y, He X, Zhou Z, Li Y, Yeung RO, Wang J, Matsushita K, Coresh J, Zhao MH, Wang H. Trends in chronic kidney disease in China. N Engl J Med. 2016;375(9):905-6. doi: 10.1056/NEJMc1602469.
» https://doi.org/10.1056/NEJMc1602469 -
4Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, Bragg-Gresham J, Balkrishnan R, Chen JL, Cope E, Eggers PW, Gillen D, Gipson D, Hailpern SM, Hall YN, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Kalantar-Zadeh K, Kovesdy CP, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, Nguyen DV, O'Hare AM, Plattner B, Pisoni R, Port FK, Rao P, Rhee CM, Sakhuja A, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, White S, Woodside K, Hirth RA. US Renal Data System 2015 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2016;67(3 Suppl 1):Svii, S1-305. doi: 10.1053/j.ajkd.2015.12.014.
» https://doi.org/10.1053/j.ajkd.2015.12.014 -
5Pofi R, Di Mario F, Gigante A, Rosato E, Isidori AM, Amoroso A, Cianci R, Barbano B. Diabetic nephropathy: focus on current and future therapeutic strategies. Curr Drug Metab. 2016;17(5):497-502. doi: 10.2174/138920021705160324165553.
» https://doi.org/10.2174/138920021705160324165553 -
6Macisaac RJ, Ekinci EI, Jerums G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis. 2014;63(2 Suppl 2):S39-62. doi: 10.1053/j.ajkd.2013.10.048.
» https://doi.org/10.1053/j.ajkd.2013.10.048 -
7Ponchiardi C, Mauer M, Najafian B. Temporal profile of diabetic nephropathy pathologic changes. Curr Diab Rep. 2013;13(4):592-9. doi: 10.1007/s11892-013-0395-7.
» https://doi.org/10.1007/s11892-013-0395-7 -
8Kolset SO, Reinholt FP, Jenssen T. Diabetic nephropathy and extracellular matrix. J Histochem Cytochem. 2012;60(12):976-86. doi: 10.1369/0022155412465073.
» https://doi.org/10.1369/0022155412465073 -
9Ding J, Sullivan DA. The effects of insulin-like growth factor 1 and growth hormone on human meibomian gland epithelial cells. JAMA Ophthalmol. 2014;132(5):593-9. doi: 10.1001/jamaophthalmol.2013.8295.
» https://doi.org/10.1001/jamaophthalmol.2013.8295 -
10Levin-Iaina N, Iaina A, Raz I. The emerging role of NO and IGF-1 in early renal hypertrophy in STZ-induced diabetic rats. Diabetes Metab Res Rev. 2011;27(3):235-43. doi: 10.1002/dmrr.1172.
» https://doi.org/10.1002/dmrr.1172 -
11Li X, Wu TT, Chen J, Qiu W. Elevated expression levels of serum insulin-like growth factor-1, tumor necrosis factor-a and vascular endothelial growth factor 165 might exacerbate type 2 diabetic nephropathy. J Diabetes Investig. 2017;8(1):108-14. doi: 10.1111/jdi.12542.
» https://doi.org/10.1111/jdi.12542 -
12Bach LA, Hale LJ. Insulin-like growth factors and kidney disease. Am J Kidney Dis. 2015;65(2):327-36. doi: 10.1053/j.ajkd.2014.05.024.
» https://doi.org/10.1053/j.ajkd.2014.05.024 -
13Strippoli GF, Bonifati C, Craig M, Navaneethan SD, Craig JC. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst Rev. 2006;(4):CD006257. doi: 10.1002/14651858.CD006257.
» https://doi.org/10.1002/14651858.CD006257 -
14Song Z, Guo Y, Zhou M, Zhang X. The PI3K/p-Akt signaling pathway participates in calcitriol ameliorating podocyte injury in DN rats. Metabolism. 2014;63(10):1324-33. doi: 10.1016/j.metabol.2014.06.013.
» https://doi.org/10.1016/j.metabol.2014.06.013 -
15Takamiya Y, Fukami K, Yamagishi S, Kaida Y, Nakayama Y, Obara N, Iwatani R, Ando R, Koike K, Matsui T, Nishino Y, Ueda S, Cooper ME, Okuda S. Experimental diabetic nephropathy is accelerated in matrix metalloproteinase-2 knockout mice. Nephrol Dial Transplant. 2013;28(1):55-62. doi: 10.1093/ndt/gfs387.
» https://doi.org/10.1093/ndt/gfs387 -
16Xu X, Xiao L, Xiao P, Yang S, Chen G, Liu F, Kanwar YS, Sun L. A glimpse of matrix metalloproteinases in diabetic nephropathy. Curr Med Chem. 2014;21(28):3244-60. doi: 10.2174/0929867321666140716092052.
» https://doi.org/10.2174/0929867321666140716092052 -
17Kong YL, Shen Y, Ni J, Shao DC, Miao NJ, Xu JL, Zhou L, Xue H, Zhang W, Wang XX, Lu LM. Insulin deficiency induces rat renal mesangial cell dysfunction via activation of IGF-1/IGF-1R pathway. Acta Pharmacol Sin. 2016;37(2):217-27. doi: 10.1038/aps.2015.128.
» https://doi.org/10.1038/aps.2015.128 -
18Singh LP, Jiang Y, Cheng DW. Proteomic identification of 14-3-3zeta as an adapter for IGF-1 and Akt/GSK-3beta signaling and survival of renal mesangial cells. Int J Biol Sci. 2006;3(1):27-39. doi:10.7150/ijbs.3.27.
» https://doi.org/10.7150/ijbs.3.27 -
19Ertürküner SP, Basar M, Tunçdemir M, Seçkin I. The comparative effects of perindopril and catechin on mesangial matrix and podocytes in the streptozotocin induced diabetic rats. Pharmacol Rep. 2014;66(2):279-87. doi: 10.1016/j.pharep.2013.09.010.
» https://doi.org/10.1016/j.pharep.2013.09.010 -
20Zhang MZ, Wang S, Yang S, Yang H, Fan X, Takahashi T, Harris RC. Role of blood pressure and the renin-angiotensin system in development of diabetic nephropathy (DN) in eNOS-/- db/db mice. Am J Physiol Renal Physiol. 2012;302(4):F433-8. doi: 10.1152/ajprenal.00292.2011.
» https://doi.org/10.1152/ajprenal.00292.2011 -
21McLennan SV, Kelly DJ, Cox AJ, Cao Z, Lyons JG, Yue DK, Gilbert RE. Decreased matrix degradation in diabetic nephropathy: effects of ACE inhibition on the expression and activities of matrix metalloproteinases. Diabetologia. 2002;45(2):268-75. doi: 10.1007/s00125-001-0730-4.
» https://doi.org/10.1007/s00125-001-0730-4
-
Financial source:
Annual Science and Technology Projects of Anhui Province (12070403059)
-
1
Research performed at Department of Nephrology, Anhui Provincial Hospital Affiliated to Anhui Medical University, Hefei 230001, China.
Publication Dates
-
Publication in this collection
2019
History
-
Received
12 Sept 2018 -
Reviewed
14 Nov 2018 -
Accepted
11 Dec 2018