ABSTRACT
Morus nigra L. (Moraceae) is a tree known as black mulberry and the leaves are used in folk medicine in the treatment of diabetes, high cholesterol and menopause symptoms. The aim of this study was to evaluate the M. nigra leaves phytochemical profile in different extractions and the hypolipidemic effect of the infusion comparing to the fenofibrate. Morus nigra infusion (MN) showed higher amounts of phenolics and flavonoids (83.85 mg/g and 79.96 µg/g, respectively), as well as antioxidant activity (83.85%) than decoction or hydromethanolic extracts. Although, decoction showed the best result for ascorbic acid (4.35 mg/100 g) than hydromethanolic or infusion (2.51 or 2.13 mg/100 g, respectively). The phenolic acids gallic, chlorogenic and caffeic and the flavonoids quercetin, rutin and catechin were found in the M. nigra extracts. Hyperlipidemic rats treated with 100, 200 or 400 mg/kg of MN decreased serum cholesterol, triglycerides and normalized lipoproteins. Furthermore, MN inhibited lipid peroxidation in liver, kidney and brain of hyperlipidemic rats. This study provides evidence that M. nigra leaves extracts are rich in polyphenols, mainly chlorogenic acid, which normalized hyperlipidemic disturbance. The results suggest a potential therapeutic effect of the M. nigra leaves infusion on dislipidemic condition and related oxidative stress.
Key words:
Morus nigra; leaves; phenolics; Triton WR-1339
INTRODUCTION
Cardiovascular disease is the leading cause of death worldwide with an increasing incidence rate (Mendis 2011MENDIS S, PUSKA P AND NORRVING B. 2011. World Health Organization, World Heart Federation & World Stroke Organization. Global atlas on cardiovascular diseases prevention and control, Geneva: World Health Organization, 166 p.). Cholesterol is a constituent of membranes and plays a role in synthesis of bile acids, hormones and vitamins. Although the hypercholesterolemic together with the hypertrigliceridemic state of the serum are both risk factors to the development of coronary heart disease and atherosclerosis progression (Lusis 2000LUSIS AJ. 2000. Atherosclerosis. Nature 407: 233-241.). On the other hand, the decrease in low density lipoprotein cholesterol (LDL) and increase in high density lipoprotein cholesterol (HDL) serum levels contribute to an anti-atherogenic condition (Lusis 2000, West et al. 1983WEST KM, AHUJA MMS AND BENNET PH. 1983. The role of circulating glucose and triglyceride concentration and their interaction with other risk factors as determinants of arterial disease in nine diabetic population samples from the WHO multinational study. Diabetes Care 6: 361-369., Assman and Nofer 2003ASSMAN G AND NOFER JR. 2003. Atheroprotective effects of high-density lipoproteins. Annu Rev Med 54: 321-341.).
Lipid-lowering substances such as statins and fibrates reduce the number of events related to cardiovascular complications, but having potential side effects and great drug dependence; many patients have been choosing alternative ways for the treatment. In addition, oxidative stress is an early event in hyperlipidemic conditions and it has been suggested that antioxidants can break a vicious cycle in the progress of the disease (Rony et al. 2014RONY KA, AJITH TA, NIMA N AND JANARDHANAN KK. 2014. Hypolipidemic activity of Phellinus rimosus against WR-1339 and high cholesterol diet induced hyperlipidemic rats. Environ Toxicol Pharm 37: 482-492.). Therefore, it has been growing steadily the interest in seeking drugs to decrease side effects and treat the disease using multiple targets; in this line, plant material is an available option.
Morus nigra L. belonging to Moraceae family usually known as black mulberry is a tree worldwide distributed and its fruits are consumed regarding its nutritional value (Gundogdu et al. 2011GUNDOGDU M, MURADOGLU F, GAZIOGLU RI AND YILMAZ H. 2011. Determination of fruit chemical properties of Morus nigra L., Morus alba L. and Morus rubra L. by HPLC. Sci Hort 132: 37-41.). The leaves are used traditionally for therapeutical purposes as for the treatment of diabetes, hypercholesterolemia, menopause and obesity (Oliveira et al. 2013OLIVEIRA ACB, OLIVEIRA AP, GUIMARÃES AL, OLIVEIRA RA, SILVA FS, REIS SAGB, RIBEIRO LAA AND ALMEIDA JRGS. 2013. Avaliação toxicológica pré-clínica do chá das folhas de Morus nigra L. (Moraceae). Rev Bras Pl Med 15: 244-249., Silva and Naves 2001SILVA CRM AND NAVES MMV. 2001. Vitamin supplementation in cancer chemoprevention. Br J Nutr 14: 135-143., Volpato et al. 2011VOLPATO GT, CALDERON IMP, SINZATO S, CAMPOS KE, RUDGE MVC AND DAMASCENO DC. 2011. Effect of Morus nigra aqueous extract treatment on the maternal-fetal outcome, oxidative stress status and lipid profile of streptozotocin-induced diabetic rats. J Ethnopharmacol 138: 691-696., Miranda et al. 2010MIRANDA MA, VIEIRA GD, ALVES MS, YAMAMOTO CH, PINHO JJRG AND SOUSA OV. 2010. Uso etnomedicinal do chá de Morus nigra L. no tratamento dos sintomas do climatério de mulheres de Muriaé, Minas Gerais, Brasil. Hosp Univ Rev 36: 61-68.). Moreover, Volpato et al. (2011) studied antihyperglicemic effect from Morus nigra leaf decoction in pregnancy diabetic rats although, obtained positive effects only in lipids levels. Additionally, Araújo et al. (2015ARAÚJO CA, LÚCIO KP, SILVA ME, ISOLDI MC, SOUZA GHB, BRANDÃO GC, SCHULTZ R AND COSTA DC. 2015. Morus nigra leaf extract improves glycemic response and redox in the liver of diabetic rats. Food Funct 6: 3490-3499.) demonstrated an increased insulinemia and improved oxidative stress state in diabetic rats treated with hydroethanolic leaf extract. Morus nigra leaves have shown evidence of anti-inflammatory, antinociceptive and hepatoprotective effects (Padilha et al. 2009PADILHA MM, VILELA FC, SILVA MJD, SANTOS MH, ALVES-DA-SILVA G AND GIUSTI-PAIVA A. 2009. Antinociceptive effect of the extract of Morus nigra leaves in mice. J Med Food 12: 1381-1385., 2010, Malhi et al. 2014MALHI TH, QADIR MI, KHAN YH AND ALI M. 2014. Hepatoprotective activity of aqueous methanolic extract of Morus nigra against paracetamol-induced hepatotoxicity in mice. Bangladesh J Pharmacol 9: 60-66.). Although, Queiroz et al. (2012QUEIROZ GT, SANTOS TR, MACEDO R, PETERS VM, LEITE MN, SILVEIRA E SÁ R AND GUERRA MO. 2012. Efficacy of Morus nigra L. on reproduction in female Wistar rats. Food Chem Toxicol 50: 816-822.), did not confirmed the estrogenic activity, the most popular use of M. nigra. Moreover, M. nigra did not exert a toxic effect on the female reproductive system or on the embryonic development of rats contributing to reduce incidence of abnormalities in offspring from diabetic dams (Volpato et al. 2011, Queiroz et al. 2012). Furthermore, Oliveira et al. (2013) have considered M. nigra leaf aqueous extract as being of low toxicity after a treatment by oral route during 30 days in rats.
Recent studies developed by Memon et al. (2010MEMON AA, MEMON N, LUTHRIA DL, BHANGER MI AND PITAFI AA. 2010. Phenolic acids profiling and antioxidant potential of mulberry (Morus laevigata W., Morus nigra L., Morus alba L.) leaves and fruits grown in Pakistan. Pol J Food Nutr Sci 60: 25-32.), Malhi et al. (2014MALHI TH, QADIR MI, KHAN YH AND ALI M. 2014. Hepatoprotective activity of aqueous methanolic extract of Morus nigra against paracetamol-induced hepatotoxicity in mice. Bangladesh J Pharmacol 9: 60-66.), Araújo et al. (2015ARAÚJO CA, LÚCIO KP, SILVA ME, ISOLDI MC, SOUZA GHB, BRANDÃO GC, SCHULTZ R AND COSTA DC. 2015. Morus nigra leaf extract improves glycemic response and redox in the liver of diabetic rats. Food Funct 6: 3490-3499.), Sánchez-Salcedo et al. (2015) with black mulberry leaves extracts using organic solvents demonstrated the richness of phenolic compounds which provides a potential antioxidant activity. Since oxidative stress has been implicating on the improvement of cardiovascular and neurodegenerative diseases this source of phenolics could be usefull therapy (Heo and Lee 2006HEO JH AND LEE CY. 2006. Phenolic phytochemicals in cabbage inhibit amyloid β protein-induced neurotoxicity. Food Sci Technol 39: 330-336., Tarozzi et al. 2013TAROZZI A, ANGELONI C, MALAGUTI M, MORRONI F, HRELIA S AND HRELIA P. 2013. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid Med Cell Longev 9: 1-10., Wiczkowski et al. 2013WICZKOWSKI W, NOWAK DS AND TOPOLSKA J. 2013. Red cabbage anthocyanins: profile, isolation, identification, and antioxidant activity. Food Res Int 51: 303-309.).
Therefore, to the best of our knowledge, this is the first study with the goals to investigate phenolics in different extracts, mainly aqueous, from black mulberry leaves (infusion, decoction and hydromethanolic). And also, to inspect the hypolipidemic and antioxidant activities from the M. nigra leaves infusion in hyperlipidemic rats, compared to the fenofibrate.
MATERIALS AND METHODS
COLLECTION, PREPARATION OF PLANT MATERIAL AND EXTRACTS
The leaves of Morus nigra L. from the city of Blumenau (Santa Catarina State, Southern Brazil - latitude 26°54’10” S, longitude 49°04’44” W) harvested in February, 2014. The species were identified, taxonomically authenticated and a voucher specimen (N° 42265) deposited at the Regional University of Blumenau’s herbarium, Santa Catarina, Brazil. The plant material was submitted to drying at 45°C with forced ventilation, grinded and then stored at -10°C.
Briefly, 2 g of milled leaves were extracted with 100 mL of boiled at 100°C distilled water resting 15 min (infusion), 100 mL of distilled water boiling for 10 min (decoction) or 100 mL of methanol: distilled water, 70%:30% (v/v) stirred for 15 min (hydromethanolic).
DETERMINATION OF BIOACTIVE COMPOUNDS AND ANTIOXIDANT CAPACITY
The concentration of total phenolics (TP) was measured using the Folin-Ciocalteu assay previously described by Singleton and Rossi (1965SINGLETON VL AND ROSSI JA. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungistic acid reagents. Am J Enol Vit 16: 144-158.), reading the absorbance at 725 nm. The TP content calculations were standardized through gallic acid (y= 0.1893x - 0.1429,r 2= 0.99) and expressed as gallic acid equivalent (GAE) mg/g dry weight. The total flavonoids (TF) quantification of extracts was performed by mixing the samples (0.5 mL) with AlCl3 (2%, w/v) and 2.5 mL ethanol. The absorbance was determined at 420 nm and the TF content was standardized through quercetin (y= 0.1755x - 0.3139, r 2= 0.99). The results expressed as quercetin equivalent (QE) µg/g dry weight (Woisky and Salatino 1998WOISKY RG AND SALATINO A. 1998. Analysis of propolis: some parameters and procedures for chemical quality control. J Apicul Res 37: 99-105.). For the total carotenoids analysis, a hexane: acetone solution (1:1, v/v) containing 100 mg of butyl hydroxytoluene (BHT) was added to 300 mg of biomass sample. After this, the absorbance was determined at 450 nm and the quantification based on the absorption coefficient (A 1% 1cm 2300, hexane - 450 nm). The results defined as β-carotene equivalent mg/g dry weight (Britton 1995BRITTON G. 1995. UV/visible spectroscopy. In: Britton G et al. (Eds), Carotenoids, spectroscopy, Birkhäuser Verlag: Basel, p. 13-62.). The quantification of ascorbic acid carried out with 20 mL of Morus nigra extracts titrated by potassium iodide solution (KIO3 0.01N). The titrations of ascorbic acid on the samples using the starch solution indicator (1%, w/v) and the results expressed as mg/100 g dry weight (Rebollo et al. 2005REBOLLO C, ROSTANI S, SANSONE S, CARRIERI R, HERNÁNDEZ V, SALHÁ B AND VIERA M. 2005. Vitamina C: una estrategia didáctica polifuncional. Ensen Cienc 23: 133-140.).
The antioxidant activity determined spectrophotometrically using the 2,2-diphenyl-1-picrylhydrazyl radical (DPPH - Sigma Chemical Co., St Louis, MO, USA). After storage at room temperature during 30 min in the dark, the absorbance of the samples was determined at 517 nm (Brand-Williams et al. 1995). DPPH radical-scavenging activity was calculated according to the following equation:
Where Ablank is the absorbance of the control reaction (containing all reagents except the test compound) and Asample is the absorbance of the test compound. All tests were performed in triplicate.
HPLC ANALYSIS
RP-HPLC was performed using a Varian ProStar230 chromatograph equipped with a C18 reverse-phase column (Agilent Technologies, California, USA, 250 mm x 4.6 mm, 5 µm), protected by a 5 µm C18 reverse-phase guard column, and a UV-visible detector (330 nm). The samples were eluted in isocratic mode at a flow rate of 0.9 mL/min, using acidified water (0.5% of formic acid):methanol (70:30, v/v) and the solvents were purchased from Tedia Brazil (Rio de Janeiro, Brazil). The chromatographic analyses lasted for 30 minutes. The phenolic compound identification was performed by comparing retention times of standard compounds, chlorogenic, gallic and caffeic acids and rutin, quercetin, catechin, purchased from Sigma Chemical Co. (St Louis, MO, USA). The injection volume was 20 μL from each extract.
ANIMALS AND EXPERIMENTAL PROTOCOL
The biological tests have been approved by the local Ethics Committee on Animal Use at Regional University of Blumenau (FURB), Protocol nr. 015/2013. Male Wistar rats were used between 9-10 weeks of age. All animals were housed in groups of four per cage, on 12 h light/dark cycle, and air temperature at 22±2°C with food and water ad libitum.
The rats were allowed to acclimatize for 1 week before the experiments and then divided into six groups (n=6-8 animals/group). Hyperlipidemic control group (HCG) was performed according to Cruz et al. (2016CRUZ AB, PITZ HS, VEBER B, BINI LA, MARASCHIN M AND ZENI ALB. 2016. Assessment of bioactive metabolites and hypolipidemic effect of polyphenolic-rich red cabbage extract. Pharm Biol 54: 3033-3039.) through a single intraperitoneal injection (i.p.) with Triton WR-1339 (400 mg/kg - Sigma Chemical Company, St Louis, MO, USA) before administration of other substances. The normal control group (NCG-distilled water), Morus nigra infusion extract (MN - 100, 200 or 400 mg/kg), or fenofibrate (FF- 65 mg/kg-EMS S/A, SP, Brazil) was given by blunt gavage twice a day for three consecutive days. In this study, the infusion extract was chosen for biological tests based on its higher phenolic content and antioxidant activity showed in the phytochemical results.
At the end of the experiment, rats fasted overnight were anesthetized by intraperitoneal (i.p.) injection of sodium thiopental (Cristália - Produtos Farmacêuticos, SP, Brazil). Blood samples were collected into tubes and centrifuged (5000 rpm, 5 min) to obtain serum for lipid profile analysis. Briefly, liver, kidneys and brain were removed, rinsed out with 0.9% cold saline, blotted with filter paper, and frozen for further estimation of lipid peroxidation. In addition, the brain was dissected obtaining cerebral cortex and hippocampus.
BIOCHEMICAL ANALYSIS
The contents of serum total cholesterol (TC), triglycerides (TG) and high-density lipoprotein (HDL-c) determined in a semiautomatic analyzer BIO-2000 (Bioplus, SP, Brazil) using commercial kits (Labtest Diagnóstica SA, Lagoa Santa, MG, Brazil) according to the manufactures’ instructions. The results of low-density lipoprotein-cholesterol (LDL) and very low-density lipoprotein cholesterol (VLDL) were estimated by the Friedewald et al. (1972FRIEDEWALD WT, LEVY RI AND FREDRICKSON DS. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499-502.) equations as the following: VLDL = TG/5 and LDL= TC - (HDL + VLDL). The atherogenic index (AT) and cardiovascular risk factor (CR) were calculated by the following equations: AT = (TC - HDL)/HDL and CR = TC/HDL according to Castelli’s indexes (Castelli et al. 1986).
EVALUATION OF LIPID PEROXIDATION
Thiobarbituric acid-reactive substances (TBARS) assay levels were determined according to the method described by Ohkawa et al. (1979OHKAWA H, OHISHI N AND YAGI K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351-358.) that measures malondialdehyde (MDA), a product of lipoperoxidation caused mainly through hydroxyl free radicals by the absorbance at 535 nm. The calibration curve developed using 1,1,3,3-tetramethoxypropane and TBARS levels calculated as nanomol of malondialdehyde formed per milligram of protein.
PROTEIN DETERMINATION
Protein was measured by Lowry et al. (1951LOWRY OH, ROSEBROUGH NJ, FARR AL AND RANDALL RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275.) method, using serum bovine albumin as standard.
STATISTICAL ANALYSIS
The chemical data expressed as mean ± S.D., biological data as mean ± S.E.M. and, both compared by one-way ANOVA followed by the Tukey test at p ≤ 0.05. The statistical package GrapPad Prism 6.0 Version for Windows (GraphPad Software, San Diego, CA, USA) was used for statistics.
RESULTS AND DISCUSSION
ANALYSIS OF TOTAL PHENOLICS, FLAVONOIDS, CAROTENOIDS, ASCORBIC ACID, AND ANTIOXIDANT CAPACITY OF Morus nigra EXTRACTS
Several studies have shown that the intake of phenolics and flavonoids can be beneficial to reducing the risk of atherosclerosis development (Costa and Martinez 1997COSTA RP AND MARTINEZ TLR. 1997. Terapia nutricional na hipercolesterolemia. Sociedade Brasileira de Cardiologia 7: 475-484.). In this context, flavonoids have been related to inhibition of LDL oxidation, platelet aggregation promoting vasodilatation, and also modification of eicosanoid synthesis (Sesso et al. 2003SESSO HD, GAZIANO JM, LIU S AND BURING JE. 2003. Flavonoid intake and the risk of cardiovascular disease in women. Am J Clin Nutr 77: 1400-1408.).
This study, to our knowledge, is the first comparing two aqueous forms and methanolic Morus nigra extracts (Table I) detecting a significant (p<0.01) higher concentration of total flavonoids from the infusion and hydromethanolic (79.96 ± 0.71 and 76.76 ± 0.78 µg/g, respectively) than the decoction (67.83 ± 1.24 µg/g). Correspondingly, the antioxidant capacity from the infusion (83.85 ± 0.99%) and hydromethanolic (81.71 ± 0.05%) extracts shared superiority to the decoction (74.37 ± 0.20%) one. The amount of total phenolics was higher in the infusion (75.86 ± 0.87 mg/g) than the hydromethanolic and decoction (51.01 ± 0.86 and 64.59 ± 0.14 mg/g, respectively). On the contrary, decoction showed better results for ascorbic acid (4.35 ± 0.13 mg/100 g) than the infusion (2.13 ± 0.14 mg/100 g) or hydromethanolic (2.51 ±0.13 mg/100 g) extracts, the same results were obtained by Guimarães et al. (2011GUIMARÃES R, BARROS L, CARVALHO AM AND FERREIRA ICFR. 2011. Infusions and decoctions of mixed herbs used in folk medicine: synergism in antioxidant potential. Phytother Res 25: 1209-1214.). In regard, Fata et al. (2016FATA NA, GEORGÉ S, ANDRÉ S AND RENARD CMGC. 2016. Determination of reaction orders for ascorbic acid degradation during sterilization using a new experimental device: the thermoresistometer Mastia®. LWT - Food Sci Technol 85: 487-492.) pointed that there are several variables to considerer besides temperature since, ascorbic acid quantity is influenced also by oxygen, light intensity, pH, water activity, presence of metallic ions and the presence of sugars (Hsu et al. 2012HSU HY, TSAI YC, FU CC AND WU JSB. 2012. Degradation of ascorbic acid in ethanolic solutions. J Agric Food Chem 60: 10696 -10701.) could be at least in part influenced the result. Regarding antioxidant effect and amounts of ascorbic acid obtained in this study, it seems that the amounts of phenolics and flavonoids were determinants while ascorbic acid did not. Finally, total carotenoids quantification revealed 12.12 ± 0.56 mg/g.
The evaluation of phenolics and antioxidant capacity found in this study was greater than demonstrated by Araújo et al. (2015ARAÚJO CA, LÚCIO KP, SILVA ME, ISOLDI MC, SOUZA GHB, BRANDÃO GC, SCHULTZ R AND COSTA DC. 2015. Morus nigra leaf extract improves glycemic response and redox in the liver of diabetic rats. Food Funct 6: 3490-3499.) also studying leaves of Morus nigra. Moreover, ascorbic acid and carotenoids could have contributed to the observed antioxidant activity showed here. According to Singh et al. (2006SINGH J, UPADHYAY AK, BAHADUR A, SINGH B, SINGH KP AND RAI M. 2006. Antioxidant phytochemicals in cabbage (Brassica oleracea L. var. capitata). Sci Hort 108: 233-237.) and Silva and Naves (2001SILVA CRM AND NAVES MMV. 2001. Vitamin supplementation in cancer chemoprevention. Br J Nutr 14: 135-143.), ascorbic acid and phenolic compounds are capable to act against superoxide and hydroxyl radicals and to reduce C-reactive protein (CRP) levels, a marker of inflammation and a predictor of heart disease (Singh et al. 2006).
RP-HPLC ANALYSIS OF PHENOLIC COMPOUNDS
The analysis identified 06 phenolic compounds in Morus nigra extracts (Figure 1), although a different phenolic profile was demonstrated for each extract. The phenolic acids present in all extracts were gallic and chlorogenic acids which the last was the major compound detected. Therefore, the constituens as quercetin, gallic and chlorogenic acids maybe involved in the higher antioxidant capacity found in the infusion and hydromethanolic extracts. The acids caffeic, chlorogenic and gallic, and the flavonoids quercetin and rutin, were already found in leaves extracts of Morus nigra (Araújo et al. 2015ARAÚJO CA, LÚCIO KP, SILVA ME, ISOLDI MC, SOUZA GHB, BRANDÃO GC, SCHULTZ R AND COSTA DC. 2015. Morus nigra leaf extract improves glycemic response and redox in the liver of diabetic rats. Food Funct 6: 3490-3499., Sanchez-Salcedo et al. 2015, Freitas et al. 2016FREITAS MM ET AL. 2016. Extracts of Morus nigra L. leaves standardized in chlorogenic acid, rutin and isoquercitrin: tyrosinase inhibition and cytotoxicity. PLoS ONE 11: 1-24.), although, catechin was not identified previously. In fact, the cinnamic acid derivatives has been thoroughly studied as anti-atherogenic agents causing alterations in cholesterol storage and transport, LDL-oxidation, and HDL particle size rearrangement (Cai et al. 2004CAI Y, LUO Q, SUN M AND CORKE H. 2004. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 74: 2157-2184., Ahmad et al. 2012AHMAD MM, MAHMOOD Q, GULZAR K, AKHTAR MS, SALEEM M AND QADIR MI. 2012. Antihyperlipidaemic and hepatoprotective activity of Dodonaea viscosa leaves extracts in alloxan-induced diabetic rabbits (Oryctolagus cuniculus). Pak Vet J 32: 50-54., Balzan et al. 2013BALZAN S, HERNANDES A, REICHERT CL, DONADUZZI C, PIRES VA, GASPAROTTO A AND CARDOZO EL. 2013. Lipid-lowering effects of standardized extracts of Ilex paraguariensis in high-fat-diet rats. Fitoterapia 86: 115-122.). Of note, the fixed wavelength at 330 nm to perform a comparison among extracts is a possible limitation of this study. In addition, further approaches needs perform co-injections and quantification of the compounds.
Chromatogram obtained by RP-HPLC (330 nm) of phenolic compounds of hydromethanolic, decoction and infusion extracts from amoreira-preta leaves (Morus nigra L.). Peaks: (1) quercetin, (2) rutin, (3) gallic acid, (4) catechin, (5) chlorogenic acid and (6) caffeic acid.
EFFECT OF Morus nigra INFUSION EXTRACT (MN) ON SERUM LIPID PROFILE IN TRITON WR-1339-INDUCED HYPERLIPIDEMIC RATS
According to Schurr et al. (1972SCHURR PE, SCHULTZ JR AND PARKINSON TM. 1972. Triton induced hyperlipidemia in rats as an animal model for screening hypolipidemic drugs. Lipids 7: 69-74.), Zarzecki et al. (2014ZARZECKI MS, ARAUJO SM, BORTOLOTTO VC, DE PAULA MT, JESSE CR AND PRIGOL M. 2014. Hypolipidemic action of chrysin on Triton WR-1339-induced hyperlipidemia in female C57BL/6 mice. Toxicol Rep 1: 200-208.) and Rony et al. (2014RONY KA, AJITH TA, NIMA N AND JANARDHANAN KK. 2014. Hypolipidemic activity of Phellinus rimosus against WR-1339 and high cholesterol diet induced hyperlipidemic rats. Environ Toxicol Pharm 37: 482-492.) the Triton WR-1339 is widely used to screening natural and chemical hypolipidemic drugs producing a hyperlipidemic condition as showed here compared to the control group (Figure 2). In agreement with Cruz et al. (2016CRUZ AB, PITZ HS, VEBER B, BINI LA, MARASCHIN M AND ZENI ALB. 2016. Assessment of bioactive metabolites and hypolipidemic effect of polyphenolic-rich red cabbage extract. Pharm Biol 54: 3033-3039.) it increased TC, TG, LDL-c and VLDL-c and, decreased HDL-c levels (p<0.001). On the other hand, MN, the selected extract in the phytochemical step, or fenofibrate treatment totally suppressed augmentation of TC and TG in hyperlipidemic rats (p<0.001). Oliveira et al. (2013OLIVEIRA ACB, OLIVEIRA AP, GUIMARÃES AL, OLIVEIRA RA, SILVA FS, REIS SAGB, RIBEIRO LAA AND ALMEIDA JRGS. 2013. Avaliação toxicológica pré-clínica do chá das folhas de Morus nigra L. (Moraceae). Rev Bras Pl Med 15: 244-249.) after treating rats during 30 days with Morus nigra extract did not find alterations in hematological and biochemical parameters including TC and TG levels considering the extract of low toxicity.
Effect of Morus nigra infusion extracts (MN) or fenofibrate (FF) on serum total cholesterol (a), triglycerides (b), HDL (c) and LDL (d) levels in Triton WR-1339-induced hyperlipemic rats (HCG). Values are expressed as means ± SEM (n=6-8). ***p<0.001 HCG compared with other groups and ###p<0.001 or #p<0.05 compared with HCG group.
As also depicted in Figure 2, only the group MN 100 demonstrated a significant decrement in LDL (p<0.05) although all doses of MN were capable to augment significantly HDL level, MN100 (p<0.001), MN200 and MN400 (p<0.05). However, FF group did not alter HDL level comparing with hyperlipidemic group (p>0.05). Finally, VLDL content (data not shown) decreased in serum of hyperlipidemic rats treated with MN extracts or FF groups (p<0.001). Previously, Volpato et al. (2011VOLPATO GT, CALDERON IMP, SINZATO S, CAMPOS KE, RUDGE MVC AND DAMASCENO DC. 2011. Effect of Morus nigra aqueous extract treatment on the maternal-fetal outcome, oxidative stress status and lipid profile of streptozotocin-induced diabetic rats. J Ethnopharmacol 138: 691-696.) showed decrease of TC, TG and VLDL levels in diabetic pregnant rats. To the best of our knowledge is the first time that Morus nigra leaves demonstrated capability to diminish TC, TG, VLDL and LDL with augmentation of HDL levels in hyperlipidemic rats. Nonetheless, popular hypolipidemic effect has been claimed for Morus nigra leaves (Volpato et al. 2011).
In this study, we identified great amounts of chlorogenic acid and quercetin in leaves of MN that could be sharing the responsibility for the hypolipidemic effect found here. Reinforcing this notion, some studies performed by Li et al. (2009LI SY, CHANG CQ, MA FY AND YU CL. 2009. Modulating effects of chlorogenic acid on lipids and glucose metabolism and expression of hepatic peroxisome proliferator-activated receptor-alpha in golden hamsters fed on high fat diet. Biomed Environ Sci 22: 122-129.) and Wan et al. (2013WAN CW, WONG CNY, PIN WK, WONG MHY, KWOK CY AND CHAN RYK. 2013. Chlorogenic acid exhibits cholesterol lowering and fatty liver attenuating properties by up-regulating the gene expression of PPAR-α in hypercholesterolemic rats induced with a high-cholesterol diet. Phytother Res 27: 545-551.) reported the modulation of lipid metabolism by chlorogenic acid decreasing TC, TG and LDL but not increasing HDL levels through up-regulating the expression of hepatic peroxissome proliferator-activated receptor (PPAR-α). Although, Nishi and Kumar (2013NISHI AA AND KUMAR P. 2013. Hypolipidemic effect of chlorogenic acid in a hypercholesterolemic rat model. Int J Pharm Bio Sci 4: 582-586.) showed increment in HDL-c level besides, the improvement on the lipid profile. Several studies claimed the positive effects of quercetin in lipid metabolism, including TC and TG reductions (Ricardo et al. 2001RICARDO KFS, OLIVEIRA TTD, NAGEM TJ, PINTO ADS, OLIVEIRA MGA AND SOARES JF. 2001. Effect of flavonoids morin; quercetin and nicotinic acid on lipid metabolism of rats experimentally fed with triton. Braz Arch Biol Technol 44: 263-267., Kamada et al. 2005KAMADA C, DA SILVA EL, OHNISHI-KAMEYAMA M, MOON JH AND TERAO J. 2005. Attenuation of lipid peroxidation and hyperlipidemia by quercitin glucoside in the aorta of high cholesterol-fed rabbit. Free Radical Res 39: 185-194., Jung et al. 2013JUNG CH, CHO I, AHN J, JEON TI AND HA TY. 2013. Quercetin reduces highfat diet induced fat accumulation in the liver by regulating lipid metabolism genes. Phytother Res 27: 139-143., Gnoni et al. 2009GNONI GV, PAGLIALONGA G AND SICULELLA L. 2009. Quercetin inhibits fatty acid and triacylglycerol synthesis in rat liver cells. Eur J Clin Invest 39: 761-768.). Furthermore, Padma et al. (2012PADMA VV, LALITHA G, SHIRONY NP AND BASKARAN R. 2012. Effect of quercetin against lindane induced alterations in the serum and hepatic tissue lipids in wistar rats. Asian Pac J Trop Biomed 2: 910-915.) demonstrated reduction in lipid levels and increasing in HDL-c in lindane-induced hyperlipidemia in rats by quercetin.
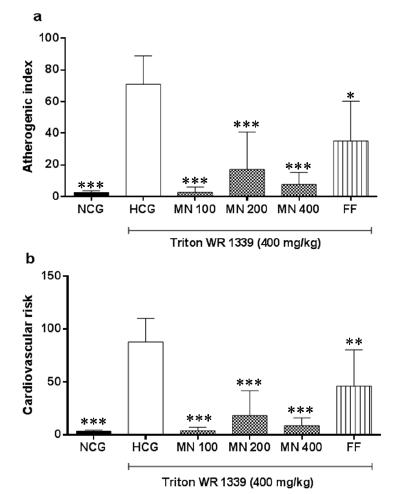
In this research, both atherogenic index (AT) and cardiac risk factor (CR), which express the risk of cardiovascular diseases, were markedly elevated in the HCG compared to NCG group. The MN was capable to decrease AT and CR (p<0.001) parameters compared with the HCG groups (Figure 3) without statistic difference among MN groups.
The atherogenic index (a) and cardiac risk factor (b) of Triton WR-1339-induced hyperlipemic rats (HCG) treated with Morus nigra infusion extract (MN) or fenofibrate (FF). Values are expressed as means ± SEM (n=6-8). ***p˂0.001 and **p˂0.01compared with HCG group.
The effect of Triton WR-1339 on lipid peroxidation in the animal model tested showed that the argumentation of serum lipids was accompanied by increase on the TBARS levels in liver, kidney, cerebral cortex and hippocampus of rats (p<0.05 and p<0.001). Also Zarzecki et al. (2014ZARZECKI MS, ARAUJO SM, BORTOLOTTO VC, DE PAULA MT, JESSE CR AND PRIGOL M. 2014. Hypolipidemic action of chrysin on Triton WR-1339-induced hyperlipidemia in female C57BL/6 mice. Toxicol Rep 1: 200-208.) showed increase of TBARS levels in liver of rats treated with Triton WR-1339. The treatment with MN extracts and FF were capable to counteract the lipid peroxidation in all structures tested significantly (Figure 4a, b, c and d). Therefore, chlorogenic acid and quercetin, both identified in the infusion of M. nigra, were capable to decrease MDA levels in serum, erythrocytes, cerebral cortex, hippocampus and liver (Jung et al. 2009, Meng et al. 2013MENG MA, CAO J, FENG Q, PENG J AND HU Y. 2013. Roles of chlorogenic acid on regulating and lipid metabolism: a review. J Based Complem Altern Med 9: 1-11., Stefanello et al. 2014, Xia et al. 2015XIA SF, XIE ZX, QIAO Y, LI LR, CHENG XR, TANG X AND LE GW. 2015. Differential effects of quercetin on hippocampus-dependent learning and memory in mice fed with different diets related with oxidative stress. Physiol Behav 138: 325-331., Imessaouedene et al. 2016).
Effect of Morus nigra infusion extract (MN) or fenofibrate (FF) on lipid peroxidation in the liver (a), kidney (b), cerebral cortex (c) and hippocampus (d) of Triton WR-1339-induced hyperlipidemic rats (HCG). Values are shown as means ± SEM (n=5). ***p<0.001 HCG compared with other groups, ###p<0.001, ##p<0.01 or #p<0.05 compared with HCG group.
CONCLUSIONS
Herein, the content of phytochemicals in Morus nigra extracts was influenced by the extraction form, therefore, revealing that the infusion is a promissory rich fount of 05 known antioxidant phenolics identified. To our knowledge, this is the first study reporting the comparison among different extracts from Morus nigra leaves, mainly aqueous and a lipid-lowering effect exhibiting an increase of HDL level, an essential and difficult parameter to rise on lipid profile. Besides, we suggest that both chlorogenic acid and quercetin, at least in part, could be responsible for the MN hypolipidemic effect. In conclusion, we demonstrated the hypolipidemic effect popularly claimed for Morus nigra leaves and we suggested a therapeutic potential usage of the infusion in dislipidemic conditions.
ACKNOWLEDGMENTS
This work was supported by grants from Universidade Regional de Blumenau (FURB), Programa Institucional de Bolsas de Iniciação Científica, Conselho Nacional de Desenvolvimento Científico e Tecnológico (PIBIC-FURB, PIBIC-CNPq) and Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC) scholarships. The authors thank the English review of Marta Helena Caetano (FURB). There is a patent request (PI10 2016 029764 8, INPI, Brazil) associated with this study.
REFERENCES
- AHMAD MM, MAHMOOD Q, GULZAR K, AKHTAR MS, SALEEM M AND QADIR MI. 2012. Antihyperlipidaemic and hepatoprotective activity of Dodonaea viscosa leaves extracts in alloxan-induced diabetic rabbits (Oryctolagus cuniculus). Pak Vet J 32: 50-54.
- ARAÚJO CA, LÚCIO KP, SILVA ME, ISOLDI MC, SOUZA GHB, BRANDÃO GC, SCHULTZ R AND COSTA DC. 2015. Morus nigra leaf extract improves glycemic response and redox in the liver of diabetic rats. Food Funct 6: 3490-3499.
- ASSMAN G AND NOFER JR. 2003. Atheroprotective effects of high-density lipoproteins. Annu Rev Med 54: 321-341.
- BALZAN S, HERNANDES A, REICHERT CL, DONADUZZI C, PIRES VA, GASPAROTTO A AND CARDOZO EL. 2013. Lipid-lowering effects of standardized extracts of Ilex paraguariensis in high-fat-diet rats. Fitoterapia 86: 115-122.
- BRAND-WILLIAMS W, CUVELIER ME AND BERSET C. 1995. Use of free radical method to evaluate antioxidant activity. Food Sci Technol 28: 25-30.
- BRITTON G. 1995. UV/visible spectroscopy. In: Britton G et al. (Eds), Carotenoids, spectroscopy, Birkhäuser Verlag: Basel, p. 13-62.
- CAI Y, LUO Q, SUN M AND CORKE H. 2004. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 74: 2157-2184.
- CASTELLI WP, GARRISON RJ, WILSON PW, ABBOTT RD, KALOUSDIAN S AND KANNEL WB. 1986. Incidence of coronary heart disease and lipoprotein cholesterol levels: the Framingham Heart Study. JAMA 256: 2835-2838.
- COSTA RP AND MARTINEZ TLR. 1997. Terapia nutricional na hipercolesterolemia. Sociedade Brasileira de Cardiologia 7: 475-484.
- CRUZ AB, PITZ HS, VEBER B, BINI LA, MARASCHIN M AND ZENI ALB. 2016. Assessment of bioactive metabolites and hypolipidemic effect of polyphenolic-rich red cabbage extract. Pharm Biol 54: 3033-3039.
- FATA NA, GEORGÉ S, ANDRÉ S AND RENARD CMGC. 2016. Determination of reaction orders for ascorbic acid degradation during sterilization using a new experimental device: the thermoresistometer Mastia®. LWT - Food Sci Technol 85: 487-492.
- FREITAS MM ET AL. 2016. Extracts of Morus nigra L. leaves standardized in chlorogenic acid, rutin and isoquercitrin: tyrosinase inhibition and cytotoxicity. PLoS ONE 11: 1-24.
- FRIEDEWALD WT, LEVY RI AND FREDRICKSON DS. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499-502.
- GNONI GV, PAGLIALONGA G AND SICULELLA L. 2009. Quercetin inhibits fatty acid and triacylglycerol synthesis in rat liver cells. Eur J Clin Invest 39: 761-768.
- GUIMARÃES R, BARROS L, CARVALHO AM AND FERREIRA ICFR. 2011. Infusions and decoctions of mixed herbs used in folk medicine: synergism in antioxidant potential. Phytother Res 25: 1209-1214.
- GUNDOGDU M, MURADOGLU F, GAZIOGLU RI AND YILMAZ H. 2011. Determination of fruit chemical properties of Morus nigra L., Morus alba L. and Morus rubra L. by HPLC. Sci Hort 132: 37-41.
- HEO JH AND LEE CY. 2006. Phenolic phytochemicals in cabbage inhibit amyloid β protein-induced neurotoxicity. Food Sci Technol 39: 330-336.
- HSU HY, TSAI YC, FU CC AND WU JSB. 2012. Degradation of ascorbic acid in ethanolic solutions. J Agric Food Chem 60: 10696 -10701.
- IMESSAOUDENE A, MERZOUK H, BERROUKECHE F, MOKHTARI N, BENSENANE B, CHERRAK S, MERZOUK SA AND ELHABIRI M. 2016. Beneficial effects of quercetin iron complexes on serum and tissue lipids and redox status in obese rats. J Nutr Biochem 29: 107-115.
- JUNG CH, CHO I, AHN J, JEON TI AND HA TY. 2013. Quercetin reduces highfat diet induced fat accumulation in the liver by regulating lipid metabolism genes. Phytother Res 27: 139-143.
- KAMADA C, DA SILVA EL, OHNISHI-KAMEYAMA M, MOON JH AND TERAO J. 2005. Attenuation of lipid peroxidation and hyperlipidemia by quercitin glucoside in the aorta of high cholesterol-fed rabbit. Free Radical Res 39: 185-194.
- LI SY, CHANG CQ, MA FY AND YU CL. 2009. Modulating effects of chlorogenic acid on lipids and glucose metabolism and expression of hepatic peroxisome proliferator-activated receptor-alpha in golden hamsters fed on high fat diet. Biomed Environ Sci 22: 122-129.
- LOWRY OH, ROSEBROUGH NJ, FARR AL AND RANDALL RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275.
- LUSIS AJ. 2000. Atherosclerosis. Nature 407: 233-241.
- MALHI TH, QADIR MI, KHAN YH AND ALI M. 2014. Hepatoprotective activity of aqueous methanolic extract of Morus nigra against paracetamol-induced hepatotoxicity in mice. Bangladesh J Pharmacol 9: 60-66.
- MEMON AA, MEMON N, LUTHRIA DL, BHANGER MI AND PITAFI AA. 2010. Phenolic acids profiling and antioxidant potential of mulberry (Morus laevigata W., Morus nigra L., Morus alba L.) leaves and fruits grown in Pakistan. Pol J Food Nutr Sci 60: 25-32.
- MENDIS S, PUSKA P AND NORRVING B. 2011. World Health Organization, World Heart Federation & World Stroke Organization. Global atlas on cardiovascular diseases prevention and control, Geneva: World Health Organization, 166 p.
- MENG MA, CAO J, FENG Q, PENG J AND HU Y. 2013. Roles of chlorogenic acid on regulating and lipid metabolism: a review. J Based Complem Altern Med 9: 1-11.
- MIRANDA MA, VIEIRA GD, ALVES MS, YAMAMOTO CH, PINHO JJRG AND SOUSA OV. 2010. Uso etnomedicinal do chá de Morus nigra L. no tratamento dos sintomas do climatério de mulheres de Muriaé, Minas Gerais, Brasil. Hosp Univ Rev 36: 61-68.
- NISHI AA AND KUMAR P. 2013. Hypolipidemic effect of chlorogenic acid in a hypercholesterolemic rat model. Int J Pharm Bio Sci 4: 582-586.
- OHKAWA H, OHISHI N AND YAGI K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351-358.
- OLIVEIRA ACB, OLIVEIRA AP, GUIMARÃES AL, OLIVEIRA RA, SILVA FS, REIS SAGB, RIBEIRO LAA AND ALMEIDA JRGS. 2013. Avaliação toxicológica pré-clínica do chá das folhas de Morus nigra L. (Moraceae). Rev Bras Pl Med 15: 244-249.
- PADILHA MM, VILELA FC, ROCHA CQ, DIAS MJ, SONCINI R, SANTOS MH, ALVES-DA-SILVA G AND GIUSTI-PAIVA A. 2010. Antiinflammatory properties of Morus nigra leaves. Phytother Res 24: 1496-1500.
- PADILHA MM, VILELA FC, SILVA MJD, SANTOS MH, ALVES-DA-SILVA G AND GIUSTI-PAIVA A. 2009. Antinociceptive effect of the extract of Morus nigra leaves in mice. J Med Food 12: 1381-1385.
- PADMA VV, LALITHA G, SHIRONY NP AND BASKARAN R. 2012. Effect of quercetin against lindane induced alterations in the serum and hepatic tissue lipids in wistar rats. Asian Pac J Trop Biomed 2: 910-915.
- QUEIROZ GT, SANTOS TR, MACEDO R, PETERS VM, LEITE MN, SILVEIRA E SÁ R AND GUERRA MO. 2012. Efficacy of Morus nigra L. on reproduction in female Wistar rats. Food Chem Toxicol 50: 816-822.
- REBOLLO C, ROSTANI S, SANSONE S, CARRIERI R, HERNÁNDEZ V, SALHÁ B AND VIERA M. 2005. Vitamina C: una estrategia didáctica polifuncional. Ensen Cienc 23: 133-140.
- RICARDO KFS, OLIVEIRA TTD, NAGEM TJ, PINTO ADS, OLIVEIRA MGA AND SOARES JF. 2001. Effect of flavonoids morin; quercetin and nicotinic acid on lipid metabolism of rats experimentally fed with triton. Braz Arch Biol Technol 44: 263-267.
- RONY KA, AJITH TA, NIMA N AND JANARDHANAN KK. 2014. Hypolipidemic activity of Phellinus rimosus against WR-1339 and high cholesterol diet induced hyperlipidemic rats. Environ Toxicol Pharm 37: 482-492.
- SÁNCHEZ-SALCEDO EM, MENA P, GARCÍA-VIGUERA C, HERNÁNDEZ F AND MARTÍNEZ JJ. 2015. (Poly) phenolic compounds and antioxidant activity of white (Morus alba) and black (Morus nigra) mulberry leaves: Their potential for new products rich in phytochemicals. J Funct Foods 18: 1039-1046.
- SCHURR PE, SCHULTZ JR AND PARKINSON TM. 1972. Triton induced hyperlipidemia in rats as an animal model for screening hypolipidemic drugs. Lipids 7: 69-74.
- SESSO HD, GAZIANO JM, LIU S AND BURING JE. 2003. Flavonoid intake and the risk of cardiovascular disease in women. Am J Clin Nutr 77: 1400-1408.
- SILVA CRM AND NAVES MMV. 2001. Vitamin supplementation in cancer chemoprevention. Br J Nutr 14: 135-143.
- SINGH J, UPADHYAY AK, BAHADUR A, SINGH B, SINGH KP AND RAI M. 2006. Antioxidant phytochemicals in cabbage (Brassica oleracea L. var. capitata). Sci Hort 108: 233-237.
- SINGLETON VL AND ROSSI JA. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungistic acid reagents. Am J Enol Vit 16: 144-158.
- STEFANELLO N, SCHMATZ R, PEREIRA LB, RUBIN MA, DA ROCHA JBT, FACCO G AND SCHETINGER MRC. 2014. Effects of chlorogenic acid, caffeine, and coffee on behavioral and biochemical parameters of diabetic rats. Mol Cell Biochem 388: 277-286.
- TAROZZI A, ANGELONI C, MALAGUTI M, MORRONI F, HRELIA S AND HRELIA P. 2013. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid Med Cell Longev 9: 1-10.
- VOLPATO GT, CALDERON IMP, SINZATO S, CAMPOS KE, RUDGE MVC AND DAMASCENO DC. 2011. Effect of Morus nigra aqueous extract treatment on the maternal-fetal outcome, oxidative stress status and lipid profile of streptozotocin-induced diabetic rats. J Ethnopharmacol 138: 691-696.
- WAN CW, WONG CNY, PIN WK, WONG MHY, KWOK CY AND CHAN RYK. 2013. Chlorogenic acid exhibits cholesterol lowering and fatty liver attenuating properties by up-regulating the gene expression of PPAR-α in hypercholesterolemic rats induced with a high-cholesterol diet. Phytother Res 27: 545-551.
- WEST KM, AHUJA MMS AND BENNET PH. 1983. The role of circulating glucose and triglyceride concentration and their interaction with other risk factors as determinants of arterial disease in nine diabetic population samples from the WHO multinational study. Diabetes Care 6: 361-369.
- WICZKOWSKI W, NOWAK DS AND TOPOLSKA J. 2013. Red cabbage anthocyanins: profile, isolation, identification, and antioxidant activity. Food Res Int 51: 303-309.
- WOISKY RG AND SALATINO A. 1998. Analysis of propolis: some parameters and procedures for chemical quality control. J Apicul Res 37: 99-105.
- XIA SF, XIE ZX, QIAO Y, LI LR, CHENG XR, TANG X AND LE GW. 2015. Differential effects of quercetin on hippocampus-dependent learning and memory in mice fed with different diets related with oxidative stress. Physiol Behav 138: 325-331.
- ZARZECKI MS, ARAUJO SM, BORTOLOTTO VC, DE PAULA MT, JESSE CR AND PRIGOL M. 2014. Hypolipidemic action of chrysin on Triton WR-1339-induced hyperlipidemia in female C57BL/6 mice. Toxicol Rep 1: 200-208.
Publication Dates
-
Publication in this collection
07 Dec 2017 -
Date of issue
Oct-Dec 2017
History
-
Received
03 Oct 2016 -
Accepted
31 Jan 2017