Abstracts
The pleural effusion, formerly called pleural spill, is an accumulation of fluid in the pleural space, as a consequence of an imbalance between the formation and reabsorption of such fluid, or due to an alteration in the drainage to lymph nodes. The purpose of this bibliographic review is to establish the importance of the use of ultrasound in pleural diffusion diagnosis. The authors discuss the use of ultrasonography in the diagnosis and therapeutic approach of this disease, and stress the importance of ultrasonography in chest diseases diagnosis, its advantages, limitations and disadvantages when compared to the common x-ray, computed tomography and physical examination. The authors also discuss the definition of pleural effusion, its physiopathology, morbidity, mortality, main causes and clinical presentation. The examination technique is systematically approached both by thoracic and abdominal pathways.
Pleural effusion; Pleural spill; Ultrasound
A efusão pleural, antigamente denominada derrame pleural, é caracterizada pelo acúmulo de líquido no espaço pleural, em decorrência do desequilíbrio entre formação e reabsorção de fluido ou por alteração na drenagem linfática. O propósito desta revisão foi estabelecer a importância da aplicação da ultra-sonografia no diagnóstico de efusão pleural. Os autores discutem a aplicação da ultra-sonografia no diagnóstico e abordagem terapêutica dessa entidade, e ressaltam sua importância nas doenças do tórax, vantagens, limitações e desvantagens em relação a radiografia simples, tomografia computadorizada e exame físico. Discutem, ainda, o conceito de efusão pleural, sua fisiopatologia, morbidade, mortalidade, principais causas e apresentação clínica. A técnica de realização do exame é sistematicamente abordada, tanto pela via torácica quanto abdominal.
Efusão pleural; Derrame pleural; Ultra-sonografia
REVIEW ARTICLE
The role of ultrasound in the assessment of pleural effusion* * Study developed at Escola de Ultra-sonografia e Reciclagem Médica de Ribeirão Preto, SP, Brazil.
Adilson Cunha FerreiraI; Francisco Mauad FilhoI; Tatiana BragaII; Glenda Downing FanstoneIII; Ivan Charbel Bumlai ChodrauiIV; Nilton OnariV
IDoctors-Professors at Escola de Ultra-sonografia e Reciclagem Médica de Ribeirão Preto
IIRadiologist-Trainee at Escola de Ultra-sonografia e Reciclagem Médica de Ribeirão Preto
IIIStudents at Faculdade de Medicina da Universidade de Ribeirão Preto
IVMD, Escola de Ultra-sonografia e Reciclagem Médica de Ribeirão Preto
Mailing address Mailing address: Prof. Dr. Adilson Cunha Ferreira Rua Manoel Ache, 980, ap. 222, Jardim Irajá Ribeirão Preto, SP, Brasil 14020-590 E-mail: acferrei@keynet.com.br
ABSTRACT
The pleural effusion, formerly called pleural spill, is an accumulation of fluid in the pleural space, as a consequence of an imbalance between the formation and reabsorption of such fluid, or due to an alteration in the drainage to lymph nodes. The purpose of this bibliographic review is to establish the importance of the use of ultrasound in pleural diffusion diagnosis. The authors discuss the use of ultrasonography in the diagnosis and therapeutic approach of this disease, and stress the importance of ultrasonography in chest diseases diagnosis, its advantages, limitations and disadvantages when compared to the common x-ray, computed tomography and physical examination. The authors also discuss the definition of pleural effusion, its physiopathology, morbidity, mortality, main causes and clinical presentation. The examination technique is systematically approached both by thoracic and abdominal pathways.
Keywords: Pleural effusion; Pleural spill; Ultrasound.
INTRODUCTION
For some time one thought that ultrasound could not be used in chest assessment. The main chest organs are filled with air which is not a good ultrasound conductor. Besides that, the ribs block ultrasound. However, ultrasound has become an invaluable resource in the assessment of abnormal chest, in which liquid and solid densities are interposed between the chest wall and the lungs, allowing excellent propagation of sound waves, making it possible to extend the use of ultrasound in the diagnosis of a number of morbidities(1).
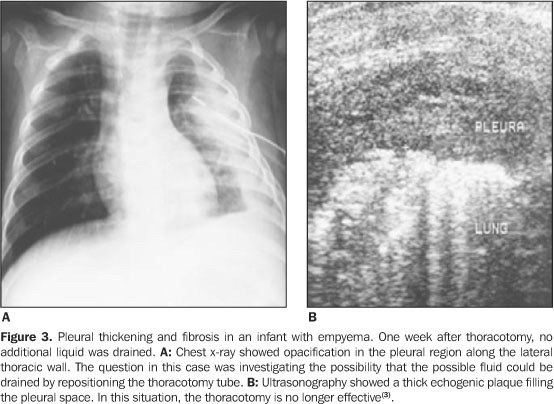
Ultrasonography can be used to clarify the nature of pleural densities, pleural effusions and pleural thickening. It can also differentiate pleural from parenchymal lesions, visualize ill parenchyma obscured by pleural effusion and detect pleural septations and other pleural abnormalities. It makes the differential diagnosis of pulmonary parenchyma diseases like consolidation, atelectasis and tumor. It differentiates cystic tumor masses from the solid ones; tumor or large or persistent pleural effusion; clarifies subpulmonary or subphrenic fluid cases, chest wall tumor mass or pleural fluid (Figure 1). It identifies the cause of unilateral elevation of the diaphragm and alteration of its motility, which can be caused by phrenic nerve paralysis, subpulmonary pleural effusion (Figure 2), subphrenic abscess, mass in the superior abdomen, diaphragmatic hernia, diaphragmatic tumor, and pulmonary volume reduction. It allows the visualization of mediastinal tumor masses, the relationship of the masses with the thymus and extension of cervical masses to the chest. It identifies the position of catheters in vessels. It diagnoses pericardial spills and identifies vascular thrombi (for example: cardiac thrombi in superior and inferior vena cava). It can identify pneumothorax. Besides establishing diagnosis, ultrasound can be used to guide thoracentesis (Figure 3), needle biopsies of peripheral and mediastinal tumor masses, and the placement of endotracheal probes(2).
Amongst the various indications and uses of the chest ultrasound, the objective of this study is reviewing its role in the pleural effusion.
PLEURAL EFFUSION
The pleural effusion occurs due to the accumulation of fluid in the pleural space as a consequence of an imbalance between the formation and fluid absorption or due to alteration in the drainage to lymph nodes. There are two types of pleural effusion(4):
1 Transudate: It occurs when there is an increase of hydrostatic pressure or a decrease of capillary oncotic pressure. As examples, one can name congestive heart failure, cirrhosis, nephrotic syndrome, peritoneal dyalisis, superior vena cava obstruction, glomerulonephritis, mixedema, pulmonary embolism, sarcoidosis and hypoalbuminemia.
2 Exsudate: It occurs due to the increase in permeability in microcirculation or alteration in the pleural space drainage to lymph nodes. As examples, one can point infectious diseases, neoplastic diseases, collagen-vascular diseases, drug-induced diseases, gastrointestinal diseases, hemothorax, chylothorax and miscellaneous (Meigs' syndrome, asbestosis, uremia, urinary tract obstruction, adult respiratory distress syndrome, abdominal surgery, yellow nail syndrome)(4).
In the United States of America pleural effusion affects 1.3 million individuals every year. The main diseases that trigger this comorbidity are heart failure decompensation (500,000), bacterial pneumonia (300,000), malignancy (200,000), pulmonary emboli (150,000), cirrhosis with ascites (50,000), pancreatitis (20,000), collagenosis and vasculitis (6,000) and tuberculosis (2,500). On the other hand, in Brazil the percentage of pleural effusion associated to tuberculosis is greater(5).
The pleural effusion morbidity and mortality are directly related to its causes, the stage of the disease at the time of the diagnosis, and to the pleural fluid biochemical finding. The morbidity and mortality rates of patients with pneumonia associated with pleural effusion are greater than in patients with pneumonia alone. The development of malignant pleural effusion is associated with a poor prognosis. The life expectancy of pleural effusion with a malignant etiology is from three to six months. Patients with pleural effusion associated with lung carcinoma and gastrointestinal tract carcinoma have a reduced life expectancy. There is significant association of malignant pleural effusion with breast and gynecologic malignancies(4).
The more commonly associated clinical manifestations are progressive dyspnea, non-productive cough and pleuritic pain. Dyspnea is the most usual finding, generally indicative of large effusions, although not superior to 500 ml. The physical examination is generally normal when there is less than 300 ml of liquid: in larger quantities, one observes massiveness, reduced vesicular murmur, decreased vocal fremitus and thoracic expansibility(4).
There are four main types of fluids in the pleural space: serous (hydrothorax), blood (hemothorax), lymph (chylothorax) and purulent (pyothorax or empyema).
ADVANTAGES OF THE ULTRASOUND IN THE PLEURAL EFFUSION DIAGNOSIS
When the ultrasound is used for the analysis and quantification of fluids in pleural effusion, it is superior to chest x-rays, being capable of correlating the effusion thickness with the real volume(5,6) (Figure 2). It allows the detection of small amounts of pleural locular fluid, with positive identification of amounts as small as 3 to 5 ml, that cannot be identified by x-rays as it is only capable of detecting volumes above 50 ml of liquid(7). Contrary to the radiological method, ultrasound allows an easy differentiation of pleural locular liquid and thickened pleura (Figure 3). It is efficient in pinpointing thoracocentesis, even in small fluid collections(7,8). The risks in resourcing only to physical examination without ultrasound guidance for puncture include: pneumothorax, hemothorax, subdiaphragmatic hematoma and subdiaphragmatic organs lacerations. The use of Ultrasonography is a very promising solution for the reduction of those possible complications(9).
When compared to computed tomography (CT), ultrasound detects the inverted diaphragm in longitudinal or sagitally oriented studies, which is not possible with CT, except for reconstruction. Ultrasound has the advantage of being a portable and practical technique, which makes it very useful in the study of infants in critical condition, whose pulmonary opacities may be mistakenly taken as pleural effusions (Figure 4)(1). The CT scan is not always an available resource, and it is expensive when compared to ultrasound, and at pediatric age range CT requires patients' sedation(8,10).
DISADVANTAGES OF ULTRASOUND IN THE PLEURAL EFFUSION
A limitation of chest ultrasound is revealed when very homogeneous and solid lesions may appear as cystic lesions. In the chest, there is no solid or cystic structure that may serve as a reference to allow such differentiation. Another difficulty with ultrasound assessment of the chest is the acoustic shade caused by a dense rib, which may induce an inattentive observer to believe that a tumor mass is anechoic. Additionally the differentiation between hemothorax and pleural effusion is difficult, except when the patient clinical history is available(1).
CHEST ULTRASOUND EXAMINATION TECHNIQUE
The patient may be in a sitting position or in supine position. The pleural space is superficial and promptly examined by ultrasound, both via direct intercostal and abdominal approaches. A high frequency linear transducer (5 to 7.5 MHz) applied directly to the chest or a sectorial or convex transducer (3.5 to 5 MHz) conducted superiorly from abdomen provides a view of the pleural space(1,5) (Figure 6).
Direct intercostal approach The pleural space is at a 1 cm depth from the rib interface. The air-filled lung, covered by the visceral pleura, is a powerful reflector of the ultrasound beam, blocking a deeper penetration of ultrasound into the chest, producing a bright linear interface that moves with respiration. The bright linear interface is the visceral pleura ultrasonographic marker. Normally, there is a thin and dark line of pleural fluid separating the parietal pleura from the visceral pleura. The parietal pleura is seen as a thin echogenic line, less distinct, in general obscured by reverberation effect. Its location is inferred based on its relationship with the ribs and the visceral pleura. The pleural fluid, in its greater part, is relatively anechoic and easily recognized as an area of echolucency separating the parietal pleura from the visceral pleura(1,5).
Abdominal approach When an image is obtained from the abdomen, the diaphragm appears as a bright and curved echogenic line, that moves with respiration. The normal diaphragm is 5mm thick and is covered by parietal pleura in its thoracic face and by the peritoneum in its abdominal face. The lung acts as a specular reflector (similar to a mirror). A specular reflection of the liver and of the spleen is seen above the diaphragm, and this sign is a definite evidence of pleural fluid absence above the diaphragm. The signs of pleural effusion in an abdominal approach include anechoic fluid below the diaphragm, visualization of the chest cavity through the fluid accumulation, liver and spleen specular reflection absence above diaphragm and in large effusions(1,5).
ULTRASONOGRAPHIC SIGNS OF PLEURAL EFFUSION
The ultrasonographic signs of pleural effusion include the detection of an anechoic space immediately deep to the thoracic walls. As the pleural effusions are sound conducting, deeply situated structures in relation to the effusions which are not normally visible, become visible when such a condition is present. Normally, when examining the thoracic wall thorough the liver, nothing is visible through it as the aerated lung interrupts the ultrasound beam. However, in the presence of pleural effusion, the posterior thoracic wall becomes visible(1,2,5).
A pleural effusion appears as a hypoechoic collection immediately above diaphragm and adjacent structures. One can separate the subjacent consolidated lung from the effusion, because the pulmonary consolidation is more dense and contains multiple aerial echogenic areas (air bronchograms) in its interior. A non-complicated effusion is totally anechoic, while a complex collection such as hemothorax or empyema has a thicker fluid with septations (Figure 5)(1,11).
The free fluid flows about the pleural space according to patient position. In dorsal decubitus, the fluid flows to the back of the liver and the lungs. If the patient is standing, the fluid flows between the lung and the diaphragm(1).
There are two findings that have proven to be predictive of pleural fluid: the presence of a definite alteration in the form of a pleural density during inspiration and expiration, and the presence of mobile septations within the pleural lesion. Presumably, septations are fibrin bundles. The back and forth movement is unequivocal evidence that the fluid has a relatively low viscosity(1,11).
Doppler can also be helpful in distinguishing a pleural effusion from a pleural thickening. When a free pleural effusion is present, there is a colored sign between the visceral and parietal pleurae or near the costophrenic angle which is related with the respiratory movements. An organized pleural thickening appears like pleural lesion with no Doppler signals(1).
Diaphragm sign When liver or spleen are used as acoustic windows and a fluid is seen adjacent to these organs, the location of the fluid is determined by reference to the position of the diaphragm. If the fluid is inside the diaphragm and centrally positioned this fluid is ascites. If the fluid is outside the diaphragm and more peripherally located, it is within the pleural space(1).
Sign of the displaced diaphragmatic crus The fluid is within the pleural space if there is interposition of fluid between the diaphragmatic crus and the vertebral column, displacing the crus and increasing its distance to the column(1).
Sign of naked area The anterior space of the liver right lobe is directly held to the posterior diaphragm without peritoneum. Therefore, the ascetic fluid in the subhepatic or subphrenic space cannot extend behind the liver up to the level of the naked area(1).
APPLICATION IN THE QUANTIFICATION
The fluid volume can be calculated by measuring the maximum perpendicular distance between the surface and the chest wall. The scan is performed with the patient in the supine position, at maximum inspiration. The measurement is made right above the diaphragm. A 20mm extension corresponds to an average volume of 380 ml (± 130 ml). A 40 mm extension corresponds to an average volume of 1,000 ml (± 330 ml) as shown on Chart 1)(2,5,6). Another way to estimate the pleural effusion is classifying it as minimal, if the hypoechoic space is seen only at the costophrenic angle; small if it covers the costophrenic angle but limited within the image formed by the transducer; moderate if the space is larger than the image but limited within two images; and large or massive if it is larger than two images formed by the transducer(3).
APPLICATION IN QUALIFYING
The sonographic spectrum of the pleural fluid is useful in differentiating transudates from exudates. Anechoic effusions represent transudative processes and exudative processes with almost the same frequency. However the echogenic liquid contains floating particulate matter, septations or fibrine filaments; or is associated with pleural nodes; or pleural thickening greater than 3 mm is an exudate. The definite diagnosis is performed by means of analysis of the fluid after thoracocentesis(11,12).
PARAPNEUMONIC EFFUSIONS
The ultrasonography is an ancillary method to infer the anatomopathologic phase of the pleural disease (parapneumonic effusion or empyema) and, consequently, it may be of help in the choice of suitable treatment. The American Thoracic Society classifies the pleural reaction to an infectious process into three consecutive anatomopathological phases: acute or exudative phase; initial, characterized by the presence of serous effusion; fibrinopurulent phase, characterized by accumulation of polymorphonuclears, fibrine and pus, with tendency to formation of pleural loculi, adherences and septations; and chronic or organization phase characterized by fibroblasts proliferation and pulmonary incarceration(12).
The ultrasonographic finding can be classified into five classes, according to the pleural effusion characteristic: class 1 free effusion; class 2 effusion with little septation; class 3 septate, thick effusion, with grumes; class 4 loculated effusion, with multiple septa, debris, pleural thickening and pulmonary consolidation areas; class 5 loculated effusion, with multiple septa and debris, pleural thickening, defined empyemic sac, pulmonary incarceration and necrosis areas of the parenchyma(12) (Figure 6).
The anatomopathological phases correlate to ultrasonographic findings. We consider as being in the acute or exudative phase, the effusions presenting ultrasonographic classification 1 or 2; in the fibrinopurulent phase are those in classifications 3 and 4; and in the chronic or fibrotic phase,those with classification 5(12).
When using the anatomopathological classification as a means to rationalize conducts, in the acute phase thoracentesis or water-seal drainage is indicated. The chronic pleural empyemas require thoracotomy for surgical decortication. When the pleural disease reaches an intermediate phase, between exudative and chronic organized, the loculation, septation and adhesion can be undone by videothoracoscopy(12,13).
FINAL CONSIDERATIONS
The ultrasonography is a modality that can be used in the pleural effusion diagnosis, particularly in neonates and infants, also allowing the differentiation between transudative effusion and exudative effusion, orienting its therapeutic ethiology. In certain situations, ultrasonography can be superior to plain chest x-ray, mainly for detection of small amounts of effusion, thoracentesis site accuracy, and differentiation of the locular pleural fluid and the thickened pleura. Main disadvantages become evident when the differentiation of very homogeneous solid lesions is necessary and also when the acoustic shadow caused by a dense rib does not allow the assessment of the adjacent image. In the parapneumonic effusion, ultrasonography is capable of effectively classifying the disease evolutive stage and may guide the therapeutic action.
REFERENCES
Received July 30, 2004.
Accepted after revision September 28, 2004.
- 1. Seibert JJ, Glaseir CM, Leithiser RE. O tórax pediátrico. In: Rumack CM, Wilson SR, Charboneau JW, editores. Tratado de ultra-sonografia. 2Ş ed. Rio de Janeiro: Guanabara Koogan, 1998;13811386.
- 2. Brant WE. Chest. In: McGahan JP, Goldberg BB, editors. Diagnostic ultrasound: a logical approach. 1st ed. Philadelphia: Lippincott-Raven, 1998;10631081.
- 3. Tsai TH, Yang PC. Ultrasound in the diagnosis and management of pleural disease. Curr Opin Pulm Med 2003;9:282290.
- 4. Silva COS, Macedo AG. Pneumologia. In: Prado FC, Ramos JA, Valle JR, editores. Atualização terapêutica. 2Ş ed. São Paulo: Artes Médicas, 2003; 14461452.
- 5. Brant WE. Tórax. In: Rumack CM, Wilson SR, Charboneau JW, editores. Tratado de ultra-sonografia. 2Ş ed. Rio de Janeiro: Guanabara Koogan, 1998;488495.
- 6. Eibenberger KL, Dock WI, Ammann ME, Dorffner R, Hormann MF, Grabenwoger F. Quantification of pleural effusions: sonography versus radiography. Radiology 1994;191:681684.
- 7. Gryminski J, Krakowa P, Lypacewicz G. The diagnosis of pleural effusion by ultrasonic and radiologic techniques. Chest 1976;70:3337.
- 8. Cardieri JMA, Rodrigues JC. Derrames pleurais. In: Schvartsman S, Schvartsman C, editores. Pronto-socorro de pediatria. 2Ş ed. São Paulo: Sarvier, 1999;286288.
- 9. Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest 2003; 123:43641.
- 10. Cirino LMI, Garcia AE, Pereira PRB, Margarido NF, Tolosa EMC. Contribuição da ultra-sonografia na orientação do tratamento cirúrgico do empiema pleural em crianças. Rev Col Bras Cir 1998;25:9195.
- 11. Kim OH, Kim WS, Kim MJ, et al. US in the diagnosis of pediatric chest diseases. RadioGraphics 2000;20:653671.
- 12. Cirino LMI, Otoch JP, Margarido NF, Pereira PRB, Tolosa EMC. Sistematização técnica da toracoscopia no empiema pleural em crianças. Rev Col Bras Cir 1995;22(Supl. 2):193.
- 13. Cirino LMI, Smaletz O, Otoch JP, et al. Análise bioquímica e tratamento cirúrgico do empiema pleural parapneumônico em crianças. Rev Med HU-USP 1997;7:2528.
Publication Dates
-
Publication in this collection
25 May 2006 -
Date of issue
Apr 2006