Abstracts
The thymic morphometry analysis was used for determining apoptosis and atrophy of the thymus of eight puppies inoculated with canine distemper virus (CDV). Three healthy dogs were used as negative controls. Sections, 5µm thick, were stained by HE and Shorr, and the latter were evaluated by morphometry. CDV nucleoprotein was detected by immunohistochemistry. Morphometric results confirmed lymphoid hypotrophy in CDV inoculated dog thymuses, more stroma, less parenchyma and higher apoptotic index/field than negative control (not inoculated) puppies. Apoptosis plays a role in the mechanism of thymus atrophy that develops in canine distemper.
puppy; apoptosis; thymus; canine distemper; immunology; virus; immunosuppression
Determinaram-se a apoptose e a atrofia no timo de oito cães novos, inoculados experimentalmente com o vírus de cinomose. Três cães saudáveis foram usados como controle negativo. Secções coradas pelo Shorr foram avaliadas por morfometria. A nucleoproteína viral foi detectada por imunoistoquímica. Os resultados morfométricos confirmaram a hipotrofia e mostraram que o timo dos cães inoculados tinha mais estroma, menos parênquima e maior índice apoptótico/campo que o dos animais-controle. Pode-se concluir que a apoptose desempenha importante papel no mecanismo de hipotrofia tímica que se desenvolve na cinomose.
cão apoptose; timo; cinomose; imunologia; vírus; imunossupressão
VETERINARY MEDICINE
Morphometric analysis of the thymus of puppies infected with the Snyder Hill Strain of canine distemper virus
Análise morfométrica do timo de cães filhotes infectados com a amostra Snyder Hill do vírus de cinomose canina
C.M. AlvesI; A.C. VasconcelosI; A.S. MartinsII; H.L. del PuertoII; F.G.A. SantosI; J.E.S. NunesI; P.P. CamposI; L. MoroI
IDepartamento de Patologia Geral - ICB UFMG Caixa Postal 486 31270-901 - Belo Horizonte, MG
IIDepartamento de Fisiologia e Biofísica ICB - UFMG
ABSTRACT
The thymic morphometry analysis was used for determining apoptosis and atrophy of the thymus of eight puppies inoculated with canine distemper virus (CDV). Three healthy dogs were used as negative controls. Sections, 5µm thick, were stained by HE and Shorr, and the latter were evaluated by morphometry. CDV nucleoprotein was detected by immunohistochemistry. Morphometric results confirmed lymphoid hypotrophy in CDV inoculated dog thymuses, more stroma, less parenchyma and higher apoptotic index/field than negative control (not inoculated) puppies. Apoptosis plays a role in the mechanism of thymus atrophy that develops in canine distemper.
Keywords: puppy, apoptosis, thymus, canine distemper, immunology, virus, immunosuppression
RESUMO
Determinaram-se a apoptose e a atrofia no timo de oito cães novos, inoculados experimentalmente com o vírus de cinomose. Três cães saudáveis foram usados como controle negativo. Secções coradas pelo Shorr foram avaliadas por morfometria. A nucleoproteína viral foi detectada por imunoistoquímica. Os resultados morfométricos confirmaram a hipotrofia e mostraram que o timo dos cães inoculados tinha mais estroma, menos parênquima e maior índice apoptótico/campo que o dos animais-controle. Podese concluir que a apoptose desempenha importante papel no mecanismo de hipotrofia tímica que se desenvolve na cinomose.
Palavras-chave: cão apoptose, timo, cinomose, imunologia, vírus, imunossupressão
INTRODUCTION
Canine distemper (CD) is an acute or subacute highly contagious febrile disease that may include respiratory, gastrointestinal and central nervous system disorders. CD has been known for centuries world wide and has been the infectious disease of dogs with the highest fatality rate only second to rabies. The disease is caused by the canine distemper virus (CDV) which belongs to the Paramyxoviridae family and to the morbillivirus genus, together with measles virus (MV), rinderpest, peste de petite ruminant, phocine distemper, dolphin and porpoise morbilliviruses (Appel and Summers, 1995). CD is an infectious disease of the Carnivora (Krakowka et al., 1985). CDV is pantropic (Krakowka et al., 1985; Krakowka et al., 1987) but has a predilection for cells of epithelial, lymphoid and nervous tissues. There is only one serotype of CDV but a variety of "biotypes" exist that vary greatly in pathogenicity and tissue tropism within the central nervous system (Mccullough et al., 1974; Summers et al., 1984).
Host susceptibility to CDV is undoubtedly one important factor on the outcome of infection (Krakowka et al., 1975). CDV affects animals of all ages, but puppies are more susceptible when maternal antibodies are lost (Appel and Summers, 1995). Virulence is another parameter that may affect the severity, extent or type of clinical disease. Certain isolates, such as Snyder Hill or R252 strain, are highly virulent and neurotropic (Greene and Appel, 1990).
The immune system, like any the other organic system, is subjected to destruction and dysfunction as a result of infection by a variety of pathogenic agents (Tizard, 2000). CDV infection causes lymphopenia and immunosuppression in dogs during the early phase of the disease (Iwatsuki et al., 1995; Moro et al., 2003b). Lymphopenia probably occurs due to the ability of the virus to replicate in and destroy the lymphoid tissues (Mccullough et al., 1974). The infection induces lymphoid cells apoptosis (Moro et al., 2003b) that can also be observed in blood smears (Moro et al., 2003a;b).
The only consistent post-mortem finding in uncomplicated CD is the thymus atrophy (Appel, 1969; Appel, 1987; Moro et al., 2003b). In some puppies, the thymus is not demonstrable grossly (Mccullough et al., 1974). In gnotobiotic dogs, at post inoculation day 6 corticomedullary ratios of thymus decrease, together with the loss of distinct demarcation between cortex and medulla. The decrease in thymic size is due both to loss of cortical thymocytes in the absence of necrosis (Krakowka et al., 1980) and inflammation, and in the presence of apoptosis (Moro et al., 2003b).
Apoptosis is an organized ATP-dependent form of cellular death which requires de novo protein synthesis and gene transcription, and is often called programmed cell death (Orlowski, 1999). Morphologically it is characterized by shrinkage of the cell, chromatin condensation or compaction against the nuclear periphery, active membrane blebbing (zeiosis), and ultimate fragmentation of the cell into membrane enclosed vesicles (apoptotic bodies) (Wyllie et al., 1980). Apoptosis has a central role in the normal development and homeostasis of all multicellular organisms. Deregulation of this process, resulting in either too much or too little cell death, can cause both developmental defects and a wide variety of disease states (Verhagen and Voux, 1999). Apoptosis has been observed in various immunosuppressive diseases of humans and animals (Ameisen and Capron, 1991; Lam and Vasconcelos, 1994; Sarli et al., 1998; Moro et al., 2003b). Ito et al. (1997) suggested that apoptosis induced in stimulated MV-infected cells could be one mechanism of immunosuppression that occurs in natural MV infection. Guo and Lu (2000) showed apoptosis in vitro caused by CDV.
The aim of this study was to demonstrate morphometrically the occurrence of hypotrophy of the thymus during the acute phase of canine distemper and the role of apoptosis in the mechanism of the hypotrophy.
MATERIALS AND METHODS
The Snyder Hill strain of CDV was kindly supplied by Dr. Max J. G. Appel (Cornell University, Ithaca, New York, 14853, USA) and was used for animal inoculation.
Eleven mongrel puppies, aged from 50 through 100 day-old, were used. The puppies from the Centro de Controle de Zoonoses, Belo Horizonte, were dewormed, fed with commercial food three times a day and water ad libitum. Eight dogs were inoculated with 250µl of Snyder Hill strain of CDV via intra peritoneal and intra nasal routes. Three puppies were kept as controls and housed in a separated isolation unit. All animals were fed with commercial ration and kept under appropriate humane methods. Dogs were euthanized and necropsied 10 days after inoculation. Representative sections from thymus and retropharingeal lymph nodes were collected and fixed in 10% phosphate-buffered formalin. Subsequently, tissues were processed and embedded in paraffin, cut at 5µm and stained with hematoxylin and eosin for histological examination. Shorr stain was also used to get a better visualization of inclusion bodies. Sections of lymph node were submitted to immunohistochemistry to detect the presence of CDV.
For immunohistochemistry, the anti-CDV nucleoprotein monoclonal antibody (MoAb) was kindly provided by Dr. Alex Walender (Canada). Slides were deparaffinized, predigested with proteinase K and reduced with sodium borohydride. Peroxidase was quenched with hydrogen peroxide. Tissues were stained with MoAb, treated with biotinylated horse anti-mouse IgG, incubated in vectastain ABC reagent and DAB peroxidase reagent1 1 Vector Laboratories. Burlingam, CA, USA. applied. Sections were counter stained with hematoxylin.
For in situ detection of DNA fragmentation, the TUNEL technique was applied for detection of genome fragmentation, using a commercial oncogene kit2 2 Oncogene Kit. Cat#QIA33 TdT FragEL TM DNA fragmentation detection kit. Oncogen Research Products, MA, USA. . The reaction was carried out as described by the manufacturer. Briefly, slides were incubated with 20µg/ml of proteinase K3 3 Sigma USA. and endogenous peroxidase was quenched with 3% H2O2 in methanol. TdT and deoxynucleotides were applied and slides placed in humid atmosphere at 37ºC for 1.5h. The reaction was stopped by blocking buffer, the slides were treated with peroxidase streptavidin conjugate plus DAB and counterstained with light green.
Microscopic images of the thymus were digitalized and submitted to morphometric analyses with the Kontron KS 300 V 2.0 software for morphometric analysis of the parenchyma and stroma. Total field area (TFA) thymic lobar areas (TLA) and empty spaces (ES) were measured in 3 random microscopic fields, magnified 40x. Interfollicular stroma area (IFS) was obtained by difference as indicated by the formula: IFS = TF-(TL+ES). The percentage of parenchyma (lobe/thymic area) and thymic stroma (stroma/thymic area) were obtained.
Sections 5µm thick were stained by Shorr4 4 Merck. Cat#9275. Darmstadt, Germany. for determining the apoptotic index. The total number of cells and the number of apoptotic cells in 35 fields (100X) per slide were counted and apoptotic indices (number of apoptotic cells/ total number of cells) were calculated.
The determination of the minimal representative number of microscopic fields to establish the cut off value to quantify apoptosis in canine thymus was reached through the analysis of the instability of the standard deviation of the mean (Sampaio, 1998; Moro et al., 2004). Firstly, a thymus of an infected dog was collected and processed routinely for histological examination and sections 5µm thick were stained by Shorr. Apoptotic indices from 300 microscopical fields (at a higher magnification - 1000x) in the same slide were registered, using an image digital analyzer. Morphometry was performed by computer using specific software5 5 Kontron KS 300 V 2.0. Zeiss, Germany. . Ten sample means of 5, 10, 15, 20, 25, 30, 35, 40, 45 and 50 random fields were set as experimental units and had their descriptive statistics calculated. Standard deviation (SD) for each sample size was plotted against its size to visualize SD trend. SD values decreased as the number of microscopic fields in the sample increased. When the oscillations of the SDs between consecutive samples were smaller than 5%, a reliable cut off was established, since the number of fields in that sample was considered representative enough to refuse a larger sampling. Such stabilization of SDs occurred at 35 fields per slide in this study. Moreover, the total number of cells and the number of apoptotic cells were counted and apoptotic indices (number of apoptotic cells/ total number of cells) were calculated. Data obtained were submitted to analysis of variance.
The areas of the parenchyma and stroma, the percentage of parenchyma (lobe/thymic area) and the thymic stroma (stroma/thymic area) were submitted to analysis of variance.
RESULTS
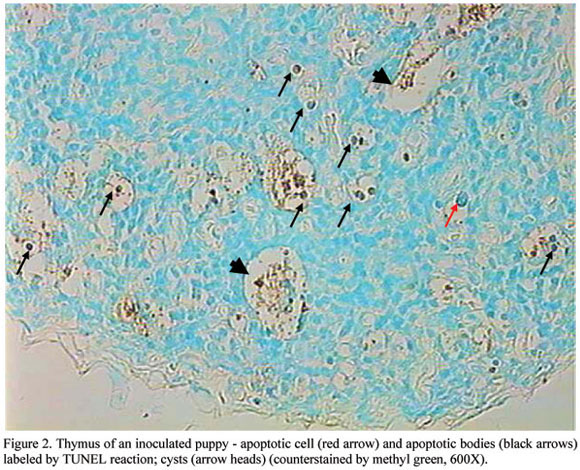
Thymus hypotrophy was evident in all inoculated puppies at the 10th day after inoculation. Thymus was reduced 5 to 6 times in volume and was reduced to remnants in some puppies. Under light microscopy, there was an absence of corticomedullary delimitation and a notorious reduction of cell population (Fig. 1B). Apoptosis was so severe that, in some cases, resulted in cysts formation (Fig. 1C; Fig. 2). Syncytia were also present (Fig. 1D). All lymph node sections were positive for CDV nucleoprotein.
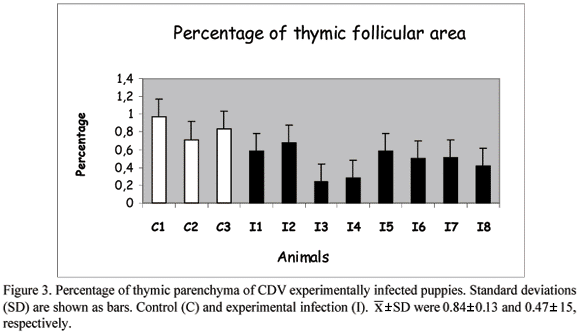
Analysis of variance showed significant difference (P<0.05) between control and inoculated groups ( ±SD) considering: [i] percentage of parenchyma (Fig. 3 - controls = 0.84±0.13; infected = 0.47±0.15) and stroma areas (Fig. 4 - controls = 0.16 %±0.13; infected = 0.52%+0.16); [ii] total number of cells (controls = 231.76± 19.1; infected = 159.18±27.43); [iii] number of apoptotic cells (controls = 18.95±3.91; infected = 44.50± 12.94) and [iv] apoptotic index (Fig. 5 - controls = 0.08±0.03; infected = 0.28±0.07).
±SD) considering: [i] percentage of parenchyma (Fig. 3 - controls = 0.84±0.13; infected = 0.47±0.15) and stroma areas (Fig. 4 - controls = 0.16 %±0.13; infected = 0.52%+0.16); [ii] total number of cells (controls = 231.76± 19.1; infected = 159.18±27.43); [iii] number of apoptotic cells (controls = 18.95±3.91; infected = 44.50± 12.94) and [iv] apoptotic index (Fig. 5 - controls = 0.08±0.03; infected = 0.28±0.07).
DISCUSSION
Thymic hypotrophy was observed, in different degrees, in all inoculated animals. This lesion is already described as characteristic for canine distemper disease by several authors (Appel, 1969; Appel, 1987; Krakowka et al., 1980). Apoptotic cells, shrunken and surrounded by a halo with condensed and fragmented nuclei, were observed either isolated or in groups in the same manner as observed before in lymph nodes (Moro et al., 2003b). Cell death developed in the absence of inflammation, which is characteristic of apoptosis (Gerschenson and Rotello, 1992; Leist and Nicotera, 1998). Morphometric results confirmed hypotrophy and showed that inoculated dogs thymuses had more stroma and less parenchyma than those of control animals. At the same time, thymuses of the inoculated dogs had less cells/field, more apoptotic cells/field and higher apoptotic index/field than control not inoculated puppies. It means that, more cells were dying through apoptosis and consequently hypotrophy was developed greatly due to this kind of cell death. Apoptosis was also observed in retropharyngeal lymph nodes as the predominant way of cell death in dogs with distemper (Moro et al., 2003b). In all normal dogs the necrosis was detected in the thymuses at the 10th day post inoculation. Several authors have reported the involvement of apoptosis in immunosuppressive diseases such as AIDS (Ameisen and Capron, 1991) and measles (Ito et al., 1997) in humans, Newcastle (Lam and Vasconcelos, 1994; Lam et al., 1995) and Gumboro (Vasconcelos and Lam, 1995) diseases in chickens and feline immunodeficiency virus infection in cats (Sarli et al., 1998). Moro et al. (2003b) showed that apoptosis was the main cause of cell death in retropharyngeal lymph nodes in conventional dogs with distemper. Additionally, there is an increment of apoptosis of peripheral blood leukocytes (Moro et al., 2003a;b).
It can be concluded that apoptosis plays a role in the mechanism of thymus hypotrophy that develops in canine distemper. Additionally, apoptosis of thymocytes might contribute greatly with the mechanism of immunosuppression that develops in this disease.
ACKNOWLEDGEMENTS
To Dr. Max J. G. Appel, for donation of the Snyder Hill strain of CDV; Dr. Alex Wandeler, for providing monoclonal antibodies; Dr. Steven Krakowka, Dr. Mike Oglesbee, Candy Glendening and Susan Ringler (Ohio State University, USA) for their assistance; Dr. Ivan B. M. Sampaio, for his helpful assistance with statistic analysis; Centro de Controle de Zoonoses - Belo Horizonte, MG, Brazil, for providing animals.
Recebido em 11 de fevereiro de 2005
Aceito em 3 de janeiro de 2006
E-mail: moroicb.ufmg.br
- AMEISEN, J.C.; CAPRON, A. Cell dysfunction and depletion in AIDS: the programmed cell death cell hypothesis. Immunol. Today, v.12, p.102-105, 1991.
- APPEL, M.J.G. (Ed.). Virus infections of vertebrates; virus infections of carnivores. In: __. Virus infections of vertebrates Amsterdam: Elsevier Science, 1987. v.1, p.133-159.
- APPEL, M.J.G. Pathogenesis of canine distemper. Am. J. Vet. Res., v.30, p.1167-1182, 1969.
- APPEL, M.J.G.; SUMMERS, B.A. Pathogenicity of morbilliviruses for terrestrial carnivores. Vet. Microbiol., v.44, p.187-191, 1995.
- GERSCHENSON, L.E.; ROTELLO, R.J. Apoptosis: a different type of cell death. Faseb J., v.6, p.2450-2455, 1992.
- GREENE, C.E.; APPLE, M.J. Canine distemper. In: GREENE, G.E. Infectious diseases of the dog and cat. Philadelphia: W.B. Saunders, 1990. p.226-241.
- GUO, A.; LU, C.P. Canine distemper virus causes apoptosis of Vero cells. J. Vet. Med .ser. B, v.47, p.183-190, 2000.
- ITO, M.; WATANABE, M.; IHARA, T. et al. Measles virus induces apoptotic cell death in lymphocytes activated with phorbol 12-myristate 13-acetate (PMA) plus calcium ionophore. Clin. Exp. Immunol, v.108, p.266-271, 1997.
- IWATSUKI, K.; OKITA, M.; OCHIKUBO, F. et al. Immunohistochemical analysis of the lymphoid organs of dogs naturally infected with canine distemper virus. J. Comp. Pathol., v.113, p.185-190, 1995.
- KRAKOWKA, S.; AXTHELM, M.K.; JOHNSON, G.C. Canine distemper virus. In: OLSEN, R.G.; KRAKOWKA, S.; BLAKESLEE, J.R. Comparative pathobiology of viral diseases Boca Raton: CRC, 1985. v.2, cap.8, p.137-164.
- KRAKOWKA, S.; COCKERELL, G.; KOESTNER, A. Effects of canine distemper virus infection on lymphoid function in vitro and in vivo Infect. Immunol., v.11, p.1069-1078, 1975.
- KRAKOWKA, S.; HIGGINS, R.J.; KOESTNER, A. Canine distemper virus: review of structural and functional modulations in lymphoid tissues. Am. J. Vet. Res., v.41, p.284-292, 1980.
- KRAKOWKA, S.; RINGLER, S.S.; LEWIS, M. et al. Immunossupression by canine distemper virus: modulation of in vitro immunoglobulin synthesis, interleukin release and prostaglandin E 2 production. Vet. Immunol. Immunopathol., v.15, p.181-201, 1987.
- LAM, K.M.; VASCONCELOS, A.C. Newcastle disease virus-induced apoptosis in chicken peripheral blood lymphocytes. Vet. Immunol. Immunopathol., v.44, p.45-56, 1994.
- LAM, K.M.; VASCONCELOS, A.C.; BICKFORD, A.A. Apoptosis as a cause of death in chicken embryos inoculated with Newcastle disease virus. Microbiol. Pathog., v.19, p.169-174, 1995.
- LEIST, M.; NICOTERA, P. Apoptosis, excitotoxicity, and neuropathology. Exp. Cell Res, v.239, 1998.
- McCULLOUGH, B.; KRAKOWKA, S.; KOESTNER, A. Experimental canine distemper virus-induced lymphoid depletion. Am. J. Pathol., v.74, p.155-170, 1974.
- MORO, L.; ALVES, C.M.; SANTOS, F.G.A. et al. Ocorrência de apoptose em leucócitos no esfregaço de sangue periférico e em sincícios na infecção in vivo pelo vírus da cinomose canina. Arq. Bras. Med. Vet. Zootec., v.55, p.110-112, 2003a.
- MORO L.; MARTINS, A.S.; ALVES, C.M. et al. Apoptosis in canine distemper. Arch. Virol., v.148, p.153-164, 2003b.
- MORO, L.; VASCONCELOS, A.C.; SANTOS, F.G.A. et al. Determination of the minimal representative number of microscopic fields to quantify apoptosis in canine lymph nodes. Arq. Bras. Med. Vet. Zootec., v.56, p.408-410, 2004.
- ORLOWSKI, R.Z. The role of ubiquitin-proteasome pathway in apoptosis. Cell Death Differ., v.6, p.303-313, 1999.
- SAMPAIO, I.B.M. Estatística aplicada à experimentação animal. Belo Horizonte: FEPMVZ, 1998. p.7-13.
- SARLI, G.; DELLA SALDA, L.; ZACCARO, L. et al. Apoptotic fraction in lymphoid tissue of FIV-infected SPF cats. Vet. Immunol. Immunopathol., v.64, p.33-34, 1998.
- SUMMERS, B.A.; GREISEN, H.A.; APPEL, M.J.G. Canine distemper encephalomyelitis: variation with virus strain. J. Comp. Pathol., v.94, p.65-75, 1984.
- TIZARD, I.R. Veterinary immunology. 6.ed. Philadelphia:Saunders, 2000.
- VASCONCELOS, A.C.; LAM, K.M. Apoptosis in chicken embryos induced by the infectious bursal disease virus. J. Comp. Pathol., v.112, p.327-338, 1995.
- VERHAGEN, A.M.; VOUX, D.L. Molecular mechanisms of apoptosis. Res. Probl. Cell Differ., v.23, p.10-24, 1999.
- WYLLIE, A.H.; KERR, J.F.R.; CURRIE, A.R. Cell death: the significance of apoptosis. Int. Rev. Cytol., v.68, p.251-305, 1980.
Publication Dates
-
Publication in this collection
06 Oct 2006 -
Date of issue
Aug 2006
History
-
Accepted
03 Jan 2006 -
Received
11 Feb 2005