Abstract
The state of Maranhão, located in northeastern Brazil, comprises three biomes: Amazonian, Caatinga, and the Cerrado. To date, 99 ant species have been recorded in the literature from the state. In the present work, we provide for the first time a profile of the ant fauna in the state based on data from the historical literature and Brazilian institutional collections. The updated records on ant diversity for the state of Maranhão revealed a total of 279 species, belonging to 71 genera and 10 subfamilies. In total, 180 species are recorded for the first time in the state, of which four species recorded for the first time in Brazil. In summary, apart from documenting the ant fauna of the region, these results provide a basis for further studies and may contribute to future conservation efforts for the biomes present in this complex landscape.
Key-Words
Distribution; Amazon; Caatinga; Cerrado; Checklist
INTRODUCTION
Understanding the distribution of species is essential to determine regional and global patterns of biodiversity (Dalzochio et al., 2018Dalzochio, M.S.; Renner, S.; Sganzerla, C.; Prass, G.; Ely, G.J.; Salvi, L.C.; Dametto, N. & Périco, E. 2018. Checklist of Odonata (Insecta) in the state of Rio Grande do Sul, Brazil with seven new records. Biota Neotropica, 18: 1-14.). In this sense, taxonomic inventories contribute to characterize areas of endemism, reveal taxonomic novelties and improve scientific collections (Moura et al., 2014Moura, C.C.M.; Moura, G.J.B.; Lisboa, E.B.F. & Luz, V.L.F. 2014. Distribuição geográfica e considerações ecológicas sobre a fauna de Testudines da Região Nordeste do Brasil. Sitientibus serie Ciencias Biologicas, 14: 1-20.; Freitas et al., 2017Freitas, M.A.; Vieira, R.S.; Entiauspe-Neto, O.M.; Sousa, S.O.; Farias, T.; Sousa, A.G. & Moura, G.J.B. 2017. Herpetofauna of the Northwest Amazon forest in the state of Maranhão, Brazil, with remarks on the Gurupi Biological Reserve. ZooKeys, 643: 141-155.). Further, the analysis of species distribution databases can help to identify gaps in sampling and species records, and can also be used in macroecological studies, species distribution modeling and to promote conservation strategies (Gasper et al., 2016Gasper, A.L.; Eisenlohr, P.V. & Salino, A. 2016. Improving collection efforts to avoid loss of biodiversity: lessons from comprehensive sampling of lycophytes and ferns in the subtropical Atlantic Forest. Acta Botanica Brasilica, 30: 166-175.).
Maranhão is a northeastern state in Brazil and comprises a total area of 329,642.170 km² (IBGE, 2018Instituto Brasileiro de Geografia e Estatística (IBGE). 2018. Maranhão. Available at: Available at: http://cidades.ibge.gov.br/brasil/ma/panorama
. Access in: 02/02/2019.
http://cidades.ibge.gov.br/brasil/ma/pan...
). Its political boundaries are the Atlantic Ocean to the north, the state of Tocantins to the south, the state of Piauí to the east, and the state of Pará to the west (Chaves et al., 2016Chaves, L.P.F.A.; Silva, R.A.; Amaral, Y.T.; Costa, M.K.L. & Siqueira, G.M. 2016. Biogeographical diversity of north mesoregion of the Maranhão state (Brazil). Journal of Geospatial Modelling, 1: 19.). The state is located in a heterogeneous landscape area under the influence of three biomes: Amazon, Cerrado, and the Caatinga. The vegetation cover - encompassing 14 different vegetation types - reflects the transition between super-humid and semi-arid climates (Santos et al., 2010Santos, M.P.D.; Cerqueira, P.V. & Soares, L.M.S. 2010. Avifauna em seis localidades no centro-sul do Estado do Maranhão, Brasil. Ornithologia, 4(1): 49-65.; IBGE, 2018Instituto Brasileiro de Geografia e Estatística (IBGE). 2018. Maranhão. Available at: Available at: http://cidades.ibge.gov.br/brasil/ma/panorama
. Access in: 02/02/2019.
http://cidades.ibge.gov.br/brasil/ma/pan...
).
Similar to other states in Brazil, Maranhão has suffered with high human impact, mainly from the early 1960s, through the construction of highways, agricultural and mining projects (Celentano et al., 2017Celentano, D.; Rousseau, G.X.; Engel, V.L.; Zelarayán, M.; Oliveira, E.C.; Araújo, A.C.M. & De Moura, E.G. 2017. Degradation of riparian forest affects soil properties and ecosystem services provision in eastern amazon of Brazil. Land Degradation & Development, 28: 482-493.). Impacts include large-scale forest conversion to pasture or by “babaçu” palm trees (Orbignya phalerata Mart.) (Santos et al., 2010Santos, M.P.D.; Cerqueira, P.V. & Soares, L.M.S. 2010. Avifauna em seis localidades no centro-sul do Estado do Maranhão, Brasil. Ornithologia, 4(1): 49-65.), and the expansion of agroindustry has converted large natural areas into grain crops (Brasil, 2009Brasil. 2009. Ministério do Meio Ambiente. Relatório técnico de monitoramento do desmatamento no bioma Cerrado, 2002 a 2008: dados revisados. Brasília: MMA, 67p. Available at: Available at: http://www.mma.gov.br/estruturas/sbf_chm_rbbio/_arquivos/relatorio_tecnico_monitoramento_desmate_bioma_cerrado_csr_ibama_2002_2008_rev_72.pdf
. Access in: 08/12/2018.
http://www.mma.gov.br/estruturas/sbf_chm...
; Santos et al., 2010Santos, M.P.D.; Cerqueira, P.V. & Soares, L.M.S. 2010. Avifauna em seis localidades no centro-sul do Estado do Maranhão, Brasil. Ornithologia, 4(1): 49-65.). In addition, other human activities, such as occupation, recreation and tourism (Chaves et al., 2016Chaves, L.P.F.A.; Silva, R.A.; Amaral, Y.T.; Costa, M.K.L. & Siqueira, G.M. 2016. Biogeographical diversity of north mesoregion of the Maranhão state (Brazil). Journal of Geospatial Modelling, 1: 19.) have also a negative impact and have caused severe loss of biodiversity, resulting in drastic changes of the landscape.
The biodiversity of Maranhão is extremely diverse (Chaves et al., 2016Chaves, L.P.F.A.; Silva, R.A.; Amaral, Y.T.; Costa, M.K.L. & Siqueira, G.M. 2016. Biogeographical diversity of north mesoregion of the Maranhão state (Brazil). Journal of Geospatial Modelling, 1: 19.; Desidério et al., 2017Desidério, G.R.; Barcelos-Silva, P.; De Souza, W.R.M.; Pes, A.M. & Azevêdo, C.A.S. 2017. Caddisflies (Insecta: Trichoptera) from Maranhão State, Northeast Region, Brazil: A new species, checklist, and new geographical records. Zootaxa, 4221: 151-171.). Compared to other Brazilian states, however, the ant diversity is poorly known. The most recent information on ant species diversity in the state recorded 99 species, belonging to 37 genera and seven subfamilies (Janicki et al., 2016Janicki, J.; Narula, N.; Ziegler, M.; Guénard, B. & Economo, E.P. 2016. Visualizing and interacting with large-volume biodiversity data using client-server web-mapping applications: The design and implementation of antmaps.org. Ecological Informatics, 32: 185-193.). This represents about ¼ of ant diversity in the state of Goiás and 35% of the ant species richness described for the state of Mato Grosso do Sul (Janicki et al., 2016Janicki, J.; Narula, N.; Ziegler, M.; Guénard, B. & Economo, E.P. 2016. Visualizing and interacting with large-volume biodiversity data using client-server web-mapping applications: The design and implementation of antmaps.org. Ecological Informatics, 32: 185-193.), two other Brazilian states comparable in size to Maranhão.
Since the end of the 20th century, collective efforts of several research groups, carring out inventories in different areas and employing complementary sampling methodologies, resulted in a significant increase in our knowledge about ant diversity in this state. Thus, the aim of this study is to present an updated list of the ant species in the state of Maranhão, considering recent field expeditions as well as material deposited in the main Brazilian ant collections. We also discuss some relevant aspects about the profile of the ant fauna, recovering the history of ant studies historically carried out in the state. Overall, our findings should be of great help in creating measures for species preservation and species recovery plans and represent the basis for future research.
MATERIAL AND METHODS
Data from collections and literature
We listed material obtained from six Brazilian ant collections (Table 1), which have historically acted as main depositary institutions for samples collected in the state of Maranhão. We also compiled data from literature, including collection events focused on partial surveys of Maranhão ant fauna (Table 2).
References and their respective areas that have records of ants collected in the state of Maranhão.
Identifications and taxonomic validation
Ants were identified by the authors of the present study using taxonomic keys, comparing specimens with myrmecological collections, or by sending them to specialists (see “Acknowledgements”). The final list containing all specimens was verified by authors of this study (JAS, LPP and RMF). Species with dubious identification were carefully examined and, when necessary, have been removed from final data set.
Distribution and maps
The biomes present in Maranhão are the Amazon Forest, characterized by tall trees and periodic to permanently flooded plains; this biome is present in the north and, essentially, in the west portion of the state. The Cerrado covers the south, central and northeast areas of the state, formed by open grasslands (Cerrado aberto) to patches of dense vegetation (Cerradão). Finally, Maranhão presents a small and fragmented portion of the Caatinga biome, in the extreme east of the state, characterized by the presence of bushy vegetation with deep roots, cacti and bromeliads (Spinelli-Araujo et al., 2016Spinelli-Araujo, L.; Bayma-Silva, G.; Torresan, F.E.; Victoria, D.; Vicente, L.E.; Bolfe, E.L. & Manzatto, C. 2016. Conservação da biodiversidade do estado do Maranhão: cenário atual em dados geoespaciais. Jaguariúna, EMBRAPA Meio Ambiente. 28p.).
We used shapefiles from the state of Maranhão made available by the Ministério do Meio Ambiente (MMA) (http://mapas.mma.gov.br/i3geo/datadownload.htm#). We used a classification in “meso-regions” pre-established by the government agency, in order to describe and discuss our results. We also used shapefiles provided by MMA for the three main biomes present in the state, to overlap sampling points and the main ecosystems in Maranhão.
For the confirmation of sampled sites (Table 3) and maps preparation, the geographical coordinates, when not available on the specimens’ label, were obtained from the IBGE (2011Instituto Brasileiro de Geografia e Estatística (IBGE). 2011. Índice de Nomes Geográficos, Escala 1:1.000.000. Base Cartográfica Contínua do Brasil ao Milionésimo. Disponível em: Disponível em: http://www.ibge.gov.br/geociencias/cartas-e-mapas/bases-cartograficas-continuas/15759-brasil.html?=&t=sobre
. Acesso em: 03/12/2018.
http://www.ibge.gov.br/geociencias/carta...
) or georeferenced using Google Earth Pro. In those cases, because we did not have access to the exact point of the sample site, we adapted a classification by the IBGE. Whenever the IBGE classified a municipality covering two biomes, we used the “transition” term after the government classification. For instance, the municipality of Imperatriz, which is classified by IBGE as “Amazon/Cerrado” biomes, becomes for the purpose of this study, “Amazon-Cerrado transition”. For specific sites and localities for which names have been historically altered, we consulted Vanzolini & Papavero (1968Vanzolini, P.E. & Papavero, N. 1968. Índice dos Topônimos Contidos na Carta do Brasil 1:1.000.000 do IBGE. São Paulo, Fundação de Amparo à Pesquisa do Estado de São Paulo. 201p.) and Vanzolini (1992Vanzolini, P.E. 1992. A Supplement to the Ornithological Gazetteer of Brazil. São Paulo, Museu de Zoologia da Universidade de São Paulo. 252p.). The geographical records were mapped using QGIS v2.18.2 (QGIS Development Team, 2019QGIS Development Team. 2019. QGIS geographic information system. Open source geospatial foundation project. Disponível em: http://qgis.org/en/site/forusers/download.html.
http://qgis.org/en/site/forusers/downloa...
).
Information from the sampled sites for the state of Maranhão. The abbreviations are as follows: (Am) Amazon, (Ce) Cerrado, (ACT) Amazon-Cerrado transition, (CCT) Cerrado-Caatinga transition. (*) For the geographic coordinates attributed in this work.
RESULTS
Based on data from Brazilian collections (Table 1) and published literature (Table 2), we recorded a total of 279 ant species for the state of Maranhão, belonging to 71 genera and 10 subfamilies (Table 4), and sampled across 65 localities (Table 3). The subfamily Myrmicinae was the most diverse, with 126 species, followed by Ponerinae (36 species), Formicinae (35 species), Dolichoderinae (27 species), Ectatomminae (25 species), Pseudomyrmecinae (16 species), Dorylinae (10 species), Amblyoponinae (2 species), and Paraponerinae and Proceratiinae (1 species each).
List of taxa recorded in the state of Maranhão and the occurrence data of the species in the literature and localities and biome present in the state. The codes of localities follow Table 3. (*) new record for Maranhão, (**) new record for Brazil, (Am) Amazon, (Ce) Cerrado, (ACT) Amazon-Cerrado transition, (CCT) Cerrado-Caatinga transition.
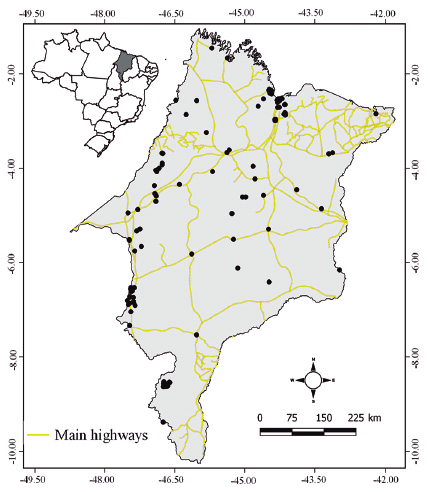
The majority of records (214 species) was concentrated along the Amazon region, followed by the Cerrado (129 species), the Amazon-Cerrado transition regions (80 species) and finally the Cerrado-Caatinga transition region where only one species was recorded (Fig. 1). A total of 180 ant species were recorded for the first time in the state, and four species were recorded for the first time in Brazil (Table 4).
Map of the state of Maranhão showing its location in Brazil. Black points indicate the sampling sites within the state that were georeferenced and recorded in the literature and collections according to biomes.
DISCUSSION
The first expeditions focused on studying the ant fauna of the state of Maranhão were performed in the late 1940s, with collections in the Cerrado areas undertaken by the myrmecologists Cincinnato Gonçalves and Walter W. Kempf. During the next three decades, collections by researchers, enthusiasts, and professional collectors had pursued the same goal - discovering new taxa and increasing the coverage of ants in scientific collections (Kempf, 1972aKempf, W.W. 1972a. Catálogo abreviado das formigas da região Neotropical (Hym. Formicidae). Studia Entomologica, 15: 1-4.). Differently, from the 1980s until the beginning of the 21st century, the main purpose of the expeditions was to carry out environmental impact assessment programs (Brandão et al., 2011Brandão, C.R.F.; Silva, R.R. & Feitosa, R.M. 2011. Cerrado ground-dwelling ants (Hymenoptera: Formicidae) as indicators of edge effects. Zoologia, 28(3): 379-387.). On the other hand, from the late 20th century, with the hiring of researchers at universities in the state of Maranhão, several expeditions have been conducted focusing on ecological studies and reporting faunal inventories (Ramos et al., 2015Ramos, A.S.J.C.; Lemos, R.N.S.; Vale, A.M.S; Batista, M.C.; Moreira, A.A.; Harada, H.Y. & Mesquita, M.L.R. 2015. Ant diversity in agro ecosystems and secondary forest. African Journal of Agricultural Research, 10: 4449-4454.; Gutiérrez et al., 2017Gutiérrez, J.A.M.; Rousseau, G.X.; Andrade-Silva, J. & Delabie, J.H.C. 2017. Taxones superiores de hormigas como sustitutos de la riqueza de especies, en una cronosecuencia de bosques secundarios, bosque primario y sistemas agroforestales en la Amazonía Oriental, Brasil. Revista de Biología Tropical, 65(1): 279-291.; Silva et al., 2017Silva, E.F.; Corá, J.E.; Harada, A.Y. & Sampaio, I.B.M. 2017. Association of the Occurrence of Ant Species (Hymenoptera: Formicidae) with Soil Attributes, Vegetation, and Climate in the Brazilian Cerrado Northeastern Region. Sociobiology, 64(4): 442-450.).
Museums, scientific collections, and historical published literature all contain important information on species distributions recorded as presence data (Newbold, 2010Newbold, T. 2010. Applications and limitations of museum data for conservation and ecology, with particular attention to species distribution models. Progress in Physical Geography, 34: 3-22.). The accuracy of the distribution data is important for several applications in biology and for species conservation planning (Graham et al., 2008Graham, C.H.; Elith, J.; Hijmans, R.J.; Guisan, A.; Townsend Peterson, A. & Loiselle, B.A. 2008. The influence of spatial errors in species occurrence data used in distribution models. Journal of Applied Ecology, 45: 239-247.). Despite the concern to accurately document of species distribution that began in the first half of the 19th century (Vanzolini, 2004Vanzolini, P.E. 2004. Episódios da Zoologia Brasílica. São Paulo, Hucitec. 212p.), for the ants this occurred in the second half of the 20th century. In the case of the records analysed in this work, the specific localities and geographic coordinates became available in the late 20th century.
Most of the ant records for the state of Maranhão remained unavailable to the specialized public for a long time, while many other records remained unidentified at a specific level. In this sense, our study has analyzed both the material deposited in Brazilian collections (Table 1) and the records in the published literature (Table 2), revealing that 64% of species were recorded in the state for the first time. Further, we made an additional effort to identify the morphospecies in ant collections. For instance, 73 ant morphospecies, belonging to 31 ant genera and two subfamilies were here identified at the specific level for the first time (Table 4).
In our data compilation, we found a number of species that were recorded for the first time in the state of Maranhão, but are widely distributed in Brazil (Janicki et al., 2016Janicki, J.; Narula, N.; Ziegler, M.; Guénard, B. & Economo, E.P. 2016. Visualizing and interacting with large-volume biodiversity data using client-server web-mapping applications: The design and implementation of antmaps.org. Ecological Informatics, 32: 185-193.), as is the case of Dolichoderus imitator Emery, 1894 and Gnamptogenys striatula Mayr, 1884, among others (Table 4). On the other hand, some hyperdiverse and taxonomically challenging genera, such as Pheidole, had a considerable increase in the number of new records. Of the 19 species of Pheidole known to the state, 12 were recorded for the first time in the state of Maranhão, and three species were recorded for the first time in Brazil.
Not surprisingly, the data obtained from the ant literature clearly indicates that taxonomy is the discipline that most contributed to the knowledge of the ant fauna in the state. This is especially true for taxonomic revisions, which deal with large numbers of specimens (e.g.,De Andrade & Baroni Urbani, 1999De Andrade, M.L. & Baroni Urbani, C. 1999. Diversity and Adaptation in the ant genus Cephalotes, past and present. Stuttgarter Beitrage zur Naturkunde Serie B, 271: 893.; Lattke, 2011Lattke, J.E. 2011. Revision of the New World species of the genus Leptogenys Roger (Insecta: Hymenoptera: Formicidae: Ponerinae). Arthropod Systematics & Phylogeny, 69: 127-264.). The high number of taxonomic publications in our survey is justified by the fact that this discipline was the first area of myrmecology to be developed in Brazil, allowing the formation of large repositories. However, although taxonomy is the discipline with the greatest number of published studies in relation to other areas, in the last 20 years the potential of ant fauna data has been explored in different study areas (Table 2).
Other factors that have contributed to increasing our knowledge of the ant fauna in the state of Maranhão are online tools, which provide high definition images of species (AntWeb, 2019AntWeb. 2019. AntWeb. Available at: Available at: http://www.antweb.org
. Access in: 03/01/2019.
http://www.antweb.org...
), taxonomic literature (Bolton, 2019Bolton, B. 2019. An online catalog of the ants of the world. Available at: Available at: http://www.antcat.org
. Access in: 10/02/2019.
http://www.antcat.org...
), geographic distribution of ant specimens (Janicki et al., 2016Janicki, J.; Narula, N.; Ziegler, M.; Guénard, B. & Economo, E.P. 2016. Visualizing and interacting with large-volume biodiversity data using client-server web-mapping applications: The design and implementation of antmaps.org. Ecological Informatics, 32: 185-193.), and general information on ant taxa (AntWiki, 2019AntWiki. 2019. AntiWiki. Available at: Available at: http://www.antwiki.org
. Access in: 19/01/2019.
http://www.antwiki.org...
). These tools facilitate the identification of specimens and provide a fast and effective access to information. In addition, the improvement and development of collection methodologies (Figueiredo et al., 2013Figueiredo, C.J.; Silva, R.R.; Munhae, C.B. & Morini, M.S.C. 2013. Fauna de formigas (Hymenoptera: Formicidae) atraídas a armadilhas subterrâneas em áreas de Mata Atlântica. Biota Neotropica, 13: 1-7.) has made the sampling more efficient.
Despite the increased understanding of biodiversity in this region, sampling coverage of ant fauna in Maranhão is strongly irregular (Fig. 1). Our study showed that the Amazon is the better sampled biome and also houses the largest number of species recorded in the state (Table 4). Most collection points are concentrated in the northern region of the state (Fig. 1), which corresponds to the Coastal region of Maranhão, with the highest population density (Chaves et al., 2016Chaves, L.P.F.A.; Silva, R.A.; Amaral, Y.T.; Costa, M.K.L. & Siqueira, G.M. 2016. Biogeographical diversity of north mesoregion of the Maranhão state (Brazil). Journal of Geospatial Modelling, 1: 19.), and where the main research centers are located.
While the Cerrado, which corresponds to the biome with the highest coverage in the state (64%) (MMA, 2011Ministério do Meio Ambiente (MMA). 2011. Plano de Ação para prevenção e controle do desmatamento e das queimadas: Cerrado. Brasília, MMA. 200p.; Stella, 2011Stella, A. 2011. Plano de prevenção e controle do desmatamento e queimadas do Maranhão. São Luís, SEMA. 120p.), remains poorly sampled with extremely sparse collections (Fig. 1). In relation to this biome, it is in the southern part of the state where most of the collection points are concentrated, which in most cases came from samples derived from environmental impact assessment programs (e.g.,Brandão et al., 2011Brandão, C.R.F.; Silva, R.R. & Feitosa, R.M. 2011. Cerrado ground-dwelling ants (Hymenoptera: Formicidae) as indicators of edge effects. Zoologia, 28(3): 379-387.).
The Amazon-Cerrado transition regions are also undersampled in the state, with few records available from taxonomic papers (Kempf, 1972aKempf, W.W. 1972a. Catálogo abreviado das formigas da região Neotropical (Hym. Formicidae). Studia Entomologica, 15: 1-4.; Brandão, 1991Brandão, C.R.F. 1991. Adendos ao catálogo abreviado das formigas da região neotropical (Hymenoptera: Formicidae). Revista Brasileira de Entomologia, 35: 319-412.) and collections. If we want to understand the association between species and forest formations it is essential to characterize species diversity in ecotones, as already observed by other groups (Santos et al., 2010Santos, M.P.D.; Cerqueira, P.V. & Soares, L.M.S. 2010. Avifauna em seis localidades no centro-sul do Estado do Maranhão, Brasil. Ornithologia, 4(1): 49-65.; Maracahipes-Santos et al., 2018Maracahipes-Santos, L.; Santos, J.O.; Reis, S.M. & Lenza, E. 2018. Temporal changes in species composition, diversity, and woody vegetation structure of savannas in the Cerrado-Amazon transition zone. Acta Botanica Brasilica, 32(2): 254-263.).
The Caatinga biome remains largely unknown in Maranhão, represented in our study by a single record in the Cerrado-Caatinga transition region (Fig. 1). Although the biome presents a small and fragmented spatial coverage (1% of the state territorial area) (Stella, 2011Stella, A. 2011. Plano de prevenção e controle do desmatamento e queimadas do Maranhão. São Luís, SEMA. 120p.), the scarcity of information about the ant fauna in the Caatinga has also been observed in other regions of Brazil (Santos et al., 1999Santos, G.M.M.; Delabie, J.H.C. & Resende, J.J. 1999. Caracterização da Mirmecofauna (Hymenoptera: Formicidae) associada à vegetação periférica de inselbergs (Caatinga-arbórea-estacional-semi-descídua) em Itatim-Bahia-Brasil. Sitientibus. Revista da Universidade Estadual de Feira de Santana, 20: 33-43.; Ulysséa & Brandão, 2013Ulysséa, M.A. & Brandão, C.R.F. 2013. Ant species (Hymenoptera, Formicidae) from the seasonally dry tropical forest of northeastern Brazil: a compilation from field surveys in Bahia and literature records. Revista Brasileira de Entomologia, 57: 217-224.; Leal et al., 2017Leal, I.R.; Leal, L.; Oliveira, F.P.; Arcoverde, G.B. & Andersen, A.N. 2017. Effects of human disturbance and climate change on myrmecochory in Brazilian Caatinga. In: Oliveira, P.S. & Koptur, S. (Eds.). Ant-plant interactions. Impacts of human on terrestrial ecosystems. Cambridge, UK, Cambridge University Press. p. 112-132.). This result illustrates the need for greater collection effort to understand and preserve biodiversity in the Caatinga and, consequently, in the state of Maranhão.
One of the main limitations of the data available to date on the ant fauna in Maranhão was a strong sampling bias, with most samples being collected near the main roads (Fig. 2). This pattern of biased sampling near highways, rivers, coasts, and cities has been reported in several taxonomic groups (Hijmans et al., 2000Hijmans, R.J.; Garrett, K.A.; Huama’n, Z.; Zhang, D.P.; Schreuder, M. & Bonierbale, M. 2000. Assessing the geographic representativeness of genebank collections: the case of Bolivian wild potatoes. Conservation Biology, 14: 1755-65.; Kadmon et al., 2003Kadmon, R.; Farber, O. & Danin, A. 2003. A systematic analysis of factors affecting the performance of climatic envelope models. Ecological Applications, 13: 853-67.; Reddy & Dávalos, 2003Reddy, S. & Dávalos, L.M. 2003: Geographical sampling bias and its implications for conservation priorities in Africa. Journal of Biogeography, 30: 1719-27.; Newbold, 2010Newbold, T. 2010. Applications and limitations of museum data for conservation and ecology, with particular attention to species distribution models. Progress in Physical Geography, 34: 3-22.; Santos & Hoppe, 2018Santos, B.F. & Hoppe, J.P.M. 2018. Filling gaps in species distributions through the study of biological collections: 415 new distribution records for Neotropical Cryptinae (Hymenoptera, Ichneumonidae). Revista Brasileira de Entomologia, 62(4): 288-291.), which is explained by the ease access, researchers’ interest in certain areas or taxa, and limited financial resources. However, further studies are required to reduce this sampling bias by using different collection methodologies and accessing previously unexplored sites.
Map of the state of Maranhão emphasizing the main highways and sampling sites of ant species within the state.
Low levels of sampling in conservation areas of the state were also observed (Fig. 3). Conservation areas (i.e., national parks, ecological stations, extractive reserves, national forests, biological reserves, among others) are of fundamental importance for biodiversity conservation (Peres, 2005Peres, C.A. 2005. Why we need mega-reserves in Amazonian forests. Conservation Biology, 19: 728-733.) and preserving ecosystem (Hallmann et al., 2017Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; Goulson, D. & de Kroon, H. 2017. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. Plos One, 12(10): e0185809.).
Map of the state of Maranhão emphasizing the Priority Conservation Areas and sampling sites of ant species within the state.
To the best of our knowledge, this is the first compilation focused on studying the ant fauna of Maranhão, one of the largest geopolitical regions of Brazil. Our study significantly increase the number of ant species recorded in the state and demonstrates the importance of carrying out planned inventories for a more detailed understanding of the regional ant fauna. Finally, our data provide the baseline information to further explore the ant fauna in Maranhão, to improve current knowledge and to accurately determine the occurrence of several species.
CONCLUSION
This paper represents an updated record of the ant species occurring in the state of Maranhão, with numbers increasing from 99 to 279 species. Further collection efforts in different biomes are essential for a better understanding of the biodiversity of the state, and for planning long-term conservation action. Ongoing studies on taxonomy, natural history, and ecology are certainly expected to contribute to this.
ACKNOWLEDGMENTS
We thank the researchers Emília Z. Albuquerque (Arizona State University), Fabrício Baccaro (Universidade Federal do Amazonas), Jacques Delabie (Comissão Executiva do Plano da Lavoura Cacaueira), Mônica Ulysséa (Museu de Zoologia da Universidade de São Paulo), Phil Ward (University of California) and, Ricardo Vicente (Universidade do Estado de Mato Grosso) for their help with the identification/confirmation of the ant species. ACF and RMF were financed by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (processes 140260/2016-1 and 1302462/2016-3, respectively). LPP, JAS and SPT were financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. TSRS was financed by CAPES (process 40001016005P5). JAMG and GXR were financed by the Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão (FAPEMA) - Finance Code 03135/13.
REFERENCES
- Andrade-Silva, J.; Pereira, E.K.C.; Silva, O.; Santos, C.L.C.; Delabie, J.H.C. & Rebelo, J.M.M. 2015. Ants (Hymenoptera: Formicidae) associated with pig carcasses in an urban area. Sociobiology, 62(4): 527-532.
- AntWeb. 2019. AntWeb. Available at: Available at: http://www.antweb.org Access in: 03/01/2019.
» http://www.antweb.org - AntWiki. 2019. AntiWiki. Available at: Available at: http://www.antwiki.org Access in: 19/01/2019.
» http://www.antwiki.org - Bolton, B. 2019. An online catalog of the ants of the world. Available at: Available at: http://www.antcat.org Access in: 10/02/2019.
» http://www.antcat.org - Borgmeier, T. 1955. Die Wanderameisen der neotropischen Region. Studia Entomologica, 3: 1-720.
- Brandão, C.R.F. 1991. Adendos ao catálogo abreviado das formigas da região neotropical (Hymenoptera: Formicidae). Revista Brasileira de Entomologia, 35: 319-412.
- Brandão, C.R.F.; Feitosa, R.M. & Diniz, J.L.M. 2015. Taxonomic revision of the Neotropical Myrmicinae ant genus Blepharidatta Wheeler. Zootaxa, 4012(1): 33-56.
- Brandão, C.R.F.; Silva, R.R. & Feitosa, R.M. 2011. Cerrado ground-dwelling ants (Hymenoptera: Formicidae) as indicators of edge effects. Zoologia, 28(3): 379-387.
- Brasil. 2009. Ministério do Meio Ambiente. Relatório técnico de monitoramento do desmatamento no bioma Cerrado, 2002 a 2008: dados revisados. Brasília: MMA, 67p. Available at: Available at: http://www.mma.gov.br/estruturas/sbf_chm_rbbio/_arquivos/relatorio_tecnico_monitoramento_desmate_bioma_cerrado_csr_ibama_2002_2008_rev_72.pdf Access in: 08/12/2018.
» http://www.mma.gov.br/estruturas/sbf_chm_rbbio/_arquivos/relatorio_tecnico_monitoramento_desmate_bioma_cerrado_csr_ibama_2002_2008_rev_72.pdf - Carvalho, A.P.R.; Silva, C.G. & Fonseca, A.R. 2011. Diversidade de formigas em um hospital público no município de Chapadinha, Maranhão, Brasil. Revista de Biologia e Ciências da Terra, 11: 67-73.
- Celentano, D.; Rousseau, G.X.; Engel, V.L.; Zelarayán, M.; Oliveira, E.C.; Araújo, A.C.M. & De Moura, E.G. 2017. Degradation of riparian forest affects soil properties and ecosystem services provision in eastern amazon of Brazil. Land Degradation & Development, 28: 482-493.
- Chaves, L.P.F.A.; Silva, R.A.; Amaral, Y.T.; Costa, M.K.L. & Siqueira, G.M. 2016. Biogeographical diversity of north mesoregion of the Maranhão state (Brazil). Journal of Geospatial Modelling, 1: 19.
- Cuezzo, F. 2000. Revisión del género Forelius (Hymenoptera: Formicidae: Dolichoderinae). Sociobiology, 35: 197-275.
- Dalzochio, M.S.; Renner, S.; Sganzerla, C.; Prass, G.; Ely, G.J.; Salvi, L.C.; Dametto, N. & Périco, E. 2018. Checklist of Odonata (Insecta) in the state of Rio Grande do Sul, Brazil with seven new records. Biota Neotropica, 18: 1-14.
- Dáttilo, W.; Vicente, R.E.; Nunes, R.V. & Carvalho, M.S.G. 2010. First Record of the Quenquém cisco-da-Amazônia Acromyrmex hystrix (Latreille) (Formicidae: Myrmicinae) for Maranhão State, Brazil. EntomoBrasilis, 3(3): 92-93.
- Dáttilo, W.; Vicente, R.E.; Nunes, R.V. & Feitosa, R.M. 2012. Influence of cave size and presence of bat guano on ant visitation. Sociobiology, 59(2): 549-559.
- De Andrade, M.L. & Baroni Urbani, C. 1999. Diversity and Adaptation in the ant genus Cephalotes, past and present. Stuttgarter Beitrage zur Naturkunde Serie B, 271: 893.
- Desidério, G.R.; Barcelos-Silva, P.; De Souza, W.R.M.; Pes, A.M. & Azevêdo, C.A.S. 2017. Caddisflies (Insecta: Trichoptera) from Maranhão State, Northeast Region, Brazil: A new species, checklist, and new geographical records. Zootaxa, 4221: 151-171.
- Dias, A.M. & Lattke, J.E. 2019. A new species and new records of minuta-group Gnamptogenys from Brazil (Hymenoptera: Formicidae). Revista Brasileira de Entomologia, 63(1): 30-34.
- Feitosa, R.M.; Brandão, C.R.F. & Dietz, B.H. 2007. Basiceros scambognathus (Brown, 1949) n. comb., with the first worker and male descriptions, and a revised generic diagnosis (Hymenoptera: Formicidae: Myrmicinae). Papéis Avulsos de Zoologia, 47(2): 31-42.
- Feitosa, R.M.; Brandão, C.R.F. & Diniz, J.L.M. 2008. Revisionary studies on the enigmatic Neotropical ant genus Stegomyrmex Emery, 1912 (Hymenoptera: Formicidae: Myrmicinae), with the description of two new species. Journal of Hymenoptera Research, 17: 64-82.
- Fernandes, I.O.; Oliveira, M.L. & Delabie, J.H.C. 2014. Description of two new species in the Neotropical Pachycondyla foetida complex (Hymenoptera: Formicidae: Ponerinae) and taxonomic notes on the genus. Myrmecological News, 19: 133-163.
- Figueiredo, C.J.; Silva, R.R.; Munhae, C.B. & Morini, M.S.C. 2013. Fauna de formigas (Hymenoptera: Formicidae) atraídas a armadilhas subterrâneas em áreas de Mata Atlântica. Biota Neotropica, 13: 1-7.
- Forel, A. 1904. Miscellanea myrmécologiques. Revue Suisse de Zoologie, 12: 1-52.
- Freitas, M.A.; Vieira, R.S.; Entiauspe-Neto, O.M.; Sousa, S.O.; Farias, T.; Sousa, A.G. & Moura, G.J.B. 2017. Herpetofauna of the Northwest Amazon forest in the state of Maranhão, Brazil, with remarks on the Gurupi Biological Reserve. ZooKeys, 643: 141-155.
- Gasper, A.L.; Eisenlohr, P.V. & Salino, A. 2016. Improving collection efforts to avoid loss of biodiversity: lessons from comprehensive sampling of lycophytes and ferns in the subtropical Atlantic Forest. Acta Botanica Brasilica, 30: 166-175.
- Gonçalves, C.R. 1942. Contribuição para o conhecimento do gênero Atta Fabr., das formigas saúvas. Boletim da Sociedade Brasileira de Agronomia, 5: 333-358.
- Gonçalves, C.R. 1947. Saúvas do sul e centro do Brasil. Boletim Fitossanitário, 2: 183-218.
- Gonçalves, C.R. 1961. O gênero Acromyrmex no Brasil (Hym. Formicidae). Studia Entomologica, 4: 113-180.
- Graham, C.H.; Elith, J.; Hijmans, R.J.; Guisan, A.; Townsend Peterson, A. & Loiselle, B.A. 2008. The influence of spatial errors in species occurrence data used in distribution models. Journal of Applied Ecology, 45: 239-247.
- Gutiérrez, J.A.M.; Rousseau, G.X.; Andrade-Silva, J. & Delabie, J.H.C. 2017. Taxones superiores de hormigas como sustitutos de la riqueza de especies, en una cronosecuencia de bosques secundarios, bosque primario y sistemas agroforestales en la Amazonía Oriental, Brasil. Revista de Biología Tropical, 65(1): 279-291.
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; Goulson, D. & de Kroon, H. 2017. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. Plos One, 12(10): e0185809.
- Hijmans, R.J.; Garrett, K.A.; Huama’n, Z.; Zhang, D.P.; Schreuder, M. & Bonierbale, M. 2000. Assessing the geographic representativeness of genebank collections: the case of Bolivian wild potatoes. Conservation Biology, 14: 1755-65.
- Instituto Brasileiro de Geografia e Estatística (IBGE). 2011. Índice de Nomes Geográficos, Escala 1:1.000.000. Base Cartográfica Contínua do Brasil ao Milionésimo. Disponível em: Disponível em: http://www.ibge.gov.br/geociencias/cartas-e-mapas/bases-cartograficas-continuas/15759-brasil.html?=&t=sobre Acesso em: 03/12/2018.
» http://www.ibge.gov.br/geociencias/cartas-e-mapas/bases-cartograficas-continuas/15759-brasil.html?=&t=sobre - Instituto Brasileiro de Geografia e Estatística (IBGE). 2018. Maranhão. Available at: Available at: http://cidades.ibge.gov.br/brasil/ma/panorama Access in: 02/02/2019.
» http://cidades.ibge.gov.br/brasil/ma/panorama - Janicki, J.; Narula, N.; Ziegler, M.; Guénard, B. & Economo, E.P. 2016. Visualizing and interacting with large-volume biodiversity data using client-server web-mapping applications: The design and implementation of antmaps.org. Ecological Informatics, 32: 185-193.
- Jesovnik, A. & Schultz, T.R. 2017. Revision of the fungus-farming ant genus Sericomyrmex Mayr (Hymenoptera, Formicidae, Myrmicinae). ZooKeys, 670: 1-109.
- Johnson, R.A. 2015. A taxonomic revision of South American species of the seed-harvester ant genus Pogonomyrmex (Hymenoptera: Formicidae). Part I. Zootaxa, 4029(1): 1-142.
- Kadmon, R.; Farber, O. & Danin, A. 2003. A systematic analysis of factors affecting the performance of climatic envelope models. Ecological Applications, 13: 853-67.
- Kempf, W.W. 1959. Insecta Amapaensia. Hymenoptera: Formicidae. Studia Entomologica, 2: 209-218.
- Kempf, W.W. 1960a. Insecta Amapaensia. Hymenoptera: Formicidae (segunda contribuição). Studia Entomologica, 3: 385-400.
- Kempf, W.W. 1960b. Estudo sôbre Pseudomyrmex I. (Hymenoptera: Formicidae). Revista Brasileira de Entomologia, 9: 5-32.
- Kempf, W.W. 1964. Uma nova Platythyrea do Brasil (Hym., Formicidae). Revista Brasileira de Entomologia, 11: 141-144.
- Kempf, W.W. 1968. Miscellaneous studies on Neotropical ants. IV. (Hymenoptera, Formicidae). Studia Entomologica, 11: 369-415.
- Kempf, W.W. 1971. A preliminary review of the ponerine ant genus Dinoponera Roger (Hymenoptera: Formicidae). Studia Entomologica, 14: 369-394.
- Kempf, W.W. 1972a. Catálogo abreviado das formigas da região Neotropical (Hym. Formicidae). Studia Entomologica, 15: 1-4.
- Kempf, W.W. 1972b. A new species of the Dolichoderine ant genus Monacis Roger, from the Amazon, with further remarks on the genus (Hymenoptera, Formicidae). Revista Brasileira de Biologia, 32: 251-254.
- Kempf, W.W. 1975. Miscellaneous studies on neotropical ants. VI. (Hymenoptera, Formicidae). Studia Entomologica, 18: 341-380.
- Lattke, J.E. 2011. Revision of the New World species of the genus Leptogenys Roger (Insecta: Hymenoptera: Formicidae: Ponerinae). Arthropod Systematics & Phylogeny, 69: 127-264.
- Leal, I.R.; Leal, L.; Oliveira, F.P.; Arcoverde, G.B. & Andersen, A.N. 2017. Effects of human disturbance and climate change on myrmecochory in Brazilian Caatinga. In: Oliveira, P.S. & Koptur, S. (Eds.). Ant-plant interactions. Impacts of human on terrestrial ecosystems. Cambridge, UK, Cambridge University Press. p. 112-132.
- Lima, W.R.S.; Marques, S.G.; Rodrigues, F.S. & Rebelo, J.M.M. 2013. Ants in a hospital environment and their potential as mechanical bacterial vectors. Revista da Sociedade Brasileira de Medicina Tropical, 46(5): 637-640.
- Longino, J.T. & Snelling, R.R. 2002. A taxonomic revision of the Procryptocerus (Hymenoptera: Formicidae) of Central America. Contributions in Science, 495: 1-30.
- Mann, W.M. 1916. The Stanford Expedition to Brazil, 1911, John C. Branner, director. The ants of Brazil. Bulletin of the Museum of Comparative Zoology, 60: 399-490.
- Maracahipes-Santos, L.; Santos, J.O.; Reis, S.M. & Lenza, E. 2018. Temporal changes in species composition, diversity, and woody vegetation structure of savannas in the Cerrado-Amazon transition zone. Acta Botanica Brasilica, 32(2): 254-263.
- Ministério do Meio Ambiente (MMA). 2011. Plano de Ação para prevenção e controle do desmatamento e das queimadas: Cerrado. Brasília, MMA. 200p.
- Monnin, R.; Ratnieks, F.L.W. & Brandão, C.R.F. 2003. Reproductive Conflict in Animal Societies: Hierarchy Length Increases with Colony Size in Queenless Ponerine Ants. Behavioral Ecology and Sociobiology, 54(1): 71-79.
- Moura, C.C.M.; Moura, G.J.B.; Lisboa, E.B.F. & Luz, V.L.F. 2014. Distribuição geográfica e considerações ecológicas sobre a fauna de Testudines da Região Nordeste do Brasil. Sitientibus serie Ciencias Biologicas, 14: 1-20.
- Newbold, T. 2010. Applications and limitations of museum data for conservation and ecology, with particular attention to species distribution models. Progress in Physical Geography, 34: 3-22.
- Pereira, E.K.C.; Andrade-Silva, J.; Silva, O.; Santos, C.L.C.; Moraes, L.S.; Bandeira, M.C.A.; Silva, C.R.R. & Rebêlo, J.M.M. 2017. Solenopsis saevissima (Smith) (Hymenoptera: Formicidae) activity delays vertebrate carcass decomposition. Sociobiology, 64: 369.
- Pereira, J.C.; Delabie, J.H.C.; Zanette, L.R.S. & Quinet, Y. 2014. Studies on an Enigmatic Blepharidatta Wheeler Population (Hymenoptera: Formicidae) from the Brazilian Caatinga. Sociobiology, 61(1): 52-59.
- Peres, C.A. 2005. Why we need mega-reserves in Amazonian forests. Conservation Biology, 19: 728-733.
- QGIS Development Team. 2019. QGIS geographic information system. Open source geospatial foundation project. Disponível em: http://qgis.org/en/site/forusers/download.html
» http://qgis.org/en/site/forusers/download.html - Ramos, A.S.J.C.; Lemos, R.N.S.; Vale, A.M.S; Batista, M.C.; Moreira, A.A.; Harada, H.Y. & Mesquita, M.L.R. 2015. Ant diversity in agro ecosystems and secondary forest. African Journal of Agricultural Research, 10: 4449-4454.
- Reddy, S. & Dávalos, L.M. 2003: Geographical sampling bias and its implications for conservation priorities in Africa. Journal of Biogeography, 30: 1719-27.
- Santos, B.F. & Hoppe, J.P.M. 2018. Filling gaps in species distributions through the study of biological collections: 415 new distribution records for Neotropical Cryptinae (Hymenoptera, Ichneumonidae). Revista Brasileira de Entomologia, 62(4): 288-291.
- Santos, G.M.M.; Delabie, J.H.C. & Resende, J.J. 1999. Caracterização da Mirmecofauna (Hymenoptera: Formicidae) associada à vegetação periférica de inselbergs (Caatinga-arbórea-estacional-semi-descídua) em Itatim-Bahia-Brasil. Sitientibus. Revista da Universidade Estadual de Feira de Santana, 20: 33-43.
- Santos, M.P.D.; Cerqueira, P.V. & Soares, L.M.S. 2010. Avifauna em seis localidades no centro-sul do Estado do Maranhão, Brasil. Ornithologia, 4(1): 49-65.
- Shoemaker, D.D.; Ahrens, M.E. & Ross, K.G. 2006. Molecular phylogeny of fire ants of the Solenopsis saevissima species-group based on mtDNA sequences. Molecular Phylogenetics and Evolution, 38: 200-215.
- Silva, E.F.; Corá, J.E.; Harada, A.Y. & Sampaio, I.B.M. 2017. Association of the Occurrence of Ant Species (Hymenoptera: Formicidae) with Soil Attributes, Vegetation, and Climate in the Brazilian Cerrado Northeastern Region. Sociobiology, 64(4): 442-450.
- Silva, G.M.; Carmo, M.S.; Moraes, L.S.; Moraes, F.C.; Barnabé, A.S. & Figueiredo, P.M.S. 2012. Formigas (Hymenoptera: Formicidae) como Vetores de Bactéria em Ambiente Hospitalar na Cidade de São Luis, Maranhão. Revista de Patologia Tropical, 41(3): 348-355.
- Silva, P.R. 2007. Biologia de algumas espécies de Blepharidatta. Biológico, 69(2): 161-164.
- Spinelli-Araujo, L.; Bayma-Silva, G.; Torresan, F.E.; Victoria, D.; Vicente, L.E.; Bolfe, E.L. & Manzatto, C. 2016. Conservação da biodiversidade do estado do Maranhão: cenário atual em dados geoespaciais. Jaguariúna, EMBRAPA Meio Ambiente. 28p.
- Stella, A. 2011. Plano de prevenção e controle do desmatamento e queimadas do Maranhão. São Luís, SEMA. 120p.
- Ulysséa, M.A. & Brandão, C.R.F. 2013. Ant species (Hymenoptera, Formicidae) from the seasonally dry tropical forest of northeastern Brazil: a compilation from field surveys in Bahia and literature records. Revista Brasileira de Entomologia, 57: 217-224.
- Ulysséa, M.A.; Prado, L.P. & Brandão, C.R.F. 2015. Type specimens of the traditional Myrmicinae (Hymenoptera: Formicidae) ant tribes deposited in the Museu de Zoologia da Universidade de São Paulo, Brazil: Adelomyrmecini, Basicerotini, Blepharidattini, Crematogastrini, Formicoxenini, Lenomyrmecini, Myrmicini, Phalacromyrmecini, Pheidolini, Stegomyrmecini, Stenammini and Tetramoriini. Papéis Avulsos de Zoologia, 55: 175-204. Disponível em: Disponível em: http://www.revistas.usp.br/paz/article/view/106374 Acesso em 25.04.2019.
» http://www.revistas.usp.br/paz/article/view/106374 - Ulysséa, M.A.; Prado, L.P. & Brandão, C.R.F. 2017. Catalogue of the Dolichoderinae, Formicinae and Martialinae (Hymenoptera: Formicidae) types deposited at the Museu de Zoologia da Universidade de São Paulo, Brazil. Papéis Avulsos de Zoologia , 57(23): 295-311. Disponível em: Disponível em: http://www.revistas.usp.br/paz/article/view/125647 Acesso em: 25.04.2019
» http://www.revistas.usp.br/paz/article/view/125647 - Vanzolini, P.E. 1992. A Supplement to the Ornithological Gazetteer of Brazil. São Paulo, Museu de Zoologia da Universidade de São Paulo. 252p.
- Vanzolini, P.E. 2004. Episódios da Zoologia Brasílica. São Paulo, Hucitec. 212p.
- Vanzolini, P.E. & Papavero, N. 1968. Índice dos Topônimos Contidos na Carta do Brasil 1:1.000.000 do IBGE. São Paulo, Fundação de Amparo à Pesquisa do Estado de São Paulo. 201p.
- Ward, P.S. 1989. Systematic Studies on Pseudomyrmecine Ants: Revision of the Pseudomyrmex oculatus and P. subtilissimus species groups with taxonomic comments on other species. Questiones Entomologicae, 25: 393-468.
- Ward P.S. 1999. Systematics, biogeography and host plant associations of the Pseudomyrmex viduus group (Hymenoptera: Formicidae), Triplaris- and Tachigali-inhabiting ants. Zoological Journal of the Linnean Society, 126: 451-540.
- Ward, P.S. 2007. The ant genus Leptanilloides: discovery of the male and evaluation of phylogenetic relationships based on DNA sequence data. Memoirs of the American Entomological Institute, 80: 637-649.
- Ward, P.S. & Downie, D.A. 2005. The ant subfamily Pseudomyrmecinae: phylogeny and evolution of big-eyed arboreal ants. Systematic Entomology, 30: 310-335.
- Watkins, J.F. 1976. The identification and distribution of New World army ants (Dorylinae: Formicidae). Waco, Texas, Baylor University Press. 102p.
- Wauters, N.; Dekoninck, W. & Fournier, D. 2018. Introduction history and genetic diversity of the invasive ant Solenopsis geminata in the Galapagos Islands. Biological Invasions, 20(11): 3207-3226.
- Wheeler, W.M. 1922. Observations on Gigantiops destructor Fabricius and other leaping ants. Biological Bulletin (Woods Hole), 42: 185-201.
- Wild, A.L. 2007. Taxonomic revision of the ant genus Linepithema (Hymenoptera: Formicidae). University of California Publications in Entomology, 126: 1-151.
- Wild, A.L. & Cuezzo, F. 2006. Rediscovery of a fossil dolichoderine ant lineage (Hymenoptera: Formicidae: Dolichoderinae) and a description of a new genus from South America. Zootaxa, 1142: 57-68.
-
Edited by: Helena Carolina Onody
-
Published with the financial support of the Committee of "Programa de Apoio às Publicações Científicas Periódicas da USP" (SIBi-USP)
Data availability
Data citations
AntWeb. 2019. AntWeb. Available at: Available at: http://www.antweb.org Access in: 03/01/2019.
Publication Dates
-
Publication in this collection
03 Oct 2019 -
Date of issue
2019
History
-
Received
22 May 2019 -
Accepted
15 July 2019