Abstracts
Hereditary spastic paraplegia (HSP) is characterized by weakness and spasticity of the lower extremities. Kjellin’s syndrome is a rare syndrome associated with HSP. The syndrome is characterized by the presence of bilateral retinal flecks, similar to the findings in Stargardt disease and fundus flavimaculatus. We report the case of a 34-year-old male who presented with complete features of Kjellin’s syndrome, with typical retinal findings observed on multimodal imaging (spectral domain optical coherence tomography [SD-OCT], near-infrared reflectance and autofluorescence imaging). The ophthalmological changes at early stages of the disease may not impair visual acuity. Therefore, the detection of central retinal degeneration requires thorough fundus examination.
Macular degeneration; Spastic paraplegia, hereditary/diagnosis; Diagnostic techniques, ophthalmological; Case reports
A paralisia espástica hereditária (HSP) é caracterizada por fraqueza e espasticidade das extremidades inferiores. A síndrome de Kjellin é uma rara associação de HSP com a presença de flecks retinianos similares aos encontrados em pacientes com doença de Stargardt ou fundus flavimaculatus. Descrevemos os achados em imagens multimodais da retina (tomografia de coerência óptica de domínio espectral [SD-OCT], reflectância próxima ao infravermelho e autofluorescência) em um paciente de 34 anos que apresenta conjunto completo de sinais e sintomas da síndrome de Kjellin. As alterações retinianas em estágios iniciais da doença podem aparecer, mesmo sem redução da acuidade visual, e por isso, para detecção da degeneração central da retina, é necessário exame minucioso do fundo de olho.
Degeneração macular; Paraplegia espástica hereditária/diagnóstico; Técnicas de diagnóstico oftalmológico; Relatos de casos
INTRODUCTION
Hereditary spastic paraplegia (HSP) is a clinically and genetically heterogeneous group of diseases involving weakness and spasticity of the lower extremities combined with additional neurological or non-neurological manifestations(11 Finsterer J, Loscher W, Quasthoff S, Wanschitz J, Auer-Grumbach M, Stevanin G. Hereditary spastic paraplegias with autosomal dominant, recessive, X-linked, or maternal trait of inheritance. J Neurol Sci. 2012;15(1-2):1-18.). There are almost 48 subtypes of HSP; however, only the 2 subtypes involving mutations of SPG11 and SPG15 are associated with Kjellin’s syndrome(11 Finsterer J, Loscher W, Quasthoff S, Wanschitz J, Auer-Grumbach M, Stevanin G. Hereditary spastic paraplegias with autosomal dominant, recessive, X-linked, or maternal trait of inheritance. J Neurol Sci. 2012;15(1-2):1-18.).
The inheritance of Kjellin’s syndrome is autosomal recessive, and the syndrome is characterized by spastic paraplegia, mental retardation, amyotrophy, thin corpus callosum, and macular dystrophy(22 Webb S, Patterson V, Hutchinson M. Two families with autosomal recessive spastic paraplegia, pigmented maculopathy, and dementia. J Neurol Neurosurg Psychiatry. 1997;63(5):628-32.). Individuals with this syndrome present with macular changes, most often described as fundus flavimaculatus or Stargardt disease-like, particularly on the basis of fluorescein angiography findings(33 Puech B, Lacour A, Stevanin G, Sautiere BG, Devos D, Depienne C, et al. Kjellin syndrome: long-term neuro-ophthalmologic follow-up and novel mutations in the SPG11 gene. Ophthalmology. 2011;118(3):564-73.).
Here we describe ophthalmological findings in a patient with Kjellin’s syndrome, extending previous reports by demonstrating retinal functional and multimodal retinal imaging studies.
CASE REPORT
A 34-year-old white male was admitted to São Paulo University Hospital in Ribeirão Preto, for investigation of spastic paraparesis. At 20 years of age, he had developed muscle weakness in the lower limbs with difficulty in walking. The symptoms progressed slowly; however, 2 years later he had lost the ability to walk unaided.
Since childhood, he had experienced learning difficulties and mild cognitive impairment combined with hypoacusis. His mother reported that he had recently displayed aggressive behavior. There was no long-term history of visual acuity impairment; however, he reported progressive worsening of vision over the past 2 years Prior to admission, there was no significant family history and the only medication was fluoxetine 20 mg per day.
Neurological examination revealed dysarthria, frontal release signs, preserved perception of touch and pain, spasticity of the lower limbs with a scissors gait, and loss of strength and muscle atrophy in the lower limbs and interosseous muscles of the hands. Laboratory studies revealed negative HIV, VDRL, FTA-abs, HTLV I/II, and hepatitis B and C serology. Vitamin B12 and folic acid levels were within normal limits.
Brain magnetic resonance imaging showed significant volumetric loss and corpus callosum atrophy. Electroneuromyography showed severe denervation of segmental cranial, cervical, thoracic, and lumbosacral muscles, suggesting diffuse lower motor neuron disease. Audiometry detected profound sensorineural hearing loss.
Ophthalmological evaluation
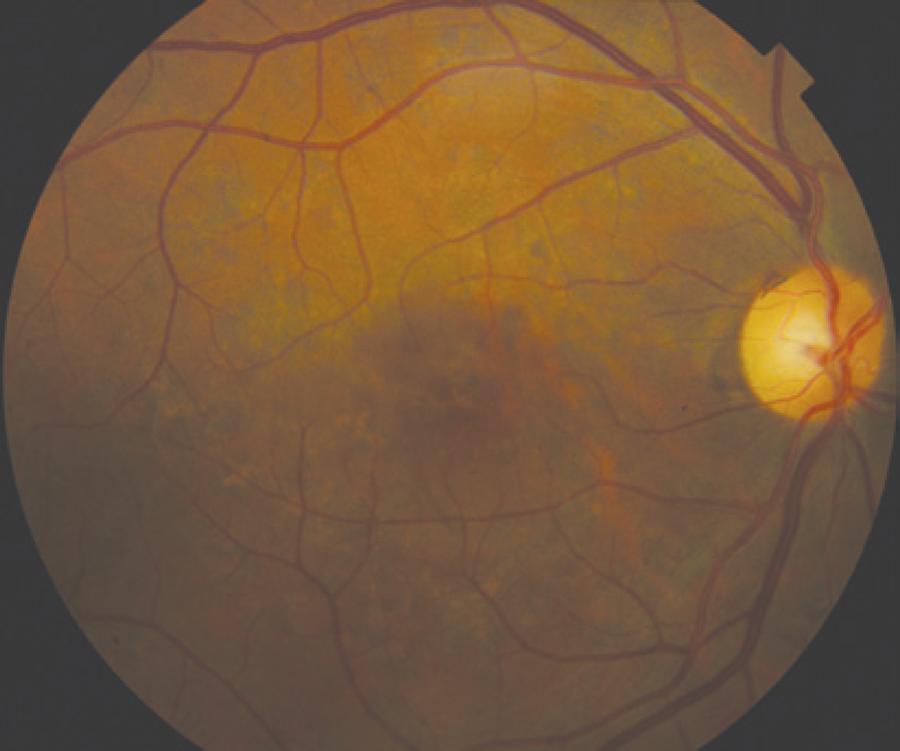
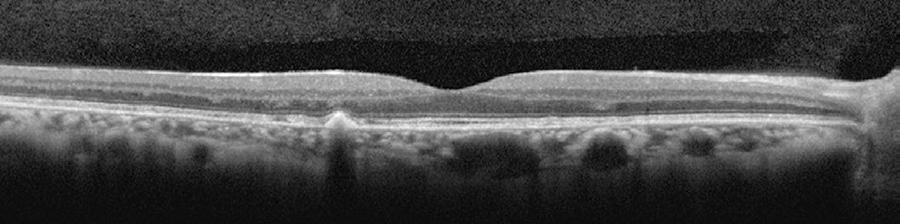
Only mild visual acuity loss was detected, with best-corrected visual acuity of 20/25 in the right eye and 20/30 in the left eye. Pupillary direct and consensual light reflexes were normal and ocular motility was preserved. Intraocular pressure was within the normal range: 14 mmHg in the right eye and 15 mmHg in the left eye (Goldmann). Slit lamp biomicroscopy revealed transparent cornea and lenses with no noteworthy structural changes. The fundus presented multiple round yellowish flecks at the level of the retinal pigment epithelium scattered at the posterior pole. Some of these flecks were elongated and showed partial confluence with neighboring flecks (Figure 1). Imaging was performed with a Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany). Areas of increased fundus autofluorescence (FA) were noted, with surrounding borders of decreased FA intensity, corresponding to the visible yellowish fundus lesions (Figure 2). Spectral domain optical coherence tomography (SD-OCT) showed elevated lesions along the retinal pigment epithelium (RPE)/ Bruch complex, preserving the external limiting membrane (Figure 3).
Color fundus photograph showing symmetric multiple uniform round yellowish lesions at the posterior pole.
Fundus autofluorescence showing high levels of autofluorescence in the center of the biomicroscopically visible flecks and surrounding halos with decreased autofluorescence signal.
Spectral domain optical coherence tomography [SD-OCT] showing elevated lesions located along the retinal pigment epithelium (RPE)/Bruch complex and preserving the external limiting membrane.
Full field and multifocal electroretinography (ERG and mfERG, respectively) were recorded and stored for offline analysis using an Espion E2 system (Diagnosys LLC, Littleton, MA, USA) in accordance with the International Society for Clinical Electrophysiology of Vision (ISCEV) guidelines. The ERG amplitude and implicit time were within normal limits; however, mfERG (Diagnosys LLC) revealed areas of amplitude reduction. Microperimetry (MAIA; CenterVue, Padova, Italy) showed test points with reduction of sensitivity but fixation was relatively stable.
DISCUSSION
The presented patient shows typical phenotypic traits of Kjellin’s syndrome
characterized by absence of the corpus callosum and macular changes. These traits are
suggestive of HSP subtypes 11 and 15, known to be associated with deformity of the
corpus callosum(11 Finsterer J, Loscher W, Quasthoff S, Wanschitz J, Auer-Grumbach M,
Stevanin G. Hereditary spastic paraplegias with autosomal dominant, recessive,
X-linked, or maternal trait of inheritance. J Neurol Sci.
2012;15(1-2):1-18.,44 Stevanin G, Azzedine H, Denora P, Boukhris A, Tazir M, Lossos A, et al.
Mutations in SPG11 are frequent in autosomal recessive spastic paraplegia with thin
corpus callosum, cognitive decline and lower motor neuron degeneration. Brain.
2008;131(Pt 3):772-84.
5 Orlen H, Melberg A, Raininko R, Kumlien E, Entesarian M, Söderberg P, et
al. SPG11 mutations cause Kjellin syndrome, a hereditary spastic paraplegia with thin
corpus callosum and central retinal degeneration. Am J Med Genet B Neuropsychiatr
Genet. 2009;5B(7):984-92.-66 Hanein S, Martin E, Boukhris A, Byrne P, Goizet C, Hamri A, et al.
Identification of the SPG15 gene, encoding spastizin, as a frequent cause of
complicated autosomal-recessive spastic paraplegia, including Kjellin syndrome. Am J
Hum Genet. 2008;82(4):992-1002.).
The natural history of macular dystrophy in Kjellin’s syndrome is such that patients rarely complain of low visual acuity unless it is associated with cataract or optic neuropathy(11 Finsterer J, Loscher W, Quasthoff S, Wanschitz J, Auer-Grumbach M, Stevanin G. Hereditary spastic paraplegias with autosomal dominant, recessive, X-linked, or maternal trait of inheritance. J Neurol Sci. 2012;15(1-2):1-18.,33 Puech B, Lacour A, Stevanin G, Sautiere BG, Devos D, Depienne C, et al. Kjellin syndrome: long-term neuro-ophthalmologic follow-up and novel mutations in the SPG11 gene. Ophthalmology. 2011;118(3):564-73.). In this context, microperimetry could be more sensitive to detect functional impairment in Kjellin’s syndrome, because preserved visual acuity is normally found if the fovea is not involved. However, microperimetry findings show a clear distinction in comparison with eccentric and unstable fixation and the loss of sensitivity found in Stargardt disease(77 Reinhard J, Messias A, Dietz K, Mackeben M, Lakmann R, Scholl HP, et al. Quantifying fixation in patients with Stargardt disease. Vision Res. 2007;47(15):2076-85.).
As expected, no changes were recorded on ERG, presumably because of the mild and focal retinal impairment typical of Kjellin’s syndrome(88 Frisch IB, Haag P, Steffen H, Weber BH, Holz FG. Kjellin's syndrome: fundus autofluorescence, angiographic, and electrophysiologic findings. Ophthalmology. 2002;109(8):1484-91.). However, there were obvious changes on mfERG, with areas of reduced amplitude bilaterally(88 Frisch IB, Haag P, Steffen H, Weber BH, Holz FG. Kjellin's syndrome: fundus autofluorescence, angiographic, and electrophysiologic findings. Ophthalmology. 2002;109(8):1484-91.).
Interestingly, some characteristics of the yellowish-white flecks interspersed with diffuse pigmented lesions found in Kjellin’s syndrome also differ from those found in Stargardt disease and fundus flavimaculatus. In Kjellin’s syndrome, the areas of increased FA are surrounded by halos of decreased FA(99 Querques G, Prato R, Coscas G, Soubrane G, Souied EH. In vivo visualization of photoreceptor layer and lipofuscin accumulation in stargardt's disease and fundus flavimaculatus by high resolution spectral-domain optical coherence tomography. Clin Ophthalmol. 2009;3:693-9.,1010 Voigt M, Querques G, Atmani K, Leveziel N, Massamba N, Puche N, et al. Analysis of retinal flecks in fundus flavimaculatus using high-definition spectral-domain optical coherence tomography. Am J Ophthalmol. 2010;150(3):330-7.).
On SD-OCT, the presence of elevated lesions along the RPE/Bruch complex, corresponding
to areas of increased FA without atrophy of the RPE/Bruch complex and the inner and
outer segment (IS/OS) layer, also differ from the findings in Stargardt disease and
fundus flavimaculatus(99 Querques G, Prato R, Coscas G, Soubrane G, Souied EH. In vivo
visualization of photoreceptor layer and lipofuscin accumulation in stargardt's
disease and fundus flavimaculatus by high resolution spectral-domain optical
coherence tomography. Clin Ophthalmol. 2009;3:693-9.
10 Voigt M, Querques G, Atmani K, Leveziel N, Massamba N, Puche N, et al.
Analysis of retinal flecks in fundus flavimaculatus using high-definition
spectral-domain optical coherence tomography. Am J Ophthalmol.
2010;150(3):330-7.-1111 Gomes NL, Greenstein VC, Carlson JN, Tsang SH, Smith RT, Carr RE, et al.
A comparison of fundus autofluorescence and retinal structure in patients with
Stargardt disease. Invest Ophthalmol Vis Sci. 2009;50(8):3953-9.).
The diagnosis of macular changes in HSP is important for the genetic evaluation because macular changes indicate association with subtypes 11 and 15. The mutations occur in KIAA 1840 (SPG11) and ZFYVE 26 (SPG15) that encode the proteins spatacsin and spastizin, respectively(11 Finsterer J, Loscher W, Quasthoff S, Wanschitz J, Auer-Grumbach M, Stevanin G. Hereditary spastic paraplegias with autosomal dominant, recessive, X-linked, or maternal trait of inheritance. J Neurol Sci. 2012;15(1-2):1-18.). These proteins are part of the AP5 membrane-trafficking complex and have been identified in animal models of photoreceptor cells(1212 Murmu RP, Martin E, Rastetter A, Esteves T, Muriel MP, El Hachimi KH, et al. Cellular distribution and subcellular localization of spatacsin and spastizin, two proteins involved in hereditary spastic paraplegia. Mol Cell Neurosci. 2011;47(3):191-202.). This suggests that the retinal changes in Kjellin’s syndrome might be the result of a metabolic defect, causing accumulation of products related to lipofuscin.
In summary, this case represents Kjellin’s syndrome with a complete SPG11 and SPG15 phenotype with macular changes. The ophthalmological changes, particularly at early stages of the disease, may not impair visual acuity but can be detected with microperimetry, careful fundus examination, and autofluorescence and/or SDOCT.
-
Funding: No specific financial support was available for this study.
ACKNOWLEDGMENT
We thank Mr. and Mrs. Balit for revising the manuscript.
REFERENCES
-
1Finsterer J, Loscher W, Quasthoff S, Wanschitz J, Auer-Grumbach M, Stevanin G. Hereditary spastic paraplegias with autosomal dominant, recessive, X-linked, or maternal trait of inheritance. J Neurol Sci. 2012;15(1-2):1-18.
-
2Webb S, Patterson V, Hutchinson M. Two families with autosomal recessive spastic paraplegia, pigmented maculopathy, and dementia. J Neurol Neurosurg Psychiatry. 1997;63(5):628-32.
-
3Puech B, Lacour A, Stevanin G, Sautiere BG, Devos D, Depienne C, et al. Kjellin syndrome: long-term neuro-ophthalmologic follow-up and novel mutations in the SPG11 gene. Ophthalmology. 2011;118(3):564-73.
-
4Stevanin G, Azzedine H, Denora P, Boukhris A, Tazir M, Lossos A, et al. Mutations in SPG11 are frequent in autosomal recessive spastic paraplegia with thin corpus callosum, cognitive decline and lower motor neuron degeneration. Brain. 2008;131(Pt 3):772-84.
-
5Orlen H, Melberg A, Raininko R, Kumlien E, Entesarian M, Söderberg P, et al. SPG11 mutations cause Kjellin syndrome, a hereditary spastic paraplegia with thin corpus callosum and central retinal degeneration. Am J Med Genet B Neuropsychiatr Genet. 2009;5B(7):984-92.
-
6Hanein S, Martin E, Boukhris A, Byrne P, Goizet C, Hamri A, et al. Identification of the SPG15 gene, encoding spastizin, as a frequent cause of complicated autosomal-recessive spastic paraplegia, including Kjellin syndrome. Am J Hum Genet. 2008;82(4):992-1002.
-
7Reinhard J, Messias A, Dietz K, Mackeben M, Lakmann R, Scholl HP, et al. Quantifying fixation in patients with Stargardt disease. Vision Res. 2007;47(15):2076-85.
-
8Frisch IB, Haag P, Steffen H, Weber BH, Holz FG. Kjellin's syndrome: fundus autofluorescence, angiographic, and electrophysiologic findings. Ophthalmology. 2002;109(8):1484-91.
-
9Querques G, Prato R, Coscas G, Soubrane G, Souied EH. In vivo visualization of photoreceptor layer and lipofuscin accumulation in stargardt's disease and fundus flavimaculatus by high resolution spectral-domain optical coherence tomography. Clin Ophthalmol. 2009;3:693-9.
-
10Voigt M, Querques G, Atmani K, Leveziel N, Massamba N, Puche N, et al. Analysis of retinal flecks in fundus flavimaculatus using high-definition spectral-domain optical coherence tomography. Am J Ophthalmol. 2010;150(3):330-7.
-
11Gomes NL, Greenstein VC, Carlson JN, Tsang SH, Smith RT, Carr RE, et al. A comparison of fundus autofluorescence and retinal structure in patients with Stargardt disease. Invest Ophthalmol Vis Sci. 2009;50(8):3953-9.
-
12Murmu RP, Martin E, Rastetter A, Esteves T, Muriel MP, El Hachimi KH, et al. Cellular distribution and subcellular localization of spatacsin and spastizin, two proteins involved in hereditary spastic paraplegia. Mol Cell Neurosci. 2011;47(3):191-202.
Publication Dates
-
Publication in this collection
Mar-Apr 2015
History
-
Received
22 Apr 2014 -
Accepted
09 July 2014