Abstract
In most countries, Chagas disease transmission control remains based on domestic insecticide application. We thus evaluated the efficacy of intra-domicile cyfluthrin spraying for the control of Triatoma dimidiata, the only Chagas disease vector in the Yucatán peninsula, Mexico, and monitored potential re-infestation every 15 days for up to 9 months. We found that there was a re-infestation of houses by adult bugs starting 4 months after insecticide application, possibly from sylvatic/peridomicile areas. This points out the need to take into account the potential dispersal of sylvatic/peridomestic adult bugs into the domiciles as well as continuity action for an effective vector control.
vector control; pyrethroides; re-colonization; peridomestic populations; sylvatic environments; Mexico
EPIDEMIOLOGY
Re-infestation of houses by Triatoma dimidiata after intra-domicile insecticide application in the Yucatán peninsula, Mexico
Eric DumonteilI, 1 1 Corresponding author. Fax: +999 923.6120. Email: oliver@tunku.uady.mx ; Hugo Ruiz-PiñaI; Eugenia Rodriguez-FélixI; Mario Barrera-PérezI; María Jesús Ramirez-SierraI; Jorge E RabinovichII; Frédéric MenuIII
ILaboratorio de Parasitología, Centro de Investigaciones Regionales "Dr. Hideyo Noguchi", Universidad Autónoma de Yucatán, Ave. Itzaes #490 x 59, 97000, Mérida, Yucatán, México
IICentro de Estudios Parasitológicos y de Vectores, Universidad Nacional de La Plata, La Plata, Argentina
IIILaboratoire de Biométrie et Biologie Evolutive, Université de Lyon I, France
ABSTRACT
In most countries, Chagas disease transmission control remains based on domestic insecticide application. We thus evaluated the efficacy of intra-domicile cyfluthrin spraying for the control of Triatoma dimidiata, the only Chagas disease vector in the Yucatán peninsula, Mexico, and monitored potential re-infestation every 15 days for up to 9 months. We found that there was a re-infestation of houses by adult bugs starting 4 months after insecticide application, possibly from sylvatic/peridomicile areas. This points out the need to take into account the potential dispersal of sylvatic/peridomestic adult bugs into the domiciles as well as continuity action for an effective vector control.
Key words: vector control - pyrethroides - re-colonization - peridomestic populations - sylvatic environments - Mexico
Chagas disease, or American trypanosomiasis, is caused by the protozoan parasite Trypanosoma cruzi, and it represents a major public health problem in Latin America, including Mexico (Dumonteil 1999). Because of difficulties associated with therapeutic treatment, and the elusive development of an effective vaccine, control of the transmission of Chagas disease remains based on vector control by insecticides (Silveira & Vinhaes 1999) and on blood bank screening (Schmuñis 1999). Vector control programs in South America have focused on the interruption of natural transmission by attacking domiciliated vector populations using pyrethroide insecticides (Silveira & Vinhaes 1999), and they have been enormously successful. The interruption of natural transmission is thus underway in the Southern Cone countries (Argentina, Brazil, Bolivia, and Chile) (WHO 1997). However, re-colonization of houses by migrating insects (from the same or other species) from sylvatic/peridomestic environments has been documented, as well as the reemergence of populations from surviving bugs (Forattini et al. 1984, Gorla 1991, Schofield 1991, Scorza et al. 1994, Gajate et al. 1996, Oliveira-Filho 1997, Dujardin et al. 1999, Almeida et al. 2000).
In the Yucatán peninsula, Mexico, the main vector of Chagas disease is Triatoma dimidiata. This species can be found in a variety of environments, including domestic, peridomestic and sylvatic environments (Zeledón 1981, Rodas et al. 1998). In a previous study, we documented strong seasonal variation in domiciliated and peridomestic populations. The analysis of the population stage structure led us to suggest that flying adults (from sylvatic or peridomestic environments) were seasonally infesting houses (Dumonteil et al. 2002). An important implication of this conclusion is that control programs may have to be designed to take this particular behaviour into account. Indeed, it is not clear whether elimination of domestic populations would be sufficient to reduce house infestation by T. dimidiata and the risk of natural transmission of Chagas disease to humans in the region.
Initial data on the control of T. dimidiata in Central America suggested that domestic populations can be effectively eliminated for up to a year with a single application of pyrethroids (Rodas et al. 1998, Acevedo et al. 2000), allowing for very cost-effective control. However, no data were available in the Yucatán peninsula, where no control program has yet been established. We thus performed a pilot study to evaluate the usefulness of a vector control program based on a similar intra-domestic insecticide application on a small number of infested houses in Yucatán, Mexico.
MATERIALS AND METHODS
Study area - Field work was carried out from November 2000 to August 2001 in the state of Yucatán, located in the Southeast of Mexico, between 87-92ºW and 17-22ºN. We selected two villages: Eknackán and Dzidzilche, where we had been working since 1999 and had observed levels of house infestation by T. dimidiata of about 20 bugs/house/year (Dumonteil et al. 2002). During our previous studies in these villages, the majority of bugs were collected between April and June, and about 80% of the houses were infested. This house infestation of T. dimidiata is characteristic of the northern part of the Yucatán peninsula, which we defined as the region with higher risk of natural transmission in the peninsula (Dumonteil et al. 2002). There were 57 and 54 registered houses in Eknakan and Dzidzilche, respectively, with 319 and 244 inhabitants (4-6 inhabitants/domicile).
Infestation monitoring before and after insecticide spraying - While most studies focus on various houses that are usually monitored only once or twice after 6 month or a year following insecticide application (Cecere et al. 1997, Diotaiuti et al. 1998, Acevedo et al. 2000), we decided to focus on four houses (two in each village) to be able to carry out a more frequent and detailed monitoring of re-infestation. We included representative houses with different characteristics as we had shown previously that there was no significant difference in apparent house infestation according to building materials (Dumonteil et al. 2002). Two houses were made of plastered stones (mampostería) with a flat cement roof, and cement floor; one house had plastered stone walls, with a galvanized tin roof and the floor was part cement and part dirt; and the fourth house had adobe walls, a thatched roof and cement floor. Doors and windows did not close effectively leaving several openings. All houses were surrounded by yards (typically 20 x 60 m) with a variety of fruit trees (banana, orange, lime, coco, mango) and free-ranging chickens and turkeys. In each village, one house was in the periphery, about 100 m from the sylvatic area, and one was more central, over 500 m away from the sylvatic area. The sylvatic area was mostly composed of bushes from abandoned areas with small patches of low forest, cultivated land (mostly henequen and corn), and a few open pastures.
House infestation was evaluated before insecticide spraying through a timed manual search for triatomines (Gürtler et al. 1999) performed by research personnel in domestic (inside houses), peridomestic (within 50 m around houses), and sylvatic habitats (more than 500 m away from the villages). Teams of two researchers performed the manual searches for 30 min in each biotope between 8:00 am and 1:00 pm. Domestic searches included walls, base of the roofs, furniture and hamocks. Peridomestic and sylvatic searches focused on animal housing (often chickens or pigs), wood piles, tree trunks, potential animal burrows or nests, piled rocks, and any other potential triatomine refuges.
Following the initial search, the interior of the four houses was sprayed with 50 mg/m2 of cyfluthrin (Solfac®, Bayer) to eliminate domestic bug populations. Such a dose of this pyrethroid was found to be effective against a wide variety of triatomine species, including T. dimidiata (Rodas et al. 1998, Oliveira-Filho 1999, Acevedo et al. 2000). To detect re-infestation by triatomines, all houses were then carefully monitored by timed manual searches as described above, every 15 days for 9 months in all three biotopes. In addition, households were asked to collect all bugs they could find between visits to improve the sensitivity of monitoring. In addition to the manual searches, mouse traps were also used for sylvatic and peridomestic biotopes (Magallon-Gastelum et al. 2001, Noireau et al. 2002). Seven to ten wire traps containing a single mouse each and partially covered with double-face adhesive tape were left overnight in each biotope, every 15 days, near potential animal burrows, piled wood or rocks, and other potential bug refuges. Bugs caught on the tape or around the trap were collected the next morning (Magallon-Gastelum et al. 2001, Noireau et al. 2002).
RESULTS
A total of 184 bugs was collected, most of them by manual collection by research personnel or the inhabitants of the premises (168 bugs) and only 16 bugs were collected with mouse traps. Eighty one were domestic, 86 peridomestic and 17 sylvatic. In the peridomicile, only seven bugs were collected with a total of 207 mouse traps over 29 nights (8% of peridomestic collections), and in the sylvatic area, nine bugs were collected with 226 traps over 29 nights (53% of sylvatic bug collections).
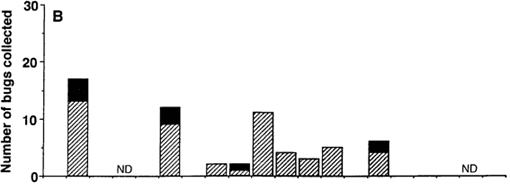
Fig. 1 shows the comparative time course of T. dimidiata collections in the three studied biotopes (domestic, peridomestic and sylvatic), before (first time point) and after insecticide spraying. Twenty one bugs were found in three of the four studied houses prior to insecticide application with a single manual search (Fig. 1A). Two weeks following insecticide application, three dead bugs were found in one of the sprayed houses of Eknackán, where we had been unable to find any bugs during our initial search. During the next four months, from mid-november to mid-march, we could not detect bugs inside the sprayed houses, indicating that domestic populations, if present, were reduced to below detectable levels by our monitoring strategy. On the other hand, a few bugs were sporadically collected in peridomestic and sylvatic areas (Fig. 1B and C ). After the first four months, live T. dimidiata began to be collected inside all sprayed houses (Fig. 1A), and domestic bugs were found at each visit from the end of March until the end of June.
Fig. 1: time course of adult (hatched bars) and larval stages (solid bars) of Triatoma dimidiata collections in domestic (A), peridomestic (B), and sylvatic (C) biotopes. Data were pooled for the four sprayed houses of Eknackán and Dzidzilche. Insecticide was applied to the domestic biotope only (arrow). ND: not done
Analysis of the population stage structure of T. dimidiata in the distincts biotopes revealed that only adult insects were collected in the domiciles, and the first larval stages were only observed four months after the initial reappearance of adults (Fig. 1A). On the other hand, larval stages were detected throughout most of the study period in peridomestic (Fig. 1B) and sylvatic populations (Fig. 1C ), suggesting that there was no marked seasonality of the reproductive cycles in these biotopes. Together, these data suggest that adult bugs migrating from nearby sites, rather than survivors of insecticide spraying, were the most likely source of new bugs in the domiciles.
DISCUSSION
Our study indicates that cyflutrin application can effectively reduce domestic populations to below detectable levels in the sprayed houses. Comparison of our data with a previous study realized during the year before insecticide spraying (Dumonteil et al. 2002) indicates that before spraying, an average of 21 bugs/house was collected in the same two villages over a one year period, while following spraying, an average of 12 bugs/house was collected over a 9 month period. As this post-spray population was exclusively composed of adults, it is more likely to be due to bugs migrating from nearby sites rather than to individuals surviving the insecticide; that is, a re-infestation process. The appearance of larval stages in the houses several months after this re-infestation by adults may indicate an attempt at colonization. This is reminiscent of our previous field observations suggesting seasonal infestation of houses by flying adults and a similar attempt at colonization that was apparently unsuccessful even in the absence of vector control. Because re-infesting bugs were removed from the domiciles at each visit, our collections may provide a rough but direct estimate of immigration rates to the domiciles. Thus, immigration of adult bugs to the domiciles during the March-June period appeared to be occurring at a rate of 2-10 bugs/house/month. This estimate is very similar to that from a population genetics study of T. dimidiata populations in Guatemala using RAPD markers, where the migration rate between houses from a same village was found to be of 9.7 bugs/generation (Dorn et al. 2003). A similar genetic analysis is currently underway to evaluate the respective participation of peridomestic and sylvatic populations to domestic (re)-infestation in the Yucatán.
The low efficacy of insecticide spraying for the long-term control of T. dimidiata in the Yucatán differs considerably from previous studies in Guatemala and Nicaragua, showing that a single insecticide application of even a small number of houses may be sufficient to control domestic T. dimidiata for up to a year (Rodas et al. 1998, Acevedo et al. 2000). This discrepancy may be due to the presence of other infested houses close to the sprayed ones that may have served as a source of re-infestation, and/or to a higher level of house colonization associated with lower dispersal of T. dimidiata in Central America compared to that of Yucatán. In fact, peridomestic and sylvatic populations are being increasingly involved in the re-infestation of domiciles following control programs (Gürtler et al. 1999), as well as in the transmission of T. cruzi to humans in the absence of house colonization by triatomines (Coura et al. 1994, 1999, 2002). As suggested by a few studies on triatomine dispersal, their flying capacity may have been somewhat underestimated (Schofield et al. 1991, 1992), and need to be assessed more carefully. A possible hypothesis for the Yucatán is that seasonal changes in blood meal availability, such as from opossums, one of the main blood source for T. dimidiata (Quintal & Polanco 1977), may induce an increase in sylvatic/peridomestic bugs foraging activity leading to a transient infestation of the domestic areas. Analysis of feeding patterns will help testing this hypothesis.
In conclusion, the control of T. dimidiata domestic populations with insecticide may require continuous actions and might be optimized by taking into account T. dimidiata dispersal. The prevention of dispersal of sylvatic/peridomestic adult bugs into domestic areas with door/window screens or bed nets may prove to be a simple, cost-effective and sustainable complementary or alternative strategy to reduce house infestation by triatomines in this region (Kroeger et al. 1999). The usefulness of such a strategy may not be limited to the Yucatán peninsula, as the transient infestation of houses by flying sylvatic adult bugs is thought to be responsible for a significant transmission of Chagas disease in several other regions (Coura et al. 1994, 1999, 2002).
ACKNOWLEDGEMENTS
To the families that actively participated in this study for their help and interest.
Received 31 July 2003
Accepted 22 March 2004
Financial support: Conacyt grant 498100-5-27865N
- Acevedo F, Godoy E, Schofield C 2000. Comparison of intervention strategies for control of Triatoma dimidiata in Nicaragua. Mem Inst Oswaldo Cruz 95: 867-871.
- Almeida CE, Vinhaes MC, Almeida JR, Silveira AC, Costa J 2000. Monitoring the domiciliary and peridomiciliary invasion process of Triatoma rubrovaria in the state of Rio Grande do Sul, Brazil. Mem Inst Oswaldo Cruz 95: 761-768.
- Cecere MC, Gürtler RE, Canale D, Chuit R, Cohen JE 1997. The role of the preidomiciliary area in the elimination of Triatoma infestans from rural Argentine communities. Rev Panam Salud Publica 1: 273-279.
- Coura JR, Barrett TV, Naranjo MA 1994. Ataque de populações humanas por triatomíneos silvestres no Amazonas: uma nova forma de transmissão da infecção chagásica? Rev Soc Bras Med Trop 27: 251-253.
- Coura JR, Junqueira AC, Boia MN, Fernandes O 1999. Chagas disease: from bush to huts and houses. Is it the case of the Brazilian Amazon? Mem Inst Oswaldo Cruz 94: 379-384.
- Coura JR, Junqueira AC, Fernandes O, Valente SA, Miles MA 2002. Emerging Chagas disease in Amazonian Brazil. Trends Parasitol 18: 171-176.
- Diotaiuti L, Azeredo BV, Busek SC, Fernandes AJ 1998. Controle do Triatoma sordida no peridomicílio rural do município de Porteirinha, Minas Gerais, Brasil. Rev Panam Salud Publica 3: 21-5.
- Dorn P, Melgar S, Rouzier V, Gutierrez A, Combe C, Rosales R, Rodas A, Kott S, Salvia D, Monroy C 2003. The Chagas vector, Triatoma dimidiata (Hemiptera:Reduviidae), is panmictic within and among adjacent villages in Guatemala. J Med Entomol 40: 436-440.
- Dujardin JP, Bermudez H, Gianella A, Cardozo L, Ramos E, Saravia R, Quiroz K, Forgues G, Carazas R, Hervas D, Chavez T, Machane M, Martínez E, Torrez M 1999. Uso de marcadores genéticos en la vigilencia entomológica de la enfermedad de Chagas. In JRA Cassab, F Noireau, G Guillén (eds), La Enfermedad de Chagas en Bolivia, EG, La Paz, Bolivia, p. 157-169.
- Dumonteil E 1999. Update on Chagas disease in Mexico. Salud Publica Mex 41: 322-327.
- Dumonteil E, Gourbiere S, Barrera-Perez M, Rodriguez-Felix E, Ruiz-Piña H, Baños-Lopez O, Ramirez-Sierra MJ, Menu F, Rabinovich JE 2002. Geographic distribution of Triatoma dimidiata and transmission dynamics of Trypanosoma cruzi in the Yucatan peninsula, Mexico. Am J Trop Med Hyg 67: 176-183.
- Forattini OP, Rabello EX, Ferreira OA, da Rocha EO, Silva E, Ferreira Santos JL 1984. Aspectos ecológicos da tripanosomiase americana. XXI. Comportamento de espécies triatomíneas silvestres na reinfestação do intra e peridomicílio. Rev Saúde Públ São Paulo 18: 185-208.
- Gajate P, Bottazzi MC, Pietrokovsky SM, Wisnivesky-Colli C 1996. Potential colonization of the peridomicile by Triatoma guasayana (Hemiptera: Reduviidae) in Santiago del Estero, Argentina. J Med Entomol 33: 635-639.
- Gorla DE 1991. Recovery of Triatoma infestans populations after insecticide application: an experimental field study. Med Vet Entomol 5: 311-324.
- Gürtler RE, Cecere MC, Canale DM, Castanera MB, Chuit R, Cohen JE 1999. Monitoring house reinfestation by vectors of Chagas disease: a comparative trial of detection methods during a four-year follow-up. Acta Trop 72: 213-234.
- Kroeger A, Ordoñez-Gonzalez J, Behrend M, Alvarez G 1999. Bednet impregnation for Chagas disease control: a new perspective. Trop Med Int Health 4: 194-198.
- Magallon-Gastelum E, Lozano-Kasten F, Flores-Perez A, Bosseno MF, Breniere SF 2001. Sylvatic triatominae of the phyllosoma complex (Hemiptera: Reduviidae) around the community of Carrillo Puerto, Nayarit, Mexico. J Med Entomol 38: 638-640.
- Noireau F, Abad-Franch F, Valente SA, Dias-Lima A, Lopes CM, Cunha V, Valente VC, Palomeque FS, de Carvalho-Pinto CJ, Sherlock I, Aguilar M, Steindel M, Grisard EC, Jurberg J 2002. Trapping Triatominae in silvatic habitats. Mem Inst Oswaldo Cruz 97: 61-63.
- Oliveira-Filho AM 1997. Uso de nuevas herramientas para el control de triatominos en diferentes situaciones entomológicas en el continente Americano. Rev Soc Bras Med Trop 30: 41-46.
- Oliveira-Filho AM 1999. Differences of susceptibility of five triatomine species to pyrethroid insecticides - Implications for Chagas disease vector control. Mem Inst Oswaldo Cruz 94 (Suppl. I): 425-28.
- Quintal RE, Polanco G 1977. Feeding preferences of Triatoma dimidiata maculipennis in Yucatan, Mexico. Am J Trop Med Hyg 26: 176-178.
- Rodas A, Mejía M, Monroy C, Tabaru Y 1998. Preliminary studies on the chemical control of Triatoma dimidiata (Reduvidae: Triatominae), the principal vector of Chagas' disease in Guatemala. Enferm Tropical Guatemala 122: 143-150.
- Schmuñis GA 1999. Prevention of transfusional Trypanosoma cruzi infection in Latin America. Mem Inst Oswaldo Cruz 94: 93-101.
- Schofield CJ 1991. Vector population responses to control interventions. Ann Soc Belg Med Trop 71 (Suppl. 1): 201-217.
- Schofield CJ, Lehane MJ, McEwan P, Catala SS, Gorla DE 1991. Dispersive flight by Triatoma sordida Trans R Soc Trop Med Hyg 85: 676-678.
- Schofield CJ, Lehane MJ, McEwen P, Catala SS, Gorla DE 1992. Dispersive flight by Triatoma infestans under natural climatic conditions in Argentina. Med Vet Entomol 6: 51-56.
- Scorza JV, Solarte Y, Moreno E 1994. The epidemiological importance of Triatoma nigromaculata (Stal, 1859) colonizing human dwellings of the Venezuelan Andes. Mem Inst Oswaldo Cruz 89: 299.
- Silveira A, Vinhaes M 1999. Elimination of vector-borne transmission of Chagas disease. Mem Inst Oswaldo Cruz 94: 405-411.
- WHO 1997. Chagas disease, Progress 1995-1996. Thirteenth programme report of the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, p. 112-123.
- Zeledón R 1981. El Triatoma dimidiata (Latreille, 1811) y su Relación con la Enfermedad de Chagas, UNED, San José, Costa Rica.
Publication Dates
-
Publication in this collection
22 July 2004 -
Date of issue
May 2004
History
-
Accepted
22 Mar 2004 -
Received
31 July 2003