ABSTRACT
The aim of this study was to characterize the tissue reactions triggered by the polypropylene mesh coated with chitosan and polyethylene glycol film, and if it’s able to prevent the formation of peritoneal adhesions. Defects in the abdominal wall of rats were induced and polypropylene meshes coated with chitosan/polyethylene glycol (CPEG group, n= 12) and uncoated (PP control group, n= 12) were implanted. On the fourth and forty-fifth postoperative day the formation of adhesion and the tissue reaction to the biomaterial was evaluated through histological and histochemical analysis. The area (P= 0.01) and severity (P= 0.002) of the adhesion was significatively less in the CPEG group. On the fourth day the foreign body reaction was less intense in CPEG group (P= 0.018) and the production of collagen fibers was more intense in this group (P= 0.041). The tissue reactions caused by the biomaterials were similar on the 45th day, with the exception of the high organization of collagen fibers in the CPEG group. The CPEG meshes did not fully prevent the formation of adhesions, but minimized the severity of the process. The foreign body reaction promoted by polypropylene meshes coated with CPEG is less intense than that triggered by uncoated polypropylene meshes.
Keywords:
foreign-body reaction; hernia; peritoneal adhesions; prosthesis; surgical mesh
RESUMO
O objetivo deste estudo foi caracterizar as reações tissulares desencadeadas pela tela de polipropileno revestida com o filme de quitosana e polietilenoglicol e verificar se ela é capaz de prevenir a formação de aderências peritoneais. Um defeito na parede abdominal dos ratos foi realizado, e as telas de polipropileno revestidas com quitosana/polietilenoglicol (grupo CPEG, n= 12) e sem revestimento (grupo controle PP, n= 12) foram implantadas. No quarto e no 45º dia pós-operatório, avaliou-se a formação de aderências e a reação tecidual ao biomaterial por análise histológica e histoquímica. A área (P= 0,01) e a severidade (P= 0,002) da aderência peritoneal foram significativamente menores no grupo CPEG no 45º dia. No quarto dia, observou-se que a reação do corpo estranho foi menor no grupo CPEG (P= 0,018), e a produção de fibras de colágeno mais intensa (P= 0,041). As reações tissulares causadas pelos biomateriais implantados foram semelhantes no 45º dia, com exceção da melhor organização das fibras colágenas no grupo CPEG. As telas CPEG não impediram completamente a formação de aderências, porém minimizaram a gravidade do processo. A reação de corpo estranho promovida por telas de polipropileno revestidas com CPEG é menos intensa do que a desencadeada por telas de polipropileno não revestidas.
Palavras-chave:
aderências peritoneais; prótese; reação de corpo estranho; tela cirúrgica
INTRODUCTION
Polypropylene meshes are among the most used prosthetic materials for the correction of abdominal wall defects (Ansaloni et al., 2009ANSALONI, L.; CATENA, F.; COCCOLINI, F. et al. Peritoneal adhesions to prosthetic materials: an experimental comparative study of treated and untreated polypropylene meshes placed in the abdominal cavity. J. Laparoendosc. Adv. Surg. Tech., v.19, p.369-374, 2009.). This biomaterial has the ability to maintain the tensile strength of the abdominal wall, having also excellent properties regarding tissue integration and handling, and low cost (Vaz et al., 2009VAZ, M.; KREBS, R.K.; TRINDADE, E.N.; TRINDADE, M.R.M. Fibroplasia after polypropylene mesh implantation for abdominal wall hernia repair in rats. Acta Cir. Bras., v.24, p.19-25, 2009.). However, its major drawback is its high adhering potential (Ansaloni et al., 2009; kist et al., 2012).
Adhesions are fibrous connections formed between the surfaces of organs and wall cavities, or both (Hammoud et al., 2004HAMMOUD, A.; GAGO, L.A.; DIAMOND, M.P. Adhesions in patients with chronic pelvic pain: a role for adhesiolysis? Fertil. Steril., v.82, p.1483-1491, 2004.), occuring as a result of a sequence of events initiated by an inflammatory reaction usually after a surgical trauma. Often adhesions increase vascular permeability, cell migration and deposition of fibrin. Normally the peritoneal fibrinolytic activity quickly removes the deposited fibrin. However, when ischemia or severe tissue damage occur, as commonly observed in surgeries for the correction of defects of the abdominal wall, physiological fibrinolysis may be insufficient. In this case, bridges of fibrin and connections between the lesions and normal tissues nearby are formed, creating an ideal framework for healing and fibrotic processes initiated by the migration of fibroblasts and neovascularization under the influence of growth factors, which results in the formation of fibrous adhesions (Rajab et al., 2010RAJAB, T.R.; CHIR, B.; WALLWIENER, M. et al. A direct comparison of seprafilm, adept, intercoat, and spraygel for adhesion prophylaxis. J. Surg. Res., v.161, p.246-249, 2010.).
In horses, adhesions are frequent after laparotomies and may be asymptomatic or cause severe complications, such as colic and intestinal obstruction. Surgical trauma is the main factor triggering adherences, with an incidence of 32.3 %, of which 84.4 % lead to intestinal obstruction in horses (Caldwell e Muller, 2010CALDWELL, F.J.; MUELLER, E. Fibrinolytic responses of the equine peritoneum to abdominal surgery, surgical trauma, and intraperitoneal sodium hyaluronate. J. Equine Vet. Sci., v.30, p.298-304, 2010.).
Strategies for reducting adhesion formation include improvement of surgical techniques, administration of drugs to minimize the inflammatory response, coating with fibrin and the use of physical barriers of low adhesiogenic potential. These techniques present some degree of success in the prevention of adhesions (Ergul e Korukluoglu, 2008ERGUL, E.; KORUKLUOGLU, B. Peritoneal adhesions: facing the enemy. Int. J. Surg., v.6, p.253-260, 2008.). In theory, materials that avoid direct contact between the injured serous surfaces in the first days after the peritoneal injury should protect the healing region, forming a physical barrier that would prevent the development of adhesions (Schnüriger et al., 2011SCHNÜRIGER, B.; BARMPARAS, G.; BRANCO, B.C. et al. Prevention of postoperative peritoneal adhesions: a review of the literature. Am. J. Surg., v.201, p.111-121, 2011.).
Chitosan is a compound obtained by the deacetylation of chitin from the exoskeleton of crustaceans and insects, and also from fungi, among other sources (Honarkar e Barikani, 2009HONARKAR, H.; BARIKANI, M. Applications of biopolymers I: chitosan. Monatsh. Chem., v.140, p.1403-1420, 2009.; Croisier e Jérôme, 2013CROISIER, F.; JÉRÔME, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J., v.49, p.780-792, 2013.). This polymer may be derivatized chemically, resulting in products with different structures, reactivities and mechanical properties, enabling the development of compounds with new characteristics, functions and applications in various areas, particularly in the biomedical field (Pillai et al., 2009PILLAI, C.K.S.; PAUL, W.; SHARMA, C.P. Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog. Polym. Sci., v.34, p.641-678, 2009.). Because it is a semi-natural nontoxic biodegradable polymer, this compound has been used safely in the form of particles, membranes and hydrogels (Honarkar e Barikani, 2009). Other studies focusing the use of chitosan derivatives aiming at the prevention of peritoneal adhesions are described in the literature (Paulo et al., 2009PAULO, N.P.; SILVA, M.S.B.; MORAES, A.M. et al. Use of chitosan membrane associated with polypropylene mesh to prevent peritoneal adhesion in rats. J. Biomed. Mater. Res. B Appl. Biomater., v.91, p.221-227, 2009.; Wei et al., 2009WEI, C.Z.; HOU, C.L; GU, Q.S. et al. A thermosensitive chitosan-based hydrogel barrier for post-operative adhesions’ prevention. Biomaterials, v.30, p.5534-5540, 2009.).
Another polymer able to minimize the development of peritoneal adhesions is polyethylene glycol (PEG) (Dunn et al., 2001DUNN, R.; LYMAN, M.D.; EDELMAN, P.G.; CAMPBELL, P.K. Evaluation of the SprayGel™ adhesion barrier in the rat cecum abrasion and rabbit uterine horn adhesion models. Fertil. Steril., v.75, p.411-416, 2001.; Ten Broek et al., 2012). It having low molecular weight, this compound may be found in the form of a viscous liquid, with water content above 90%. High molecular weight PEGs, on the other hand, have the aspect of waxes. This synthetic compound does not favor the growth of bacterial cultures and may act as a physical barrier, promoting the isolation of tissue injury to adjacent tissue. It remains in the body during the critical period of development of adhesions (from five to seven days), and, afterwards, it is hydrolyzed and absorbed (Dunn et al., 2001).
The aim of this research was to determine whether polypropylene meshes coated with chitosan films associated with polyethylene glycol are effective in preventing peritoneal adhesions in rats with surgically promoted abdominal defects, characterizing the tissue reactions 4 and 45 days after implantation.
MATERIALS AND METHODS
This study was approved by the Ethics Committee on Animal Research of the Federal University of Goiás (protocol number 165/2010). A total of 24 Wistar rats at four months of age were divided into two groups (n= 12 each), according to the biomaterial to be implanted: CPEG group (polypropylene mesh coated with chitosan and polyethylene glycol) and PP group (uncoated polypropylene mesh), the positive control group for adhesion formation. Each group was divided into two subgroups (n= 6) which were submitted to macroscopic, histological and histochemical evaluation at days 4 and 45.
Polypropylene meshes (Intracorp®) with 163g/m² were cut into 5cm x 5cm samples which were then coated on one side with a film of chitosan and polyethylene glycol according to Rammazzina (2011RAMMAZZINA, W.A. Recobrimento de tela de polipropileno com quitosana e polietileno glicol por deposição via electrospinning. 2011. 121f. Dissertação (Mestrado em Desenvolvimento de Processos Biotecnológicos) - Faculdade de Engenharia Química, Universidade de Campinas, Campinas, SP.).
The polypropylene meshes, coated and not with chitosan and polyethylene glycol, were analyzed regarding their visual aspect, their surface and cross section morphology by scanning electron microscopy (SEM), Leo 440i, thickness (through measurements with a Digimess micrometer) and behavior with respect to swelling and stability (in terms of weight loss) in the presence of simulated body fluid (SBF) according to Rammazzina (2011RAMMAZZINA, W.A. Recobrimento de tela de polipropileno com quitosana e polietileno glicol por deposição via electrospinning. 2011. 121f. Dissertação (Mestrado em Desenvolvimento de Processos Biotecnológicos) - Faculdade de Engenharia Química, Universidade de Campinas, Campinas, SP.). The meshes were cut in 20mm x 16mm samples and hydrated in Ringer’s lactate solution for 15 minutes before surgery.
The animals received 2.5mg/kg of xylazine and 5.0mg/kg of morphine sulfate administered intramuscularly. The ventral region of the abdomen was shaved and submited to antisepsis. Isoflurane was administered in a diluent flow of O2 at 0.5 L/min for anesthetic induction and maintenance without gases rebreathing using masks for rodents.
A median retroumbilical skin incision was performed, followed by the divulsion of the subcutaneous tissue on the right side. Later, an excision on aponeurosis block, muscles and peritoneum of around 1cm was performed in the region of the aponeurosis of the external fascia of the rectus and external oblique abdominal. For the reconstruction of the abdominal wall, the biomaterial randomly chosen of each animal was inserted into the intraperitoneal space and attached using four equidistant points (simple separate pattern) with 4-0 polypropylene suture; skin closure was also perfomed with the same surgical wire with simple continuous pattern.
Postoperatively, the animals received 5mg/kg of morphine sulfate subcutaneously every 8h for analgesia during 72h. Euthanasia was performed at four and 45 days. After the euthanasia, skin folding of the abdominal wall was performed, followed by the incision of the abdominal muscles in "U" shape. The implant site of each animal was photographed with a digital camera (DSC-W130, Sony) in a static position of 15cm and adherence formation was measured in percentage in relation to mesh area with the software Image J version 1.36b (National Institutes of Health, USA). The scores used to determine the type of adhesion are described in: 0, absence of adherence; 1, adherence of omentum in the suture zone; 2, omental adherence to the material surface below 50%; 3, omental adherence to the material surface above 50%; 4, visceral adherence on the suture zone; 5, visceral adherence on the material surface.
For histopathological and histochemical evaluations, a fragment of the tissue formed in the implantation site encompassing the abdominal wall, mesh and adherence was collected from each animal and fixed in 10% buffered formalin for 24 hours, followed by the routine processing for coloration with hematoxylin and eosin (HE) and picrosirius red. The samples stained with HE and picrosirius red were analyzed with an optical microscope and an optical microscope with polarized light, respectively, with 40x magnification, by a single evaluator. Two regions of each sample were analyzed: the region near the monofilament of the mesh and the interface region between the mesh and the abdominal wall, with five fields randomly chosen of each region to represent the results.
The indicators of inflammation and repair process including polimorphonuclear cells, mononuclear cells, fibrin and angiogenesis were classified qualitatively in: 0, absent; 1, slight, present at less than 25% of the field; 2 moderate, present in 26% to 50% of the field; 3, severe, present in 51% to 100% of the field. The presence of giant cells were classified in: 0, absent; 1, slight, presence of 1 to 2 surrounding the implant; 2, moderate, presence of 3 to 4 surrounding the implant; 3, severe, presence of more than 4 surrounding the implant. The images of 10 fields of each slide were recorded photographically at random during the analysis the samples with a polarized light microscope, in regions similar to the ones evaluated using the HE stain, for quantitative assessment of the area occupied by collagen fibers through the Image J software.
For the statistical analysis of the quantitative variables ANOVA test was used. For qualitative variables, the nonparametric Mann-Whitney test was used. The XLSTAT program (version 2011.1.01) was used for all statistical analysis, with P< 0.05.
RESULTS
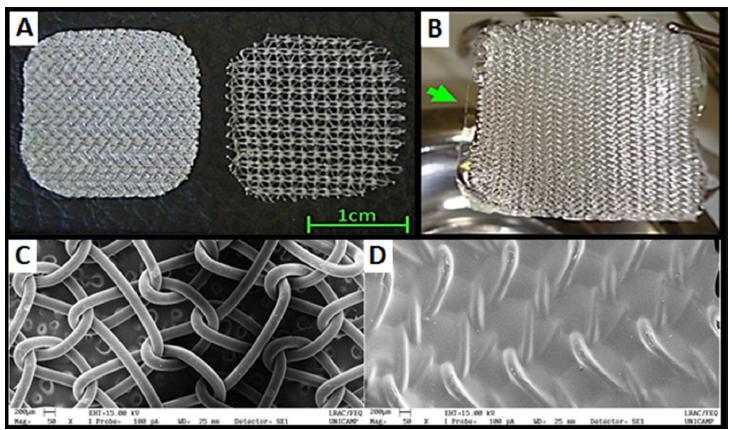
The coating of the PP meshes with the film consisting of CPEG appeared to be homogeneous and free of macroscopic irregularities. After the drying process, the coated mesh had a tendency to wind, showing increased stiffness. The hydration of the CPEG in lactate ringer’s solution before surgery restored the flat and malleable aspect of the mesh (Figure 1A and 1B). The analysis by SEM of uncoated and coated meshes showed that the coating consists of a dense layer of material deposited throughout the polypropylene mesh, covering its entire surface (Figure 1C and 1D). The thickeness, their capacity to absorb simulated body fluid and their stability of the biomaterials in it for seven days are shown in Table 1.
Typical aspect of the biomaterials: A, CPEG coated mesh before hydration (right), uncoated polypropylene mesh (left); B, the film of chitosan/polyethylene glycol obtained by hydration with ringer-lactate solution in the CPEG coated mesh (arrow); morphology of the PP (C) meshe and CPEG mesh (D) analyzed by scanning electron microscopy in 50x magnifation.
Thickness, SBF absorption capacity after 24 h in 37°C and stability regarding mass loss of coated and uncoated meshes after 7 days of exposure to the same solution
The macroscopic analysis showed differences in the healing process at the site of the induced defect in the abdominal wall between the groups after four days of implantation. The surgical wounds of the abdominal wall of animals in the CPEG group showed closure of the abdominal defect by granulation tissue in all animals (Figure 2).
Macroscopic appearance of abdominal wounds in Wistar rats four days after surgery: A, abdominal wounds with formation of granulation tissue, CPEG group (arrow); B, abdominal wounds with defect still quite evident, PP group (arrow).
All animals developed peritoneal adhesions, which varied in intensity according to the biomaterial employed. In the CPEG group, the average adhesion area on the biomaterial was significantly smaller than PP group in both evaluation periods, as well as the severity of adhesions (Table 2).
Peritoneal adhesion score and measured area of the implanted biomaterial involved by peritoneal adhesions in wistar rats in the fourth and the 45th postoperative day
There were no diference regarding histological finds, except for the presence of polimornuclear cells and fibrin that was moderate in CPEG group (P= 0,02 and P= 0,025 respectively), the presence of the mononuclear cells that was moderate to intense in PP group at the fourth day (P= 0,018) and the angiogenesis was slight in this period. After 45 days of the implantation of CPEG and PP meshes it was possible to observe neoperitonium on the surface of the meshes in adhesion-free areas without the presence of fibrin. The variables polymorphonuclear and mononuclear cells, giant cells, and angiogenesis was tipicaly slight at 45 day postoperative.
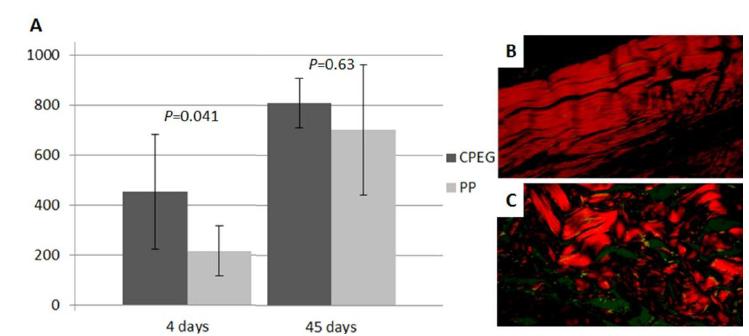
The amount of total collagen fibers was higher in the CPEG group on the fourth day post-implant than in the PP group (P= 0.041). The amount of total collagen fibers represented by the area measured 45 days after the surgery showed no difference between groups (Figure 3A). However, the collagen fibers were more organized in the CPEG group (Figure 3B and 3C).
Area occupied by collagen fibers in megapixels (MP) (Y axis) at the site of implantation of CPEG and PP meshs in Wistar rats on the fourth and the 45th postoperative day (X axis) (A). Sample of the CPEG group indicating better targeting of type I collagen fibers in red after 45 days os impalantation (B) and PP group with collagen fibers less organized (C), both in picrosirius stainning, 40x.
DISCUSSION
The coating layer of CPEG mesh would probably reduce in thickness with time due to solubilization of PEG in the abdominal cavity and also to the enzymatic degradation of chitosan by lysozyme. The improved absorption of fluids of physiological characteristics contributes to increasing the biomaterial thickness, but also enhances its flexibility, potentially enabling better accommodation of the device in vivo. The in vitro stability of the coating with respect to weight loss during incubation in conditions similar to those of the physiological environment for 7 days is high, indicating that probably within a week after implantation, encompassing, then, the most critical period regarding adhesion formation (Mettler et al., 2004METTLER, L.; AUDEBERT, A.; LEHMANN-WILLENBROCK, E. et al. A randomized, prospective, controlled, multicenter clinical trial of sprayable, site-specific adhesion barrier system in patients undergoing myomectomy. Fertil. Steril., v.82, p.398-404, 2004.; Rajab et al., 2010RAJAB, T.R.; CHIR, B.; WALLWIENER, M. et al. A direct comparison of seprafilm, adept, intercoat, and spraygel for adhesion prophylaxis. J. Surg. Res., v.161, p.246-249, 2010.), appropriate integrity of the chitosan-PEG coating would still be observed.
The coating of PP meshes with CPEG films performed in the present work resulted in devices similar to composite meshes, which have one side composed of a material preferably macroporous that faces the abdominal wall to maintain its strength, and the other side composed of a microporous or laminated material which should face the abdominal viscera to prevent the formation of adhesions (Araújo et al., 2010ARAÚJO, U.R.M.F.; CZECZKO, N.G.; DEALLARMI, A. et al. The choice of the mesh to use in the intraperitoneal position in the surgical repair of abdominal wall defects. Arq. Bras. Cir. Dig., v.23, p.118-121, 2010.). In the present study, the laminar side of the biomaterial was equivalent to the chitosan/polyethylene glycol film, and the macroporous side to the polypropylene mesh itself. The material attained had adequate characteristics regarding aspect, morphology, thickness, body fluid absorption and stability in body conditions.
After four days of surgery, the presence of the CPEG film on the surface of the meshes was macroscopically visible in the abdominal wounds, but after 45 days, incorporation and mesotelization of the biomaterial were noticed. This latter finding would not impair adhesion prevention, as the CPEG film was still present during the minimum period necessary to prevent the development of adhesions, which occurs in the first days after implantation (Mettler et al., 2004METTLER, L.; AUDEBERT, A.; LEHMANN-WILLENBROCK, E. et al. A randomized, prospective, controlled, multicenter clinical trial of sprayable, site-specific adhesion barrier system in patients undergoing myomectomy. Fertil. Steril., v.82, p.398-404, 2004.; Rajab et al., 2010RAJAB, T.R.; CHIR, B.; WALLWIENER, M. et al. A direct comparison of seprafilm, adept, intercoat, and spraygel for adhesion prophylaxis. J. Surg. Res., v.161, p.246-249, 2010.).
The proliferative phase of wound repair is macroscopically characterized by the presence of granulation tissue and wound contraction, prevailing between the 7th and 14th day of healing (Sahota et al., 2004SAHOTA, P.S.; BURN, J.L.; BROWN, N.J.; MACNEIL, S. Approaches to improve angiogenesis in tissue-engineered skin. Wound Repair Regen., v.12, p.635-642, 2004. ). However, in the present work, these findings were observed in the CPEG group four days after the surgical procedure. This early presence of apparent granulation tissue may be attributed to the healing properties of chitosan, mainly to stimulation of fibroblast proliferation (Alekseeva et al., 2010ALEKSEEVA, T.P.; RAKHMETOVA, A.A.; BOGOSLOVSKAYA, O.A. et al. Wound healing potential of chitosan and N-sulfosuccinoyl chitosan derivatives. Izv. Akad. Nauk. Ser. Biol., v.37, p.339-345, 2010.).
The mechanisms proposed to minimize adhesion formation by the use of chitosan is it activity as a physical barrier between the peritoneal injury and abdominal structures, and its healing properties (Wei et al., 2009WEI, C.Z.; HOU, C.L; GU, Q.S. et al. A thermosensitive chitosan-based hydrogel barrier for post-operative adhesions’ prevention. Biomaterials, v.30, p.5534-5540, 2009.). Polyethylene glycol prevents adhesions due to its capacity to effectively remain in the injury site during the critical period of adhesion formation before being completely absorbed and to associate with high amounts of water (Dunn et al., 2001DUNN, R.; LYMAN, M.D.; EDELMAN, P.G.; CAMPBELL, P.K. Evaluation of the SprayGel™ adhesion barrier in the rat cecum abrasion and rabbit uterine horn adhesion models. Fertil. Steril., v.75, p.411-416, 2001.; Mettler et al., 2004METTLER, L.; AUDEBERT, A.; LEHMANN-WILLENBROCK, E. et al. A randomized, prospective, controlled, multicenter clinical trial of sprayable, site-specific adhesion barrier system in patients undergoing myomectomy. Fertil. Steril., v.82, p.398-404, 2004.; Quinino et al., 2013QUININO, R.M.; ARAÚJO-FILHO, I.; LIMA, F.P. et al. Adhesion prevention in reabsorbable polyethylene glycol hydrogel (Coseal®) coated polypropylene mesh in rabbits. Acta. Cir. Bras., v.28, p.807-814, 2013. ). The water binding property increases lubricity, facilitating the sliding effect between the biomaterial and the abdominal structures, which in turn promotes the isolation of the injury site from adjacent tissues.
The presence of large numbers of polymorphonuclear cells observed in the group CPEG on the fourth day may be attributed to stimulation of migration of this type of cell by chitosan, as described by Ueno et al. (2001UENO, H.; MORI, T.; FUJINAGA, T. Topical formulations and wound healing applications of chitosan. Adv. Drug. Deliv. Rev., v.52, p.105-115, 2001.). Four days after surgery, mononuclear cells were found in lower amounts in the CPEG group in both areas of evaluation, mainly in the region of the mesh monofilaments. At least two hypotheses might explain this finding. First, the CPEG mesh possibly triggered lower foreign body reaction than the PP group due to the presence of chitosan, which, according to Shi et al. (2006SHI, C.; ZHU, Y.; RAN, X. et al. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J. Surg. Res., v.133, p.185-192, 2006.), cause less reaction to the foreign body. Second, chitosan minimized the adhesion of macrophages to the polypropylene surface, similarly to what was described by Zhou et al. (2008ZHOU, J.; LIWSKI, R.S.; ELSON, C.; LEE, T.D.G. Reduction in postsurgical adhesion formation after cardiac surgery in a rabbit model using N,O-carboxymethyl chitosan to block cell adherence. J. Thorac. Cardiovasc. Surg., v.135, p.777-783, 2008.), although the exact mechanism of this property is not clarified.
The presence of giant cells in both groups was predominantly mild. The monofilament of the biomaterials had dimensions too large to be capable of being degraded through the phagocytic activity of isolated macrophages, therefore the presence of giant cells around the monofilament may be potentially attributed to the fusion of macrophages to originate multinuclear cells whose function is to degrade these larger structures. Giant multinucleated cells formed by the fusion of macrophages are able to process particles larger than 10μm by the release of enzymes and these cells are also associated to the release of pro-inflammatory cytokines (Xia e Triffitt, 2006XIA, Z.; TRIFFITT, J.T. A review on macrophage responses to biomaterials. Biomed. Mater., v.1, p.R1-R9, 2006.).
Despite the larger amount of fibrin in the CPEG group, formation of severe adhesions was minimal, what may be credited to the chitosan/polyethylene glycol film role as a physical barrier between the wound site and the abdominal structures, so that the lesion is not directly in contact with the abdominal cavity, as in other barrier systems made of chitosan (Paulo et al., 2009PAULO, N.P.; SILVA, M.S.B.; MORAES, A.M. et al. Use of chitosan membrane associated with polypropylene mesh to prevent peritoneal adhesion in rats. J. Biomed. Mater. Res. B Appl. Biomater., v.91, p.221-227, 2009.; Wei et al., 2009WEI, C.Z.; HOU, C.L; GU, Q.S. et al. A thermosensitive chitosan-based hydrogel barrier for post-operative adhesions’ prevention. Biomaterials, v.30, p.5534-5540, 2009.).
Although it is reported that chitosan stimulates angiogenesis (Ueno et al., 2001UENO, H.; MORI, T.; FUJINAGA, T. Topical formulations and wound healing applications of chitosan. Adv. Drug. Deliv. Rev., v.52, p.105-115, 2001.), in the present work no difference regarding angiogenesis was noticed between the groups analysed at the fourth day. This effect may depend on the properties of the chitosan used, such as the molecular weight and the deacetylation degree, and also on its formulation (Şenel e McClure, 2004ŞENEL, S.; McCLURE, S.J. Potential applications of chitosan in veterinary medicine. Adv. Drug. Deliv. Rev., v.56, p.1467-1480, 2004.).
The amount of collagen fibers in the CPEG group may be due to the healing capacity of chitosan. As described by Ueno et al. (2011), this polymer can stimulate fibroblast proliferation and collagen production. However, the PP group showed a larger amount of fibroblasts compared to CPEG group in the mesh interface zone/abdominal wall and showed lower production of collagen. It can be inferred that chitosan has triggered a greater response only to the production of collagen by fibroblasts in the CPEG group. The best Histoarchitecture of collagen fibers observed in the CPEG group, may be attributed to chitosan's ability to promote tridmensional protein matrix for tissue growth as described by Muzzarelli et al. (1998).
Finally, it is emphasized that the approach of clinical use of polypropylene meshes coated with a film of chitosan combined with polyethylene glycol is significantly more advantageous from the viewpoint of material.
CONCLUSIONS
The polypropylene meshes coated with the film of chitosan and polyethylene glycol is not able to fully prevent the formation of peritoneal adhesions, they significantly minimize the severity of the process. The foreign body reaction promoted by polypropylene meshes coated with CPEG is less intense than that triggered by uncoated polypropylene meshes in the acute phase of inflammation and tissue repair.
ACKNOWLEDGEMENTS
The authors acknowledge the support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), Pró-Reitoria de Pesquisa e Pós-Graduação da Universidade Federal do Pará (PROPESP/UFPA) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). The aid of Ana Kelly Girata with membrane preparation and of Dr. Andréa C. K. Bierhalz with the SEM analysis are also acknowledged.
REFERENCES
- ALEKSEEVA, T.P.; RAKHMETOVA, A.A.; BOGOSLOVSKAYA, O.A. et al. Wound healing potential of chitosan and N-sulfosuccinoyl chitosan derivatives. Izv. Akad. Nauk. Ser. Biol., v.37, p.339-345, 2010.
- ANSALONI, L.; CATENA, F.; COCCOLINI, F. et al. Peritoneal adhesions to prosthetic materials: an experimental comparative study of treated and untreated polypropylene meshes placed in the abdominal cavity. J. Laparoendosc. Adv. Surg. Tech., v.19, p.369-374, 2009.
- ARAÚJO, U.R.M.F.; CZECZKO, N.G.; DEALLARMI, A. et al. The choice of the mesh to use in the intraperitoneal position in the surgical repair of abdominal wall defects. Arq. Bras. Cir. Dig., v.23, p.118-121, 2010.
- CALDWELL, F.J.; MUELLER, E. Fibrinolytic responses of the equine peritoneum to abdominal surgery, surgical trauma, and intraperitoneal sodium hyaluronate. J. Equine Vet. Sci., v.30, p.298-304, 2010.
- CROISIER, F.; JÉRÔME, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J., v.49, p.780-792, 2013.
- DUNN, R.; LYMAN, M.D.; EDELMAN, P.G.; CAMPBELL, P.K. Evaluation of the SprayGel™ adhesion barrier in the rat cecum abrasion and rabbit uterine horn adhesion models. Fertil. Steril., v.75, p.411-416, 2001.
- ERGUL, E.; KORUKLUOGLU, B. Peritoneal adhesions: facing the enemy. Int. J. Surg., v.6, p.253-260, 2008.
- HAMMOUD, A.; GAGO, L.A.; DIAMOND, M.P. Adhesions in patients with chronic pelvic pain: a role for adhesiolysis? Fertil. Steril., v.82, p.1483-1491, 2004.
- HONARKAR, H.; BARIKANI, M. Applications of biopolymers I: chitosan. Monatsh. Chem., v.140, p.1403-1420, 2009.
- KIST, C.; MANNA, B.B.; MONTES, J.H.M. et al. Comparative study of intraperitoneal adhesions associated with the use of meshes of polypropylene and polypropylene coated with omega-3 fatty acid. Rev. Col. Bras. Cir., v.39, p.201-206, 2012.
- METTLER, L.; AUDEBERT, A.; LEHMANN-WILLENBROCK, E. et al. A randomized, prospective, controlled, multicenter clinical trial of sprayable, site-specific adhesion barrier system in patients undergoing myomectomy. Fertil. Steril., v.82, p.398-404, 2004.
- MUZZARELLI, R.; BALDASSARRE, V.; CONTI, F. et al. Biological activity of chitosan: ultrastructural study. Biomaterials, v.9, p.247-252, 1988.
- PAULO, N.P.; SILVA, M.S.B.; MORAES, A.M. et al. Use of chitosan membrane associated with polypropylene mesh to prevent peritoneal adhesion in rats. J. Biomed. Mater. Res. B Appl. Biomater., v.91, p.221-227, 2009.
- PILLAI, C.K.S.; PAUL, W.; SHARMA, C.P. Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog. Polym. Sci., v.34, p.641-678, 2009.
- QUININO, R.M.; ARAÚJO-FILHO, I.; LIMA, F.P. et al. Adhesion prevention in reabsorbable polyethylene glycol hydrogel (Coseal®) coated polypropylene mesh in rabbits. Acta. Cir. Bras., v.28, p.807-814, 2013.
- RAJAB, T.R.; CHIR, B.; WALLWIENER, M. et al. A direct comparison of seprafilm, adept, intercoat, and spraygel for adhesion prophylaxis. J. Surg. Res., v.161, p.246-249, 2010.
- RAMMAZZINA, W.A. Recobrimento de tela de polipropileno com quitosana e polietileno glicol por deposição via electrospinning. 2011. 121f. Dissertação (Mestrado em Desenvolvimento de Processos Biotecnológicos) - Faculdade de Engenharia Química, Universidade de Campinas, Campinas, SP.
- SAHOTA, P.S.; BURN, J.L.; BROWN, N.J.; MACNEIL, S. Approaches to improve angiogenesis in tissue-engineered skin. Wound Repair Regen., v.12, p.635-642, 2004.
- SCHNÜRIGER, B.; BARMPARAS, G.; BRANCO, B.C. et al. Prevention of postoperative peritoneal adhesions: a review of the literature. Am. J. Surg., v.201, p.111-121, 2011.
- ŞENEL, S.; McCLURE, S.J. Potential applications of chitosan in veterinary medicine. Adv. Drug. Deliv. Rev., v.56, p.1467-1480, 2004.
- SHI, C.; ZHU, Y.; RAN, X. et al. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J. Surg. Res., v.133, p.185-192, 2006.
- TEN BROEK, R.P.; KOK-KRANT, N.; VERHOEVE, H.R. et al. Efficacy of polyethylene glycol adhesion barrier after gynecological laparoscopic surgery: results of a randomized controlled pilot study. Gynecol. Surg., v.9, p.29-35, 2012.
- UENO, H.; MORI, T.; FUJINAGA, T. Topical formulations and wound healing applications of chitosan. Adv. Drug. Deliv. Rev., v.52, p.105-115, 2001.
- VAZ, M.; KREBS, R.K.; TRINDADE, E.N.; TRINDADE, M.R.M. Fibroplasia after polypropylene mesh implantation for abdominal wall hernia repair in rats. Acta Cir. Bras., v.24, p.19-25, 2009.
- WEI, C.Z.; HOU, C.L; GU, Q.S. et al. A thermosensitive chitosan-based hydrogel barrier for post-operative adhesions’ prevention. Biomaterials, v.30, p.5534-5540, 2009.
- XIA, Z.; TRIFFITT, J.T. A review on macrophage responses to biomaterials. Biomed. Mater., v.1, p.R1-R9, 2006.
- ZHOU, J.; LIWSKI, R.S.; ELSON, C.; LEE, T.D.G. Reduction in postsurgical adhesion formation after cardiac surgery in a rabbit model using N,O-carboxymethyl chitosan to block cell adherence. J. Thorac. Cardiovasc. Surg., v.135, p.777-783, 2008.
Publication Dates
-
Publication in this collection
10 Oct 2019 -
Date of issue
Jul-Aug 2019
History
-
Received
15 Dec 2016 -
Accepted
15 Aug 2018