Abstracts
The increasing consumption of soybeans due to its bioactive compounds has attracted interest in describing the grain's constituents and variation during processing. Phytate has been the aim of much research since it chelates essential minerals but also has beneficial antioxidant effects. This study evaluated the variation of phytate, calcium, zinc, and iron during soaking and cooking of soybeans. The phytate: Zn and phytate: Fe molar ratios were determined in order to estimate the bioavailability of these minerals. Six food-type varieties were used: BR 36, BRS 213, BRS 216, BRS 232, BRS 155, and Embrapa 48. The samples were soaked in water 3:1 (w/w) for 12 hours at room temperature and cooked. Cooking time was determined by modeling the softening of each variety using fractional conversion. Water content, phytate, and minerals were determined in raw, soaked and cooked samples. The water content of raw grains for all varieties was 9.9 g.100 g-1 increasing to a range of 58.1-63.7 g.100 g-1 after soaking and 63.1-66.0 g.100 g-1 after cooking. Soaking caused a significant reduction in phytate (23-30%), but cooking caused no additional reduction. The phytate: Zn molar ratio was 20 indicating that zinc absorption could be impaired, while the phytate: Fe molar ratio was 8, below the level of compromising absorption
zinc; calcium; iron; phytic phosphorus
O aumento de consumo de soja devido à presença de compostos bioativos despertou o interesse em descrever os constituintes dos grãos e variações durante o processamento. Fitato tem sido bastante pesquisado, pois quela minerais essenciais, mas tem efeitos benéficos como antioxidante. Este trabalho avaliou a variação em fitato, cálcio, zinco e ferro durante embebição e cocção de sojas. As razões molares de fitato: Zn e fitato: Fe foram determinadas para estimar biodisponibilidade dos minerais. Seis variedades de soja para consumo humano foram avaliadas: BR 36, BRS 213, BRS 216, BRS 232, BRS 155 e Embrapa 48. As amostras foram maceradas em água 3:1 (p/p) por 12 horas em temperatura ambiente e cozidas. O tempo de cocção foi determinado modelando o amaciamento de cada variedade usando conversão fracionada. Umidade, fitato e minerais foram determinados em soja crua, macerada e cozida. Umidade dos grãos crus em todas as variedades foi de 9,9 g.100 g-1, aumentando para entre 58,1 e 63,7 g.100 g-1 após maceração e entre 63,1 e 66 g.100 g-1 após cocção. A maceração causou redução significativa de fitato (23-30%), mas a cocção não causou redução adicional. A razão molar de fitato: Zn foi 20, indicando que a absorção de zinco pode ser afetada, enquanto a de fitato: Fe foi 8, menor que o nível de comprometimento de absorção
zinco; cálcio; ferro; fósforo fítico
ORIGINAL
Effect of soaking and cooking on phytate concentration, minerals, and texture of food-type soybeans
Efeito de maceração e cocção sobre concentração de fitato, minerais e textura em sojas para consumo humano
Elisa Noemberg Lazzari KarkleI; Adelaide BeleiaII,* * A quem a correspondência deve ser enviada
IDepartment Grain Science and Industry, Kansas State University, 201, Shellenberg Hall, Manhattan, Ks 66506, USA
IIDepartamento de Ciência e Tecnologia de Alimentos, Universidade Estadual de Londrina - UEL, CEP 96051-970, Londrina - PR, Brasil, E-mail: beleia@uel.br
ABSTRACT
The increasing consumption of soybeans due to its bioactive compounds has attracted interest in describing the grain's constituents and variation during processing. Phytate has been the aim of much research since it chelates essential minerals but also has beneficial antioxidant effects. This study evaluated the variation of phytate, calcium, zinc, and iron during soaking and cooking of soybeans. The phytate: Zn and phytate: Fe molar ratios were determined in order to estimate the bioavailability of these minerals. Six food-type varieties were used: BR 36, BRS 213, BRS 216, BRS 232, BRS 155, and Embrapa 48. The samples were soaked in water 3:1 (w/w) for 12 hours at room temperature and cooked. Cooking time was determined by modeling the softening of each variety using fractional conversion. Water content, phytate, and minerals were determined in raw, soaked and cooked samples. The water content of raw grains for all varieties was 9.9 g.100 g-1 increasing to a range of 58.1-63.7 g.100 g-1 after soaking and 63.1-66.0 g.100 g-1 after cooking. Soaking caused a significant reduction in phytate (23-30%), but cooking caused no additional reduction. The phytate: Zn molar ratio was 20 indicating that zinc absorption could be impaired, while the phytate: Fe molar ratio was 8, below the level of compromising absorption.
Keywords: zinc; calcium; iron; phytic phosphorus.
RESUMO
O aumento de consumo de soja devido à presença de compostos bioativos despertou o interesse em descrever os constituintes dos grãos e variações durante o processamento. Fitato tem sido bastante pesquisado, pois quela minerais essenciais, mas tem efeitos benéficos como antioxidante. Este trabalho avaliou a variação em fitato, cálcio, zinco e ferro durante embebição e cocção de sojas. As razões molares de fitato: Zn e fitato: Fe foram determinadas para estimar biodisponibilidade dos minerais. Seis variedades de soja para consumo humano foram avaliadas: BR 36, BRS 213, BRS 216, BRS 232, BRS 155 e Embrapa 48. As amostras foram maceradas em água 3:1 (p/p) por 12 horas em temperatura ambiente e cozidas. O tempo de cocção foi determinado modelando o amaciamento de cada variedade usando conversão fracionada. Umidade, fitato e minerais foram determinados em soja crua, macerada e cozida. Umidade dos grãos crus em todas as variedades foi de 9,9 g.100 g-1, aumentando para entre 58,1 e 63,7 g.100 g-1 após maceração e entre 63,1 e 66 g.100 g-1 após cocção. A maceração causou redução significativa de fitato (23-30%), mas a cocção não causou redução adicional. A razão molar de fitato: Zn foi 20, indicando que a absorção de zinco pode ser afetada, enquanto a de fitato: Fe foi 8, menor que o nível de comprometimento de absorção.
Palavras-chave: zinco; cálcio; ferro; fósforo fítico.
1 Introduction
The approval by the Food and Drug Administration in the United States in 1999 of the health claim for the relationship between consumption of soy protein and decreased risk of coronary heart disease led to an increase in the consumption of soy products. Special varieties of soybeans have been developed for food use aiming at better flavor characteristics (mainly by reducing the levels of lipoxygenase), yellow hulls and light colored hylum, reduced cooking time, and size according to a specific use (YOKOMIZO; VELLO, 2000; DESTRO et al., 2002; CARRÃO-PANIZZI et al., 2002a, 2002b).
Phytate is present in soybeans in concentrations up to 2.3% of the grain's dry weight. Its chemical structure makes this molecule an active chelator of minerals, and it is also an antioxidant. Phytates are known for causing mineral deficiency in populations that have grain-based diets, and it is also a concern in specific groups that have marginal mineral intake, such as infants, teenagers, pregnant women, and the elderly (WEAVER; KANNAN, 2002). The molar ratio phytate:mineral seems to be important to estimate the absorption, especially of iron and zinc.
Despite its possible deleterious effects on human nutrition, phytate is also an effective antioxidant. The effects of phytate in reducing cholesterol levels and as an anti-cancer agent have received substantial attention (JENAB; THOMPSON, 2002). Since there are both deleterious and positive effects on human health, there is a clear importance of knowing the bioactive compounds present in soy, along with their interaction and modifications during food processing. The aim of this work was to conduct a mass balance study of phytates and selected minerals of nutritional importance during soaking and cooking of six varieties of food-type soybeans and to determine texture variation during domestic processing. Cooking time was determined after modeling the cooking kinetics of each variety. Molar ratios of phytate:iron and phytate:zinc were determined for the cooked beans as a way to estimate bioavailability.
2 Material and methods
2.1 Material
The following food-type soybean varieties were used: Embrapa 48, BR 36, BRS 213, BRS 216, BRS 232, and BRS 155. The tests were conducted approximately 10 months after harvest, which occurred in 2005 in the city of Londrina - PR.
2.2 Methods
Determination of cooking time
Samples were soaked in water 3:1 (w/w) in closed containers for 12 hours (room temperature). The grains were drained and placed in a beaker with boiling water (8:1 w/w). Every 10 minutes (from t = initial to t = 200 minutes), 10 intact grains were removed from the cooking media and cooled. The grains were evaluated for hardness using a texture analyzer (Stable Micro Systems, TA.XT2i, Surrey, England) with a flat 25 mm diameter cylinder probe. The conditions (adapted from COELHO, 2004) were: test speed of 2 mm/s, force of 0.05 N, and compression of 57% (3.2 mm for all varieties except for BRS 216, for which 2.0 mm was used). The maximum force of resistance to compression of an individual grain represents its hardness. All varieties were soaked, cooked, and evaluated in duplicate. Boiling water was added to the beakers as needed during the cooking period.
The hardness data collected were treated using the fractional conversion methodology proposed by Rizvi and Tong (1997), which describes the softening of the vegetables under prolonged cooking as a first order reaction. The hardness after cooking for 200 minutes was considered the residual texture. The fractional conversion was determined using the following Equation 1:
where: f = fractional conversion; H0 = hardness at t = 0; Ht = hardness at t; H = residual hardness.
= residual hardness.
The plot of logarithm of (1- f) versus time represents the softening of each variety. Using the first order equation, the time to achieve hardness value of 23 N was calculated. The optimum hardness was based on the results found by Shimelis and Rakshit, (2005) and Coelho (2004) for instrumental and sensory hardness of different types of beans since no reference was found for soybeans under similar test conditions.
Soaking and cooking
A sample of 60 g of each variety was soaked in tap water 3:1 for 12 hours, at room temperature. The grains were drained, a sample of the soaking water was collected, and 50 g of the soaked soybeans were separated for analysis. The remaining grains were placed in a beaker with boiling water (8:1 w/w) and cooked for the previously determined cooking time.
Analysis
The content of water, phytate, and minerals were determined in triplicates in raw, soaked, and cooked samples (the cooking procedure was conducted in duplicates). The water content of the grains was determined by drying the samples at 105 ºC until constant weight (INSTITUTO... 1985). Phytate was extracted and quantified using the ferric chloride precipitation method of Thompson and Erdman (1982). After precipitation and mineralization, the phosphorus content of the samples was determined according to Chen, Toribara and Warner (1956), and the phytate concentration was calculated considering 28.2% phosphorus in the molecule.
Soybeans were examined for calcium (Ca), zinc (Zn), and iron (Fe) using plasma emission spectrometry (Thermo Jarrell Ash Corporation, Plasma ICAP 61E Waban, United States). Total solids in soaking and cooking media were determined by drying a 10 mL sample at 60 ºC until dryness.
The molar ratios phytate:Zn and phytate:Fe were calculated based on 1 kg of the cooked sample according to the following Equation 2:
where: PA = calculated phytate content; MWPA = PA molecular weight (660 Da); Min = mineral content (zinc or iron); MWminute = mineral molecular weight (Zn = 65 Da; Fe = 56 Da)
The software Statistica 6.0 (StatSoft, Tulsa, United States) was used to obtain the fractional conversion plots and equations and to perform ANOVA and Tukey test among the varieties and processing steps.
3 Results and discussion
3.1 Cooking time
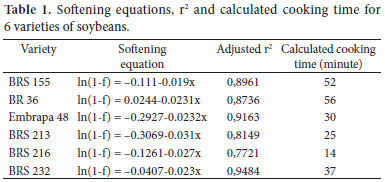
Using the fractional conversion technique, a softening equation for each variety was determined and the cooking time was calculated (Table 1). All varieties adjusted well to the first order equation, with r2 above 0.77. Cooking time varied from 14 to 56 minutes (varieties BRS 216 and BR 36, respectively), with mean cooking time of 36 minutes. The variety BRS 216 had the least initial hardness and was the fastest cooking, while BR 36 had the highest initial hardness and took longer to cook. Another difference between the varieties was the weight value of 100 grains, BRS 216 the smallest grain (7.2 g ± 0.17) and BR 36 the largest grain ( 22.5 g ± 0.07). Among all the varieties, there was no influence of grain size in the cooking time. Varieties BRS 155 cooked in 52 minutes with 15.1 g.100 grains-1, while BR 36 cooked in 56 minutes with weight of 22.5 g.100 grains-1.
The results are similar to those reported by Meneguce et al. (2004) for 34 genotypes of soybeans using a Mattson cooker device. The authors reported cooking time of 25-67 minutes, with a mean of 38 minutes.
3.2 Effect of soaking and cooking on water content, solid loss, and phytic acid
The medium water content of the grains before processing was of 9.9 g.100 g-1, with non-significant variation among the varieties. Soaking caused moisture to rise to an average of 61.5 g.100 g-1 (Table 2), with great difference among varieties (range: 58.1 for BR 36 to 63.7 g.100 g-1 for BRS 216). All varieties had an additional increase in moisture after cooking (63.1 for BRS 232 to 66 g.100 g-1 for BRS 155). The varieties with higher moisture after soaking also reached higher water content after cooking (BRS155, BRS213 and BRS216).
The phytate content of the raw samples ranged from 1.19 g.100 g-1 for BRS 155, the lowest and the only different from the other samples, to 1.81 g.100 g-1 for BRS 213. During the soaking and cooking processes the mean loss of solids into water was of 4.5 and 13.1g.100 g-1, respectively. This loss represents mainly carbohydrates leading to a concentration of the remaining components. Due to this, the variation of phytate (Table 3) and mineral contents (Table 4) is better studied using the mass-balance approach.
When considering the loss in dry matter, soaking caused a reduction in phytate of 23% in variety BRS 232 and up to 30% in variety BRS 155. Such a reduction was slightly higher than that found by Lestienne et al. (2005a), an average of 23%, and by Beleia, Ida and Lethi (1990), 22%.
There was no loss of total phosphorus during processing, but only of phytic phosphorus, thus the phytates were probably hydrolyzed to inorganic P, which remained in the grain. Lestienne et al. (2005b) found an increase of inorganic P in soaked soybeans but not in the soaking media. The authors also detected very little phytase activity in the soaking media suggesting that the phytate is hydrolyzed but the products remain in the grain.
Cooking did not promote additional reduction of phytate for any of the samples (Table 3). Since the samples were dropped into boiling water, it can be assumed that the phytase was immediately inactivated and since there was no reduction, lixiviation was also very limited. Cheryan (1980) explained that during cooking, phytate can form insoluble salts with calcium and magnesium instead of soluble salts with potassium.
3.3 Effect of soaking and cooking on mineral content and estimated bioavailability
Soaking under the conditions used in this study did not affect the calcium content of the soybeans, and cooking had no influence on calcium for varieties BR 36, BRS 213, BRS 216, and BRS 155. This result agrees with the findings by Salunkhe and Deshpande (1991) and Duhan, Khetarpaul and Bishnoi (2002), who reported total retention of calcium for different types of beans. The only explanation for the calcium increase of varieties Embrapa 48 and BRS 232 is that the use of tap water (seeking to simulate household cooking) might have had some influence.
According to Kamchan et al. (2002), the bioavailability of calcium in cooked soybeans is approximately of 11%, the authors claim that phytate is the main cause of such low bioavailability. In the present study, no pattern was found regarding phytate and calcium contents for all processing steps. Considering the portion size for soybeans stipulated by the Brazilian Agency (ANVISA) of 60 g of dry beans (BRASIL, 2003), the amount of calcium provided by the cooked samples would range from 99 to 172 mg (Embrapa 48 and BRS 155, respectively - Table 4). This represents 10 -17% of the Adequate Ingestion of this nutrient established by the Institute of Medicine (INSTITUTE..., 1997), which is 1000mg/day for adults age 19-50. This recommendation is based on an average calcium bioavailability of 25% for most foods. A bioavailability of 11% implies that the portion should be almost doubled to truly provide 10-17% of the Daily Adequate Ingestion.
Like what was found for calcium, soaking and cooking had little effect on zinc content. This can be due to the strong associating of this mineral to protein in the cells of the cotyledon of the grain (WEAVER; KANNAN, 2002). The same result for soaked soybeans was found by Lestienne et al. (2005a, 2005b).
According to Weaver and Kannan (2002), zinc forms the most stable complex with phytate, and thus it is the most affected mineral in terms of bioavailability. Various authors consider that a molar ratio phytate: Zn of 10 is the limit for optimal absorption (LESTIENNE et al., 2005b; ADEYEYE et al., 2000). In the cooked soybean samples, the calculated mean molar ratio was 20 (Table 5), with all varieties above 10, this suggests that the phytate present in cooked soybeans could impair the absorption of zinc present in the grain and contribute to zinc deficiency. It is worth mentioning though, that in a normal diet, where many different foods are consumed at the same time, this molar ratio can easily be altered. According to Weaver and Kannan (2002), other factors that influence absorption are the pH changes within the gastrointestinal tract and the dilution caused by endogenous secretions.
The iron content of the soybeans was not affected by processing, except for variety BRS 155, which showed an increase after soaking but returned to the initial concentration after cooking. This behavior was seen for all varieties; however, it was only significant for BRS 155. Since tap water was used, it could explain the source of iron, but it was not retained in the cooked grain.
It is considered that diets with phytate:Fe molar ratio greater than 14 can be a cause of iron deficiency (LESTIENNE et al., 2005b; ADEYEYE et al., 2000). All samples were below this figure. Therefore, it is likely that iron absorption is not significantly impaired by the phytate content of the cooked soybean. However, the same considerations made above regarding zinc absorption are also valid for iron.
4 Conclusions
Since soaking promoted significant phytate reduction, it is an effective treatment when the intention is to reduce phytate levels, which can be needed for population groups with marginal mineral intake. If the intention is to benefit from the antioxidant activity of the phytate, then soybeans should be cooked without soaking since cooking did not promote additional reduction in the phytate content. Calcium, zinc, and iron contents were not affected by soaking and cooking under the conditions adopted in this study. Although no calcium was lost, the low bioavailability implies the consumption of twice the recommended portion of a significant mineral intake. According to the molar ratios phytate:Zn and phytate:Fe in the cooked grains, iron absorption is not affected, but zinc absorption could be impaired.
Acknowledgment
The authors are grateful for the financial support provided by CNPq for granting a master's program scholarship.
Recebido para publicação em 15/1/2009
Aceito para publicação em 8/7/2009 (004020)
- ADEYEYE, E. I. et al. Calcium, zinc and phytate interrelationships in some foods of major consumption in Nigeria. Food Chemistry, v. 71, n. 4, p. 435-441, 2000.
- BELEIA, A.; IDA, E. I.; LETHI, T. T. Distribuição de fósforo e ácido fítico durante o processamento de extrato hidrossolúvel de soja. Arquivos de Biologia e Tecnologia, v. 33, n. 3, p. 623-629, 1990.
- BRASIL. Agência Nacional de Vigilância Sanitária - ANVISA. Resolução RDC nº 359, de 23 de dezembro de 2003. Aprova Regulamento Técnico de porções de alimentos embalados para fins de rotulagem nutricional. Diário Oficial [da] República Federativa do Brasil, Poder Executivo, Brasília, DF, 26 dez. 2003.
- CARRÃO-PANIZZI, M. C. et al. BRS 213: nova cultivar de soja para alimentação humana. In: CONGRESSO BRASILEIRO DE SOJA E MERCOSOJA, 2., 2002, Londrina. Anais... Londrina: Embrapa Soja, 2002a. Documentos 181, p. 201.
- CARRÃO-PANIZZI, M. C. et al. BRS 216: nova cultivar de soja para alimentação humana. In: CONGRESSO BRASILEIRO DE SOJA E MERCOSOJA, 2., 2002, Londrina. Anais... Londrina: Embrapa Soja, 2002b. Documentos 181, p. 202.
- CHEN JUNIOR, P. S.; TORIBARA, T. S; WARMER, H. Microdetermination of phosphorus. Analytical Chemistry, v. 28, n. 11, p. 1756-1758, 1956.
- CHERYAN, M. Phytic acid interactions in food systems. Critical Reviews in Food Science and Nutrition, v. 13, n. 4, p. 297-335, 1980.
- COELHO, S. R. M. Alterações químicas, físico-químicas e físicas durante o envelhecimento natural e acelerado de feijão comum (Phaseolus vulgaris L.). 2004. 235 f. Tese (Doutorado em Ciência de Alimentos)-Universidade Estadual de Londrina, Londrina, 2004.
- DESTRO, D. et al. Agronomic and chemical characterization of soybean genotypes for human consumption. Crop Breeding and Applied Biotechnology, v. 2, n. 4, p. 599-608, 2002.
- DUHAN, A. D.; KHETARPAULl, N.; BISHNOI, S. Content of phytic acid and HCl extractability of calcium, phosphorus and iron as affected by various domestic processing and cooking methods. Food Chemistry, v. 78, n. 1, p. 9-14, 2002.
- INSTITUTE OF MEDICINE - IOM. Dietary reference intakes: for calcium, phosphorus, magnesium, vitamin D and fluoride. Washington, DC: National Academy, 1997.
- INSTITUTO ADOLFO LUTZ - IAL. Normas analíticas do IAL São Paulo: IAL, 1985.
- JENAB, M.; THOMPSON, L. U. Role of phytic acid in cancer and other diseases. In: REDDY, N. R.; SATHE S. K. (Eds.). Food phytates Florida: CRC, 2002. p. 225-248.
- KAMCHAM, A. et al. In vitro calcium bioavailability of vegetables, legumes and seeds. Journal of Food Composition and Analysis, v. 17, n. 3-4, p. 311-320, 2002.
- LESTIENNE, I. et al. Effects of soaking whole cereal and legume seeds on iron, zinc and phytate contents. Food Chemistry, v. 89, n. 3, p. 421-425, 2005a.
- LESTIENNE, I. et al. The effects of soaking of whole, dehulled and ground millet and soybean seeds on phytate degradation and Phy:Fe and Phy:Zn molar ratios. International Journal of Food Science and Technology, v. 40, n. 4, p. 391-399, 2005b.
- MENEGUCE, B. et al. Performance of cultivars and pure lines of food-type soybean to the trait grain cooking time WORLD SOYBEAN RESEARCH CONFERENCE, 7.; INTERNATIONAL SOYBEAN PROCESSING AND UTILIZATION CONFERENCE, 4.; CONGRESSO BRASILEIRO DE SOJA, 3., 2004, Londrina. Londrina: Embrapa Soybean, p. 63, 2004.
- RIZVI, A. F.; TONG, C. H. Fractional conversion for determining texture degradation kinetics of vegetables. Journal of Food Science, v. 62, n. 1. p. 1-7, 1997.
- SALUNKHE, D. K.; DESHPANDE, S. S. Legumes. In: DESHPANDE, U. S.; DESHPANDE, S. S. Foods of plant origin New York: AVI, 1991. p. 230-261.
- SHIMELIS, E. A.; RAKSHIT, S. K. Proximate composition and physico-chemical properties of improved dry bean (Phaseolus vulgaris L.) varieties grown in Ethiopia. LWT- Food Science and Technology, v. 38, n. 4, p. 331-338, 2005.
- THOMPSON, D. B.; ERDMAN, J. W. Phytic acid determination in soybeans. Journal of Food Science, v. 47, n. 2, p. 513-517, 1982.
- WEAVER, C. M.; KANNAN, S. Phytate and mineral bioavailability. In: REDDY, N. R.; SATHE, S. K. (Eds.). Food phytates Florida: CRC, 2002. p. 211-223.
- YOKOMIZO, G. K.; VELLO, N. A. Coeficiente de determinação genotípica e de diversidade genética em topocruzamentos de soja tipo alimento com tipo grão. Pesquisa Agropecuária Brasileira, v. 35, n. 11, p. 2223-2228, 2002.
Publication Dates
-
Publication in this collection
26 Jan 2011 -
Date of issue
Dec 2010
History
-
Accepted
08 July 2009 -
Received
15 Jan 2009