Abstracts
6-phosphofructo-1-kinase (phosphofructokinase; PFK) activity from Rhodnius prolixus, a haematophagous insect which is usually a poor flyer, was measured and compared in two metabolically active tissues - flight muscle and fat body. The activity of this important regulatory glycolytic enzyme was much more pronounced in muscle (15.1 ± 1.4 U/mg) than in fat body extracts (3.6±0.4 U/mg), although the latter presented higher levels of enzyme per protein content, as measured by western-blotting. Muscle extracts are more responsible than fat body to ATP and fructose 6-phosphate, both substrates of PFK. Allosteric regulation exerted by different effectors such as ADP, AMP and fructose 2,6-phosphate presented a singular pattern for each tissue. Optimal pH (8.0-8.5) and sensitivity to pH variation was very similar, and citrate was unable to inhibit PFK activity in both extracts. Our results suggest the existence of a particular PFK activity for each tissue, with regulatory patterns that are consistent with their physiological roles.
phosphofructokinase; metabolism; insect
A atividade da fosfofrutocinase (PFK) de Rodnius prolixus, um inseto hematófago, o qual vôa somente pequenas distâncias, foi medida e comparada em dois tecidos metabolicamente ativos - músculo de asa e corpo gorduroso. A atividade desta importante enzima glicolítica regulatória foi muito mais pronunciada em músculo de asa (15,1 ±1,4 U/mg) do que em extrato de corpo gorduroso (3,6 ±0,4 U/mg) embora este último tenha apresentado níveis mais altos da enzima por quantidade de proteína, como medido por western-blotting. Extratos de músculo foram mais responsivos do que corpo gorduroso para ATP e frutose-6-fosfato, ambos substratos da PFK. A regulação alostérica exercida por diferentes efetores tais como ADP, AMP, frutose-2,6-bisfosfato apresentou um padrão singular para cada tecido. O pH ótimo (8,0-8,5) e a sensibilidade a variações de pH, foram muito similares e o citrato foi incapaz de inibir a atividade da PFK em ambos os extratos. Nossos resultados sugerem a existência de uma atividade particular da PFK para cada tecido com padrões regulatórios que são consistentes com suas funções fisiológicas.
fosfotrutocinase; metabolismo; inseto; glicólise; músculo; corpo gorduroso
BIOMEDICAL AND MEDICAL SCIENCES
Allosteric regulation of 6-phosphofructo-1-kinase activity of fat body and flight muscle from the bloodsucking bug Rhodnius prolixus
Gutemberg G. AlvesI, II; Monica M. Marinho-CarvalhoI, II; Georgia C. AtellaII; Mario A.C. Silva-NetoII; Mauro Sola-PennaI
ILaboratório de Enzimologia e Controle do Metabolismo (LabECoM), Departamento de Fármacos, Faculdade de Farmácia Universidade Federal do Rio de Janeiro, 21941-590 Rio de Janeiro, RJ, Brasil
IIInstituto de Bioquímica Médica, Programa de Biologia Molecular e Biotecnologia Universidade Federal do Rio de Janeiro, 21941-590 Rio de Janeiro, RJ, Brasil
ABSTRACT
6-phosphofructo-1-kinase (phosphofructokinase; PFK) activity from Rhodnius prolixus, a haematophagous insect which is usually a poor flyer, was measured and compared in two metabolically active tissues flight muscle and fat body. The activity of this important regulatory glycolytic enzyme was much more pronounced in muscle (15.1 ± 1.4 U/mg) than in fat body extracts (3.6±0.4 U/mg), although the latter presented higher levels of enzyme per protein content, as measured by western-blotting. Muscle extracts are more responsible than fat body to ATP and fructose 6-phosphate, both substrates of PFK. Allosteric regulation exerted by different effectors such as ADP, AMP and fructose 2,6-phosphate presented a singular pattern for each tissue. Optimal pH (8.0-8.5) and sensitivity to pH variation was very similar, and citrate was unable to inhibit PFK activity in both extracts. Our results suggest the existence of a particular PFK activity for each tissue, with regulatory patterns that are consistent with their physiological roles.
Key words: phosphofructokinase, metabolism, insect.
RESUMO
A atividade da fosfofrutocinase (PFK) de Rodnius prolixus, um inseto hematófago, o qual vôa somente pequenas distâncias, foi medida e comparada em dois tecidos metabolicamente ativos - músculo de asa e corpo gorduroso. A atividade desta importante enzima glicolítica regulatória foi muito mais pronunciada em músculo de asa (15,1 ±1,4 U/mg) do que em extrato de corpo gorduroso (3,6 ±0,4 U/mg) embora este último tenha apresentado níveis mais altos da enzima por quantidade de proteína, como medido por western-blotting. Extratos de músculo foram mais responsivos do que corpo gorduroso para ATP e frutose-6-fosfato, ambos substratos da PFK. A regulação alostérica exercida por diferentes efetores tais como ADP, AMP, frutose-2,6-bisfosfato apresentou um padrão singular para cada tecido. O pH ótimo (8,0-8,5) e a sensibilidade a variações de pH, foram muito similares e o citrato foi incapaz de inibir a atividade da PFK em ambos os extratos. Nossos resultados sugerem a existência de uma atividade particular da PFK para cada tecido com padrões regulatórios que são consistentes com suas funções fisiológicas.
Palavras-chave: fosfotrutocinase, metabolismo, inseto, glicólise, músculo, corpo gorduroso.
INTRODUCTION
The flight muscle of insects is described as a highly oxidative tissue when compared to the skeletal muscle found in other animals, and some insects present a 50-100 fold increase on their glycolytic rate upon the initiation of flight (Weis-Fough 1952, Krammer and Heinrich 1978, Wegener 1996). In insects such as Rhodnius prolixus, which presents relatively sedentary habits, although capable of sustained flight for periods longer than 1 hour (Ward and Baker 1982, Ward et al. 1982), carbohydrates may represent important substrates for oxidation on flight muscle. The majority of the carbohydrates from this tissue comes from glycogenolysis, as well as from the haemolymph, being originated directly from diet or released by the fat body (Candy and Kilby 1959, Candy et al. 1997). The fat body seems to accumulate functions of both liver and adipose tissue (Keeley 1985). This tissue represents the main storage of amino acids, glucose, trehalose and lipids in insects, and may be responsible for some degree of homeostasis of these metabolites on haemolymph (Becker et al. 2001).
6-phosphofructo-1-kinase (PFK; phosphofructokinase; ATP:D-fructose-6-phosphate-1-trans-ferase; EC2.7.1.11) catalyzes the phosphorylation of fructose-6-phosphate to fructose-1,6-bisphosphate and is a key enzyme on the regulation of glycolysis. Consequently, its activity might reflect on carbohydrate metabolism in both flight muscle and fat body. In diverse living systems, including insects, this enzyme is known to be modulated by ATP, ADP, AMP and fructose 2,6-bisphosphate, among several other effectors (for a review see Uyeda 1979, Newsholme and Leech 1983, Schirmer and Evans 1990). Khoja (1991) has shown that fructose 2,6-bisphosphate is one of the most potent activators of PFK activity in the flight and leg muscles of in the insect Poekilocerus bufonius, as well as in other of its tissues, such as the hindgut and midgut. However, several reports indicate that some well known modulators of mammal PFK activity, such as  and citrate, may have none or little effect on the insect enzyme (Walker and Bailey 1969, Newsholme et al. 1977, Leite et al. 1988, Khoja et al. 1990).
and citrate, may have none or little effect on the insect enzyme (Walker and Bailey 1969, Newsholme et al. 1977, Leite et al. 1988, Khoja et al. 1990).
In order to provide more information on the carbohydrate metabolism in insects, in this study we compare the PFK activity of extracts obtained from both flight muscle and fat body from the blood-feeding insect Rhodnius prolixus, and their regulatory behavior related to the most important effectors with physiological relevance on these tissues.
MATERIALS AND METHODS
MATERIALS
ATP, ADP, AMP, Tris, fructose 6-phosphate, fructose 2,6-bisphosphate, NADH and the coupled enzymes aldolase, triose-phosphate isomerase and a-glycerophosphate dehydrogenase were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Anti-mouse secondary IgG for the western blotting assay was obtained from Santa Cruz (CA, USA). Other reagents were of the higher purity available.
OBTAINING FAT BODY AND FLIGHT MUSCLE HOMOGENATES
Adult male Rhodnius prolixus kept in our departamental colony at 28ºC, 70% humidity and fed at 28 days intervals were used in this study. Ten days after feeding, fat body and flight muscle from 10 individuals were dissected, weighted and homogenized with a glass homogenizer in a solution containing 50 mM Tris-HCl,30 mM KF, 4 mM EDTA and 15 mM 2-mercaptoethanol, pH 7.5. The preparation was then centrifuged at 6400 rpm and the protein content of the supernatant was measured according to Lowry et al. (1951).
ASSAY OF 6-PHOSPHOFRUCTO-1-KINASE ACTIVITY
PFK activity from fat body and flight muscle homogenates were measured as previously described (Sola-Penna et al. 2002, Meira et al. 2005) in a reaction media containing 50 mM Tris-HCl pH 7.4, 0,2 mM NADH,10 mM MgCl2, ATP and fructose 6-phosphate in the concentrations indicated in the figures, plus the coupled enzymes aldolase (0.25 U/mL), triose phosphate isomerase (1 U/mL) and a-glycerophosphate dehydrogenase (4 U/mL). Reaction was started by the addition of a volume of homogenate containing 10 µg of protein, and the oxidation of NADH was monitored by 10 minutes in a spectrophotometer at 340 nm, and the linear phase was identified for each experiment. A molar extinction coefficient of 6,220 ×106 M cm2 was used for the calculation of the fructose 1,6-bisphosphate concentration.
WESTERN BLOTTING
Polyclonal antibody raised against mammalian PFK was obtained in our laboratory (Meira et al. 2005) by the subcutaneous injection of 45 days old rats weighting approximately 100 g with 250 µL of a solution containing 1 mg purified rabbit skeletal muscle PFK and complete Freund's adjuvant. A second immunization was made 15 days after by the injection of 70µL of incomplete Freund's adjuvant and 1 mg/mL PFK. IgG was colleted from 30 mL blood extracted at day 45, after centrifugation by 30 minutes at 1500 rpm. For the western blotting, samples of fat body and flight muscle homogenates containing 150 µg of protein were separated with SDS-PAGE (10%) and transferred to a nitrocellulose membrane, as confirmed by staining with Ponceau Red. The membrane was blocked, washed and incubated with 1:1000 purified anti-PFK IgG and 1:800 anti-mouse secondary antibody (Santa Cruz, CA, USA). The membrane was developed with BCIP/NBT developer (Meira et al. 2005). Rabbit muscle PFK and a set of molecular mass standards was from Sigma Chemicals Co. (St. Louis, MO, USA) were used in order to determine the molecular mass of R. prolixus PFK, in a parallel SDS-PAGE.
KINETIC AND STATISTICAL ANALYSIS
Kinetic parameters for the substrate curves were calculated by non-linear regression using the software SigmaPlot (Systat, CA, USA). Presented values are the mean ± standard errors of the parameters calculated fitting the equations bellow to the experimental data for, at least, 4 independent experiments.
where V is the enzyme rate at a given substrate concentration (S), K0.5 is the apparent affinity constant and n is the cooperativity index.
where V is the enzyme rate at a given substrate concentration (S), Ki is the apparent inhibition constant and n is the cooperativity index.
Statistical differences were calculated by Student's t-test using the software SigmaStat (Systat, CA, USA). A P < 0.05 was considered as statistically different for all experiments.
RESULTS
EFFECTS OF PH
The PFK activity from R. prolixus is dependent on the H+ concentration on both tissues studied. Figure 1A shows the activity of the enzyme measured in different pH, at fixed ATP and fructose-6-phosphate concentrations (2 and 5 mM, respectively). The activity on both extracts, after normalized by the maximal activities obtained (Fig. 1B), presented a very similar response to pH, with maximal activity found at pH values above 8 (8.2 muscle, 8.4 fat body), and a pronounced decrease at pH values lower than 7. At pH 7.4, both tissues presented more response to allosteric regulation than inoptimal pH, as previously tested, and then it was used in the experiments of allosteric regulation presented in this paper.
KINETIC BEHAVIOR
In order to compare the 6-phosphofructo-1-kinase activity from R. prolixus flight muscle and fat body we measured the kinetic parameters for the two enzyme substrates, fructose-6-phosphate and ATP. The kinetic parameters were determined measuring the initial velocity of enzyme catalysis in the function of substrate concentration. All velocity measurements were performed following the product formation in the function of time. The slope of product formation during the initial linear phase of the reaction was used to determine initial velocity, and is expressed as U/mg. One U was considered as the formation of one µmol product per minute. Figure 2 shows the curves of the initial velocity of PFK versus fructose-6-phosphate concentration for the both tissues. The enzyme velocity responds in an allosteric manner to fructose-6-phosphate and the parameters of the equation 1 were best fitted to the experimental data. The flight muscle presents a higher specific activity of the enzyme (p < 0.05), comparing to the fat body homogenates (15.14 ±0.63 U/mg and 5.3 ±0.7 U/mg, respectively; P < 0.05, Student's t-test). In addition, we observed that the K for fructose-6-phosphate is lower in the flight muscle comparing to the fat body (0.81 ±0.11 mM and 1.61 ±0.25 mM, respectively; P < 0.05, Student's t-test).
Figure 3A shows that the enzyme responds in avery similar pattern to lower concentrations (up to 3 mM) of its other substrate, ATP. In both extracts, the enzyme initial velocity responds in an allosteric manner to ATP. The parameters shown in Table I, obtained with the best fitting of experimental data to equation 1, revealed that the muscle homogenate, once again with a higher specific PFK activity, also has the lower K0.5 for this substrate (0.68 ±0.29 U/mg versus 0.95 ±0.51 U/mg, for the fat body homogenate; P < 0.05, Student's t-test). At higher concentrations (Fig. 3B), ATP becomes a potent inhibitor of PFK activity on both tissues, although with more pronounced effects on fat body extracts. In this tissue, 6 mM ATP reduced PFK activity to 25% of its maximal velocity, while the muscle homogenate, submitted to the same conditions, presented about 40% of the V. However, the best fitting of experimental data to equation 2 revealed a lower Ki for ATP at higher concentrations to the muscle enzyme, as shown in Table I. Although both extracts presented some degree of cooperativity toward ATP, we were unable to identify significant differences between them.
RESPONSE TO ALLOSTERIC REGULATION
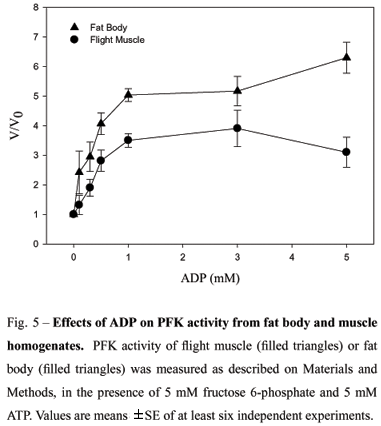
In different tissues, PFK is submitted to a tight regulation, as a result of interactions between substrates and several allosteric inhibitors and activators. Citrate is described as potentiating the inhibitory effects of ATP in several animal tissues, providing an explanation to the glucose-fatty acid-ketone body cycle. In R. prolixus, both muscle and fat body homogenates presented no change on their PFK activity (Fig. 4) as a function of increasing sodium citrate concentrations. However, other allosteric effectors, such as nucleotides, were able to markedly affect PFK activity. Figure 5 shows that ADP is able to increase the enzyme activity on both extracts, but at different potencies. At 5 mM ADP, fat body PFK activity was 6 times higher than that observed in the absence of effector, compared to an activation of 3 times found in the muscle.
Differences between fat body and flight muscle became evident again when the allosteric regulator studied was AMP (Fig. 6). This effector was able to activate PFK on both extracts, but the pattern found was dependent on the conditions tested. Figure 6A shows the effects of different AMP concentrations on the PFK activity of fat body homogenate where it can be seen that AMP was much more potent as activator at an inhibitory ATP concentration (5 mM) than when the substrate was present at a saturating stimulatory concentration (2 mM; P < 0.05, Student's t-test). However, the same experiment performed with the flight muscle homogenates showed that AMP was able to activated PFK, but no differences were observed when the experiment was performed at different ATP concentrations (Fig. 6B; P > 0.05, Student's t-test).
This dependency on ATP concentration was also found on the effects of fructose-2,6-bisphosphate, one of the most potent activators of PFK already described. At the saturating stimulatory ATP concentration (2 mM), fructose-2,6-bisphosphate was unable to activate the muscle homogenate activity (Fig. 7A, circles), while a small but significant activation (P < 0.05, Student's t-test) was observed in the fat body (Fig. 8A, circles). However, at the inhibitory ATP concentration (5 mM), fructose-2,6-bisphosphate promotes a very potent activation of the enzyme in both tissues (Fig. 7A and 8A for flight muscle and fat body, respectively). For better visualization of the fructose-2,6-bisphosphate activation on PFK activity of the insect tissues, the data presented on panels A of figures 7 and 8 were recalculated relative activation (Fig. 7B and 8B). As it can be seen, this activation is more pronounced on fat body (10 times) than in flight muscle (4 times).
WESTERN-BLOTTING ANALYSIS
As it is shown in Figure 9, probing insect tissues extracts with antibodies against muscle PFK from rabbit caused the recognition of a specific band after electrophoresis. Although both lanes presented staining, it was markedly higher in fat body homogenates, in all western-blotting assays performed. By comparison, and the electrophoretic mobility with several standard proteins and rabbit muscle PFK, we were able to calculate to these bands a molecular mass of 86 kDa per monomer (data not shown).
DISCUSSION
It is reasonable to consider carbohydrate oxidation as an important metabolic process in the flight muscle of insects, especially those used to short-range flight activity, and that a tight glycolytic control of PFK activity would then be required on this tissue. It has been already shown in one such insect, Rhodnius prolixus, a significant decrease on muscle glycogen storage during flight (Ward et al. 1982).
According to our data, PFK activity in the flight muscle of R. prolixus is highly responsive to allosteric regulation. ATP presents inhibitory effects at concentrations above 3 mM, higher than those described as inhibitory for other insects (Walker and Bailey 1969, Holden and Storey 1993), but with an estimated Ki within the near-physiological range described previously (Wegener et al. 1991, Wegener 1996). It is possible that, in muscle, PFK activity remains mostly inhibited, and dependant on the activation promoted by other effectors. 31P NMR-spectroscopy studies in vivo with Locusta migratoria indicated the absence of greater alterations of ATP content on the flight muscle during flight (Wegener et al. 1991). However, our data shows that, at least in vitro, several effectors such as ADP, AMP and fructose-2,6-bisphosphate are able to activate flight muscle PFK even in the presence of inhibitory ATP concentrations. Curiously, in our experiments fructose-2,6-bisphosphate showed little or no effects at lower ATP concentrations, suggesting that the mechanism of activation by the compound is counteracting the inhibitory effects of ATP.
A similar pattern was found on the fat body homogenates, although with some singular characteristics. This tissue presented higher calculated K0.5 to the substrates, and higher Ki to the inhibition by ATP, when compared to muscle. While the highest levels of relative PFK activity were always obtained with muscle homogenates, the total extent of activation promoted by ADP, AMP and fructose-2,6-bisphosphate was markedly higher on fat body. This sensitivity to allosteric effectors suggests a tightly regulated glycolytic pathway, which may be consistent with physiologic roles of the fat body. Some insects are extremely dependent on meal carbohydrates to maintain trehalose levels on the haemolymph (Thompson et al. 2001) while haematophagous insects such as R. prolixus (exposed to poor carbohydrate, blood-based diets) may rely on the direct synthesis at the fat body. This process, known as trehalogenesis, requires the regulation of several enzymes, including glycogen phosphorylase, fructose-1,6-bisphosphatase and PFK (Becker et al. 1996). Our data suggests fructose-2,6-bisphosphate as a strong candidate for short-term regulation, as it is able to increase several times PFK activity on fat body extracts, depending on concentration. Also, the fructose 2,6-bisphosphate levels on fat body may be altered in physiological events such as fasting and re-feeding (Meyer-Fernandes et al. 2001). This activator may represent a target to the regulation induced by several hormones, including octopamine and hypertrehalosemic hormones.
Citrate, a well known modulator of this enzyme in vertebrates (Newsholme et al. 1977) was unable to exert the inhibition of PFK activity in both tissues. This insensitivity to citrate was previously described in other studies with insects (Walker and Bailey 1969, Newsholme et al. 1977, Leite et al. 1988, Khoja et al. 1990), although slightly effects were described in some reports (Khoja 1991, Holden and Storey 1993). Within the fat body, the lack of response to citrate may be correlated with the possibility of attaining higher taxes of de novo synthesis of lipids from carbohydrates. In the flight muscle, the absence of the Randle cycle is less relevant if flight activity remains dependent on the oxidation of carbohydrates, although Ward et al. (1982) have shown evidences that R. prolixus depends on fatty acid oxidation during long-term flight. In that case, other mechanisms would be required to decrease the levels of carbohydrate oxidation on this tissue - such as lowering the levels of fructose-2,6-bisphosphate, for example.
It is described that PFK presents higher allosteric responses at higher H+ concentrations, in pH values much lower than the optimal, and known as regulatory pH. Even though we did not investigate the allosteric alterations induced by pH, our data have shown that, at fixed ATP and fructose-6-phosphate concentrations, both homogenates presented very similar alterations on PFK activity. This suggests that possible alterations on K0.5 and Ki for these substrates expected at different pH values may have similar rates in fat body and flight muscle. However, it is not clear if the pH sensitivity found on the flight muscle presents any physiological relevance, since flight on insects is described as a highly oxidative activity (Ford and Candy 1972, Candy et al. 1997, Suarez 2000), with little or no production of lactate (the main responsible for pH alterations in muscle). In Rhodnius and other Hemiptera the transverse tracheolar system forms large air cavities among the mitochondria, which accounts for an efficient oxygen supply (Wigglesworth and Lee 1982). It remains to be told if alterations on pH or lactate concentration really occur on R. prolixus flight muscle.
In our work, we could not correlate the markedly higher PFK activity found on flight muscle to greater protein contents. On the contrary, the polyclonal anti-rabbit muscle PFK IgG detected lower levels of PFK staining in muscle homogenates than those found in fat body in all western blotting experiments. Since both PFK activity and PFK content were assayed in the same kind of homogenate and normalized with the homogenate protein content, our results strongly suggests that a more active type of enzyme is found on flight muscle, presenting kinetic and allosteric properties differing from those found in fat body.
There are three different isoforms of PFK monomers already described - M (muscle), L (liver) and F (fibroblast). In insects, the presence of different isozymic forms of PFK remains to be determined. In this work, antibodies were raised against rabbit skeletal muscle PFK, described as composed solely by the M isoform. However, all isoforms present high levels of similarity, what leads to the production of polyclonal IgG unable to sort them. Consequently, it is possible, but not probable, that some kind of PFK subunit remains undetected in our experiment. However, it becomes clear that, regardless of those possibilities, both fat body and flight muscle have singular profiles of glycolytic regulation, which might be consistent with their specific role in several physiological conditions, such as fasting or flight.
ACKNOWLEDGMENTS
This work is part of the doctoral work of GGA, and was supported by grants from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Fundação Ary Frausino/Fundação Educacional Charles Darwin (FAF/FECD Programa de Oncobiologia), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Programa de Núcleos de Excelência (PRONEX), and Programa de Apoio ao Desenvolvimento Científico e Tecnológico (PADCT).
Manuscript received on November 6, 2005; accepted for publication on November 7, 2005; presented by LUCIA MENDONÇA PREVIATO
Correspondence to: Mauro Sola-Penna
E-mail: maurosp@ufrj.br
- BECKER A, SCHLODER P, STEELE JE AND WEGENER G. 1996. The regulation of trehalose metabolism in insects. Experientia 52: 433-439.
- BECKER A, LIEWALD JFJ, STYPA H AND WEGENER G. 2001. Antagonistic effects of hypertrehalosemic neuropeptide on the activities of 6-phosphofructo-1-kinase and fructose-1,6-bisphosphatease in cockroach fat body. Insect Biochem Mol Biol 31: 381-392.
- CANDY DJ AND KILBY BA. 1959. Site and mode of trehalose biosynthesis in the locust. Nature 183: 1594-1595.
- CANDY DJ, BECKER A AND WEGENER G. 1997. Coordination and integration of metabolism in insect flight. Comp Biochem Physiol 117B: 497-512.
- FORD WCL AND CANDY DJ. 1972. The regulation of glycolysis in perfused locust flight muscle. Biochem J 130: 1101-1112.
- HOLDEN CP AND STOREY KB. 1993. Regulation of phosphofructokinase and the control of cryoprotectant sybthesis in a freeze-avoiding insect. Can J Zool 71: 1895-1899.
- KEELEY LL. 1985. In: COMPREHENSIVE INSECT PHYSIOLOGY, BIOCHEMISTRY AND PHARMACOLOGY, KERKUT GA AND GILBERT LI (Eds), Pergamon Press, London 3: 211-248.
- KHOJA SM. 1991. A comparison of the allosteric properties of 6-phosphofructo-1-kinase from the flight muscle, leg muscle, mid-gut and hind-gut of the grasshopper, Poekilocerus bufonius Comp Biochem Physiol 99B: 833-837.
- KHOJA SM, AL-ROBAI AA AND AL-FIFI ZI. 1990. Regulatory properties of 6-phosphofructo-1-kinase of the midgut of the grasshopper. Poekilocerus bufinius Insect Biochem 20: 443-449.
- KRAMMER AE AND HEINRICH B. 1978. Insects flight metabolism. Adv Insect Physiol 13: 133-228.
- LEITE A, NETO JA, LEYTON JF, CRIVELLARO O AND ELDORRY HA. 1988. Phosphofructokinase from bumblebee flight muscle. J Biol Chem 263: 17527-17533.
- LOWRY OH, ROSENBROUGH NJ, FARR AL AND RANDALL RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275.
- MEIRA DD, MARINHO-CARVALHO MM, TEIXEIRA CA, VEIGA VF, DA POAIN AT, HOLANDINO C, FREITAS MS AND SOLA-PENNA M. 2005. Clotrimazole decreases human breast cancer cells viability through alterations in cytoskeleton-associated glycolytic enzymes. Mol Genet Metab 84: 354-362.
- MEYER-FERNANDES JR, CLARK CP, GONDIM K AND WELLS MA. 2001. Fat body fructose-2,6-bisphosphate content and phosphorylase activity correlate with changes in hemolymph glucose concentration during fasting and re-feeding in larval Manduca sexta Insect Biochem Mol Biol 31: 165-170.
- NEWSHOLME EA AND LEECH AR. 1983. In: BIOCHEMISTRY FOR THE MEDICAL SCIENCES, J Wiley & Sons, Chichester, New York, NY, USA, p. 300335.
- NEWSHOLME EA, SUGDEN PH AND WILLIAMS T. 1977. Effect of citrate on the activities of 6-phosphofructokinase from nervous and muscle tissues from different animals and its relationships to the regulation of glycolysis. Biochem J 166: 123-129.
- SCHIRMER T AND EVANS PR. 1990. Structural basis of the allosteric behavior of phosphofructokinase. Nature 343: 140-145.
- SOLA-PENNA M, SANTOS AC, ALVES GG, EL-BACHA T, FABER-BARATA J, PEREIRA MF, SEREJO FC, DA POIAN AT AND SORENSON M. 2002: A radioassay for phosphofructokinase-1 activity in cell extracts and purified enzyme. J Biochem Biophys Meth 50: 129-140.
- SUAREZ RK. 2000. Energy Metabolism during insect flight: biochemical design and physiological performance. Physiol Biochem Zool 73: 765-771.
- THOMPSON SN, REDAK RA AND WANG L-W. 2001. Altered dietary nutrient intake maintains metabolic homeostasis in parasitized larvae of the insect Manduca sexta L. J Exp Biol 204: 4065-4080.
- UYEDA K. 1979. Phosphofructokinase. Adv Enzymol 48: 193-244.
- WALKER PR AND BAILEY E. 1969. A comparison of the phosphofructokinase of the fat body and flight muscle of the adult male desert locust. Biochem J 111: 365-369.
- WARD JP AND BAKER PS. 1982. The tethered flight performance of a laboratory population of Triatoma infestans Klug, (Hemiptera: Reduviidae). Bull Ent Res 19: 425-429.
- WARD JP, CANDY DJ AND SMITH SN. 1982. Lipid storage and changes during flight by triatomine bugs (Rhodnius prolixus and Triatoma infestans). J Insect Physiol 28: 527-529.
- WEGENER G. 1996. Flying insects: model systems in exercise physiology. Experientia 52: 404-412.
- WEGENER G, MICHEL R AND NEWSHOLME EA. 1986. Fructose 2,6-bisphosphate as a signal for changing from sugar to lipid oxidation during flight in locusts. FEBS Lett 201: 129-132.
- WEGENER G, BOLAS NM AND THOMAS AAG. 1991. Locust flight metabolism studied in vivo by P NMR spectroscopy. J Comp Physiol B 161: 247-256.
- WEIS-FOUGH T. 1952. Fat combustion and metabolic rate of flying locusts (Schistocerca gregaria Forskal). Phil Trans R Soc Sec B 237: 1-36.
- WIGGLESWORTH VB AND LEE WM. 1982. The supply of oxygen to the flight muscles of insects: a theory of tracheole physiology. Tissue Cell 14: 501-18.
Publication Dates
-
Publication in this collection
26 Mar 2007 -
Date of issue
Mar 2007
History
-
Accepted
07 Nov 2005 -
Received
06 Nov 2005