ABSTRACT
Widely distributed organisms face different ecological scenarios throughout their range, which can potentially lead to micro-evolutionary differentiation at specific localities. Mating systems of animal pollinated plants are supposed to evolve in response to the availability of local pollinators, with consequent changes in flower morphology. We tested the relationship among pollination , mating system, and flower morphology over a large spatial scale in Brazilian savannas using the tree Curatella americana (Dilleniaceae). We compared fruit set with and without pollinators in the field, and analyzed pollen tube growth from self- and cross-pollinated flowers in different populations. Populations with higher natural fruit set also had lower fruit set in bagged flowers, suggesting stronger barriers to self-fertilization. Furthermore, higher levels of autogamy in field experiments were associated with more pollen tubes reaching ovules in self-pollinated flowers. Morphometric studies of floral and leaf traits indicate closer-set reproductive organs, larger stigmas and smaller anthers in populations with more autogamy. We show that the spatial variation in mating system, flower morphology and pollination previously described for herbs also applies to long-lived, perennial tropical trees, thus reemphasizing that mating systems are a population-based attribute that vary according to the ecological scenario where the plants occur.
Keywords:
Cerrado savanna; mating system; pollination biogeography; reproductive assurance; self-compatibility
Introduction
The interactions established between organisms can vary greatly throughout space and time (Thompson 2005Thompson JN. 2005. The geographic mosaic of coevolution. Chicago, University of Chicago Press.). For instance, plants can interact with contrasting assemblages of pollinators throughout their geographical range (Gómez et al. 2014Gómez JM, Munoz-Pajares AJ, Abdelaziz M, Lorite J, Perfectti F. 2014. Evolution of pollination niches and floral divergence in the generalist plant Erysimum mediohispanicum. Annals of Botany 113: 237-249. ; Herrera 2005Herrera CM. 2005. Plant generalization on pollinators: species property or local phenomenon? American Journal of Botany 92: 13-20. ; Waser et al. 1996Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. 1996. Generalization in pollination systems, and why it matters. Ecology 77: 1043-1060. ). The resulting pollination interactions can vary from specialist to generalist for both plants and animals, and may have strong implications for the evolution of plant mating systems (Gómez 2002Gómez JM. 2002. Generalización en las interacciones entre plantas y polinizadores. Revista Chilena de Historia Natural 75: 105-115.; Dart et al. 2012Dart SR, Samis KE, Austen E, Eckert CG. 2012. Broad geographic covariation between floral traits and the mating system in Camissoniopsis cheiranthifolia (Onagraceae): multiple stable mixed mating systems across the species’ range? Annals of Botany 109: 599-611.; Barrett 2013Barrett SCH. 2013. The evolution of plant reproductive systems: how often are transitions irreversible? Proceedings of the Royal Society B: Biological Sciences 280: 20130913. doi: 10.1098/rspb.2013.0913
https://doi.org/10.1098/rspb.2013.0913...
). Historically, plant mating systems were considered an attribute of the species (Brys & Jacquemyn 2011Brys R, Jacquemyn H. 2011. Variation in the functioning of autonomous self-pollination, pollinator services and floral traits in three Centaurium species. Annals of Botany 107: 917-925. ; Rosas-Guerrero et al. 2014Rosas-Guerrero V, Aguilar R, Martén-Rodríguez S, et al. 2014. A quantitative review of pollination syndromes: do floral traits predict effective pollinators? Ecology Letters 17: 388-400.), even though their lability between populations was pointed out over 40 years ago (Stebbins 1974Stebbins GL. 1974. Flowering plants: evolution above the species level. Cambridge, Harvard University Press.). Therefore, the mating system may vary within a species’ range due to trade-offs between genetic variability, promoted by cross-pollination, and reproductive assurance from self-pollination, which vary in response to different ecological scenarios throughout space and time (Barrett et al. 1989Barrett SCH, Morgan MT, Husband BC. 1989. The dissolution of a complex genetic polymorphism: the evolution of self-fertilization in tristylous Eichhornia paniculata (Pontederiaceae). Evolution 43: 1398-1416. ; Moeller 2006Moeller DA. 2006. Geographic structure of pollinator communities, reproductive assurance, and the evolution of self-pollination. Ecology 87: 1510-1522. ; Opedal et al. 2016Opedal ØH, Albertsen E, Armbruster WS, Pérez-Barrales R, Falahati-Anbaran M, Pélabon C. 2016. Evolutionary consequences of ecological factors: pollinator reliability predicts mating-system traits of a perennial plant. Ecology Letters 19: 1486-1495. ). For example, some herb species with mixed mating systems show different levels of autogamy throughout their ranges (Kalisz & Vogler 2003Kalisz S, Vogler DW. 2003. Benefits of autonomous selfing under unpredictable pollinator environments. Ecology 84: 2928-2942.; Moeller 2006Moeller DA. 2006. Geographic structure of pollinator communities, reproductive assurance, and the evolution of self-pollination. Ecology 87: 1510-1522. ).
To rely on a mixed mating system, and oscillate between the two extremes of selfing and outcrossing as the environment demands, may be a consequence of the reproductive assurance hypothesis proposed by Darwin (1876Darwin C. 1876. The effects of cross and self fertilisation in the vegetable kingdom. London, John Murray.), which was later re-elaborated (Baker & Stebbins 1965Baker HG, Stebbins GL. 1965. The genetics of colonizing species: proceedings of the first international union of biological sciences symposia on general biology. 1st. edn. New York, Academic Press Inc.; Baker 1967Baker HG. 1967. Support for Baker’s law-as a rule. Evolution 21: 853-856.). According to this hypothesis, mechanisms ensuring autogamy should be favoured in areas with low pollinator availability (Lloyd 1992Lloyd DG. 1992. Self- and cross-fertilization in plants. II. The selection of self-fertilization. International Journal of Plant Sciences 153: 370-380.; Herlihy & Eckert 2002Herlihy CR, Eckert CG. 2002. Genetic cost of reproductive assurance in a self-fertilizing plant. Nature 416, 320-323. ). On the other hand, shorter-lived plants experience stronger selection for reproductive assurance (more prone to selfing), as they usually only experience one or few reproduction events early in life (Moeller et al. 2017Moeller DA, Runquist RDB, Moe AM, et al. 2017. Global biogeography of mating system variation in seed plants. Ecology Letters 20: 375-384. ).
Besides spatially structured processes, past climate dynamics and stability have also affected species distribution and diversity patterns (Svenning & Skov 2007Svenning JC, Skov F. 2007. Ice age legacies in the geographical distribution of tree species richness in Europe. Global Ecology and Biogeography 16: 234-245. ; Cárdenas et al. 2011Cárdenas ML, Gosling WD, Sherlock SC, Poole I, Pennington RT, Mothes P. 2011. The response of vegetation on the Andean flank in western Amazonia to pleistocene climate change. Science 331: 1055-1058.; Sandel et al. 2011Sandel B, Arge L, Dalsgaard B, et al. 2011. The influence of Late Quaternary climate-change velocity on species endemism. Science 334: 660-664. ; Kissling et al. 2012Kissling WD, Dormann CF, Groeneveld J, et al. 2012. Towards novel approaches to modelling biotic interactions in multispecies assemblages at large spatial extents. Journal of Biogeography 39: 2163-2178.), population demography and genetic structure (Grazziotin et al. 2006Grazziotin FG, Monzel M, Echeverrigarauy S, Bonato SL. 2006. Phylogeography of the Bothrops jararaca complex (Serpentes: Viperidae): past fragmentation and island colonization in the Brazilian Atlantic Forest. Molecular Ecology 15: 3969-3982.; Cabanne et al. 2007Cabanne GS, Santos FR, Miyaki CY. 2007. Phylogeography of Xiphorhynchus fuscus (Passeriformes, Dendrocolaptidae): vicariance and recent demographic expansion in southern Atlantic forest. Biological Journal of the Linnean Society 91: 73-84.), and the structure of mutualistic plant-pollinator assemblages (Dalsgaard et al. 2011Dalsgaard B, Magård E, Fjeldså J. 2011. Specialization in plant-hummingbird networks is associated with species richness, contemporary precipitation and quaternary climate-change velocity. PLOS ONE 6: e25891. https://doi.org/10.1371/journal.pone.0025891
https://doi.org/10.1371/journal.pone.002...
; 2013Dalsgaard B, Trøjelsgaard K, González AMM. 2013. Historical climate-change influences modularity and nestedness of pollination networks. Ecography 36: 1331-1340.). Therefore, just as the lack of reproductive partners during range expansion or small population size may currently favour individuals with higher levels of autogamy (Ivey & Carr 2012Ivey CT, Carr DE. 2012. Tests for the joint evolution of mating system and drought escape in Mimulus. Annals of Botany 109: 583-598. ; Levin 2012Levin DA. 2012. Mating system shifts on the trailing edge. Annals of Botany 109: 613-620.; Hargreaves & Eckert 2013Hargreaves AL, Eckert CG. 2013. Evolution of dispersal and mating systems along geographic gradients: implications for shifting ranges. Functional Ecology 28: 5-21.; Griffin & Willi 2014Griffin PC, Willi Y. 2014. Evolutionary shifts to self-fertilisation restricted to geographic range margins in North American Arabidopsis lyrata. Ecology Letters 17: 484-490. ), this could have happened over geological time.
Changes in mating systems are usually associated with variation in a group of floral traits (Berg 1960Berg RL. 1960. The ecological significance of correlation pleiades. Evolution 14: 171-180. ; Armbruster et al. 1999Armbruster WS, Stilio VSD, Tuxill JD, Flores TC, Runk JLV. 1999. Covariance and decoupling of floral and vegetative traits in nine Neotropical plants: a re-evaluation of Berg’s correlation-pleiades concept. American Journal of Botany 86: 39-55.). For example, transitions to higher levels of autogamy are usually related to reduction in flower size and herkogamy (the separation between male and female reproductive organs within a flower - Wyatt 1988Wyatt R. 1988. Phylogenetic aspects of the evolution of self-pollination. In: Gottlieb LD, Jain SK. (eds.) Plant evolutionary biology. London, Springer p. 109-131), faster flower development time (Armbruster et al. 2002Armbruster WS, Mulder CPH, Baldwin BG, Kaliz S, Wessa B, Nute H. 2002. Comparative analysis of late floral development and mating-system evolution in tribe Collinsieae (Scrophulariaceae s.l.). American Journal of Botany 89: 37-49. ; Mazer et al. 2004Mazer SJ, Paz H, Bell MD. 2004. Life history, floral development, and mating system in Clarkia xantiana (Onagraceae): do floral and whole-plant rates of development evolve independently? American Journal of Botany 91: 2041-2050. ), and flowering earlier in the season (Mazer et al. 2004Mazer SJ, Paz H, Bell MD. 2004. Life history, floral development, and mating system in Clarkia xantiana (Onagraceae): do floral and whole-plant rates of development evolve independently? American Journal of Botany 91: 2041-2050. ; Martin & Willis 2007Martin NH, Willis JH. 2007. Ecological divergence associated with mating system causes nearly complete reproductive isolation between sympatric Mimulus species. Evolution 61: 68-82). Other flower traits, such as the reduction of rewards and attractiveness in the display (Ornduff 1969Ornduff R. 1969. Reproductive biology in relation to systematics. Taxon 18: 121-133. ; Dudley et al. 2007Dudley LS, Mazer SJ, Galusky P. 2007. The joint evolution of mating system, floral traits and life history in Clarkia (Onagraceae): genetic constraints vs. independent evolution. Journal of Evolutionary Biology 20: 2200-2218. ), are hypothesised to follow the previously mentioned primary modifications. Most research has focused on herb species, which are known to consistently present lower outcrossing rates (Moeller et al. 2017Moeller DA, Runquist RDB, Moe AM, et al. 2017. Global biogeography of mating system variation in seed plants. Ecology Letters 20: 375-384. ). Therefore, the life history (annual, perennial, etc.) and growth form (herb, vine, tree, etc.) are the main predictors of outcrossing rates among flowering plants (Moeller et al. 2017Moeller DA, Runquist RDB, Moe AM, et al. 2017. Global biogeography of mating system variation in seed plants. Ecology Letters 20: 375-384. ). To the best of our knowledge, only one studied has described the geographical variation in the mating system and flower morphology for a non-herbaceous species, in this case a vine (Opedal et al. 2016Opedal ØH, Albertsen E, Armbruster WS, Pérez-Barrales R, Falahati-Anbaran M, Pélabon C. 2016. Evolutionary consequences of ecological factors: pollinator reliability predicts mating-system traits of a perennial plant. Ecology Letters 19: 1486-1495. ).
In a recent and extensive review of mating system variation among populations, the authors described such scenario as a widespread phenomenon weakly related to phylogeny or pollination mode (Whitehead et al. 2018Whitehead MR, Lanfear R, Mitchell RJ, Karron JD. 2018. Plant mating systems often vary widely among populations. Frontiers Ecology and Evolution. 6: 38.). They concluded that ecological studies about the processes generating interpopulational variations in mating systems are still needed. With the aim of clarifying how pollinator availability relates to mating system and flower morphology in perennial plants, we studied populations of a widely distributed Neotropical tree Curatella americana (Dilleniaceae) (Ratter et al. 2003Ratter JA, Bridgewater S, Ribeiro JF. 2003. Analysis of the floristic composition of the Brazilian cerrado vegetation III: comparison of the woody vegetation of 376 areas. Edinburgh Journal of Botany 60: 57-109. ). Focusing on populations in three disjunct regions of savanna in Brazil, we tested the following hypotheses: 1. Effective pollinator availability (mean visitation frequency of pollinators per flower) is positively correlated to natural fruit set and inversely related to autogamy; 2. Smaller populations, or those at the distribution edge, will have higher levels of autogamy; 3. Populations with smaller flowers and less herkogamy will have higher levels of autogamy.
Materials and methods
Study sites
We studied ten populations of the Neotropical savanna tree Curatella americana L. (Fig. 1), regarding pollinators, floral biology and morphology, mating system, and pollen tube growth under controlled pollination. Populations 5-10 (Fig. 1) were in the large continuous area of savanna (Cerrado) located in Central Brazil, hereafter called Southern populations. Populations 1-3 were in the disjunct area of Roraima state, hereafter referred to as Northern populations and population 4 was in a fragment of savanna surrounded by the Amazon forest, hereafter called Santarém. Populations 9 and 10 (Jatai and Caldas Novas; Fig. 1) belong to the Southern region, located at one of the southern edges of the C. americana distribution in South America, but are still connected to the large continuous area of the distribution. These two populations therefore experience different scenarios when compared to the disjunct areas, as they are edge populations with much greater area. Such difference is important to note when considering the findings of this study (see Results).
Map of the original area of Brazilian Cerrado (Neotropical Savanna) and indications of the studied populations. Populations 1, 2 and 3 are included in the region called Northern Cerrado, 4 is in Santarém, and 5-10 are in the Southern Cerrado region.
Floral biology and autogamy were studied in all populations, but morphology for the purpose of morphometric analysis was studied in five populations from all the regions (2, 3, 4, 5 and 6; Fig. 1). The closest populations were 100 km apart and the furthest were more than 2,700 km apart from each other.
Floral biology
Floral biology measurements sensitive to dehydration were carried out in situ or, in the case of herkogamy, from pictures of the flowers in standard position using a fixed holder connected to the camera (Canon EOS Kiss X5). Time, sequence, and duration of anthesis, flower longevity, stigma receptivity, odour emission (presence of osmophores), and pollen availability (time of pollen presentation) were observed and described following Dafni et al. (2005Dafni A, Kevan PG, Husband BC. 2005. Practical pollination biology. Cambrigde, Enviroquest Ltd.). Receptivity was assessed by dropping hydrogen peroxidase onto the stigmas and checking for bubbles. The location of osmophores was visually assessed after immersing flowers in a solution of neutral red for five minutes.
Flowers were collected from five populations (2, 3, 4, 5 and 6; Fig. 1) and fixed in 70 % alcohol for linear or size measurements in the lab. The length of stamens (total and anther) and size of pistil (total length -ovary+style+stigma- and stigma area) were measured using a digital calliper. Measurements of shape of petal, sepal, ovary, and leaf (as a control) were obtained from pictures and analysed using geometric morphometrics (Bookstein 1991Bookstein FL. 1991. Morphometric tools for landmark data: geometry and biology. Cambridge, Cambridge University Press.). Landmarks and semi-landmarks were placed on scanned structures using the software TPS Dig (Rohlf 2010Rohlf FJ. 2010. TpsDig. Stony Brook/ New York, Department of Ecology and Evolution, State University of New York at Stony Brook.). Semi-landmarks were used to define the borders of the structures when a homologous landmark was not available (Fig. S1 in supplementary material). We used three leaves per individual and 10 individuals in each population to analyze leaf morphology. For flower measurements, we sampled one flower per individual and 15 individuals per population. Qualitative variations in flower morphology were observed and noted in the field.
Pollinator importance
To check for fruit set with (open, natural fruit set, or NF hereafter) and without pollinators (autogamy or AU hereafter), we bagged inflorescences (minimum 20 flowers each) from at least 10 individuals per population (AU) and marked and left an equal amount of flowers open to visitation (NF). We used selection of linear models to test the effect of pollinator visitation (measured as the mean number of large-sized bee visits per flower) on both natural pollination and autogamy treatments. Visitation rates were measured during the flowering time, observing one individual tree (or a branch in large flower displays) per day and counting the number of flowers and the frequency of visitation for ten minutes each half hour, throughout the flower’s lifetime. In each population at least five individuals were observed, resulting in a minimum of 20 observation hours per population. Pollinator counting and observations were carried out at the peak of the flowering season in each population, and this period was different in each geographic region. Within the same region, observations were concentrated in periods of about ten days. Afterwards, we moved to the next population, and only returned to the populations to collect fruit after a month. Total number of visits to flowers from each individual plant was divided by the total number of flowers observed, to calculate the mean visitation rate per flower on each day. The population average of visitation rate for the observed flowers was then used to predict natural fruit set (NF) and autogamy (AU) in each area. During the other twenty minutes of observation we recorded visitors’ behaviour on flowers and observed their potential as pollinators by looking for contact between insects and reproductive structures.
To check for possibile self-pollen deposition, we also bagged flowers one day before anthesis and then collected one stigma per flower as soon as the flower opened and another stigma four hours later. Each flower had two equal stigmas, which made the test perfectly paired. The stigmas were placed directly on a slide with stained glycerine jelly, covered with a cover slip, and pollen grains were counted under light microscopy. The difference in the number of pollen grains was tested by a paired t test. To test for possible accidental pollen deposition during manipulation, some stigmas were manipulated and the second stigma was removed 10 minutes later. Since the number of pollen grains on stigmas was not different between these two treatments, we assumed that accidental deposition had a minor effect and would not change the experimental results.
Pollen tubes
Pollen tubes were observed to identify possible mechanisms operating at the pistil level, related to the different pollination treatments in the studied populations. In order to check e pollen tube growth through the style, we collected ten hand-pollinated pistils (five cross and five self-pollinated) from ten individuals using exactly the same protocol in each population. The pistils were fixed in FAA (Formalin-Acetic acid-Alcohol) + ethanol 50 %, 24 hours after hand pollination. The preparation followed the technique proposed by Martin (1959Martin FW. 1959. Staining and observing pollen tubes in the style by means of fluorescence. Stain Technology 34: 125-128. ) and pistils were stained with a solution of aniline blue. Since pistils were covered by trichomes, we pre-treated them in a solution of sodium hypochlorite (10 %) and distilled water to facilitate trichome removal. Even after such treatment it was still very difficult to count individual pollen tubes, therefore, results were assessed qualitatively through visual comparisons of the ten slides from each population under fluorescence microscopy.
Morphometric analysis
We separated the form of all the landmark configurations into shape and size using geometric morphometrics (Bookstein 1991Bookstein FL. 1991. Morphometric tools for landmark data: geometry and biology. Cambridge, Cambridge University Press.). Size was measured as centroid size (CS), the square root of the sum of the squared distances of each landmark from the centroid, or gravity centre, of the landmark configuration. Size variation among populations and regions was tested using mixed models, comparing a set of three models: one with region, another with population, and a null model with only the intercept as a fixed effect. Individual was included as a random effect in all models. Models were compared using AICc (Burnham & Anderson 2002Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. 2nd. edn. New York, Springer.).
To measure shape, all configurations were scaled to unit CS, and superimposed by a generalized least squares (GLS) Procrustes procedure. Given the presence of semi-landmarks, a sliding procedure minimizing the GLS residuals was used, as the exact location of the semi-landmarks along the structure outline was arbitrary. A mean shape was calculated and the differences between its landmarks and the landmarks of each individual were the residuals of the GLS procedure. To test for differences in shape among the regions, we ran a Procrustes Analysis of Variance (ANOVA) using a hierarchically nested design, with replications (repeated measurement of the landmarks from leaves, petals, etc.) nested within individuals, populations, and regions. Differences in shape among individuals, populations, and regions were visualized by doing a Principal Components Analysis on the superimposed shapes. All geometric morphometrics analysis were done using the geomorph package in R (Adams & Otarola-Castillo 2013Adams DC, Otarola-Castillo E. 2013. Geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods in Ecology and Evolution 4: 393-399. ).
For the univariate flower morphology measurements (number of stamens, stigma area, anther size, gynaecium, and androecia length) we used a one-way ANOVA, and when results were significant, the means were further differentiated using a Tukey multiple comparisons test (at p ≤0.05). All analyses were carried out using the R software environment (R Development Core Team 2014R Development Core Team 2014. R: A language and environment for statistical computing. Vienna, R Foundation for Statistical Computing.).
Results
Floral biology
Basic flower biology was observed in the individuals where the experiments were performed in all populations. In total, about 68,000 flowers were manipulated and at least 680,000 were observed. Curatella americana flowered from June to September in the Southern Cerrado (populations 5-10; Fig. 1), from September to October in Santarém (population 4), and from October to November in the Northern Cerrado (populations 1-3). Individual trees mass-flowered, producing all of their flowers within a short period of two weeks. Flowers are pentamerous, actinomorphic and open (plate type), grouped in panicles, white to the human eye, and UV absorbing (Fig. 2). Their odour is sweet and basic staining of the flowers indicated that osmophores are located at the margin of the petals. Flowers open between 04:30h and 06:30h and last for about seven hours. Pollen started to be released between 30 minutes to an hour after flower opening. The stigma was receptive for the entire period flowers were open. In the North, we registered one individual showing functional pistils and only rudiments of anthers without pollen, behaving as a female plant. On the other hand, in Central Brazil (Nova Xavantina) we found two individuals with staminate and hermaphrodite flowers (andromonoecious).
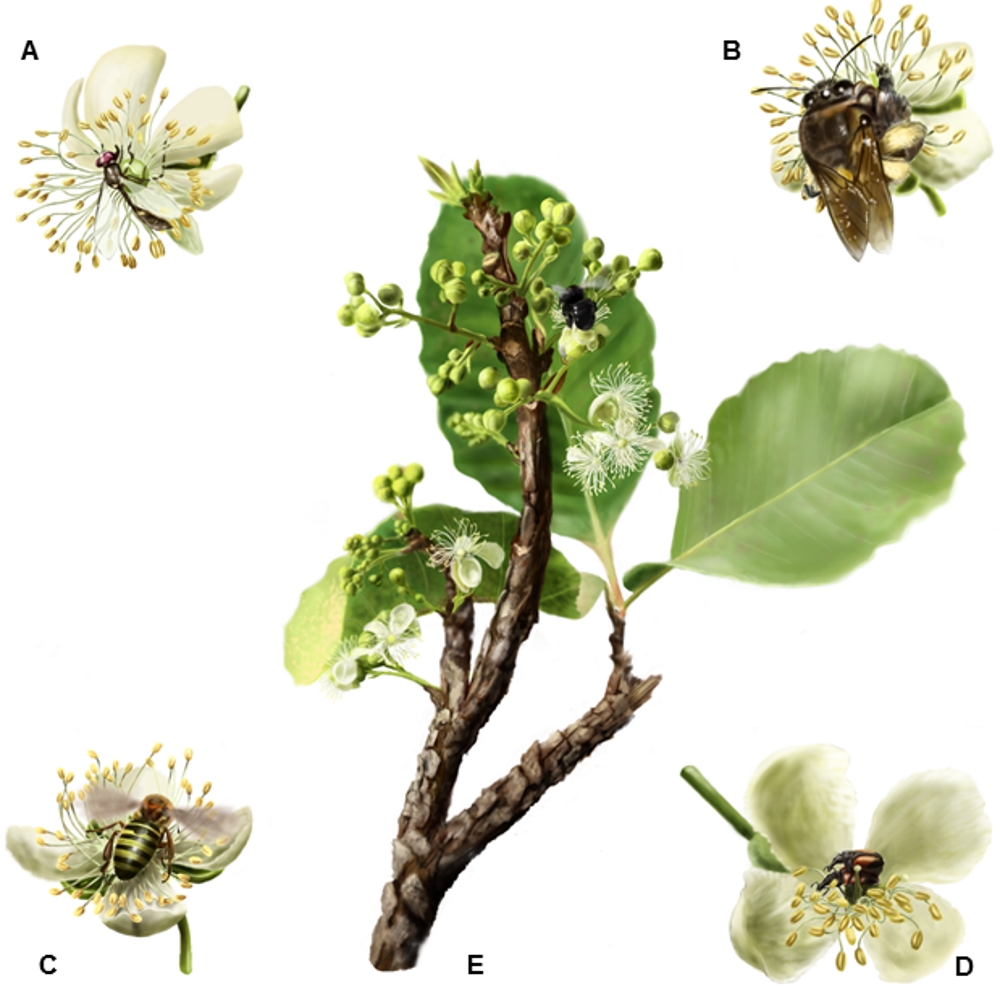
Inflorescence of Curatella americana and flower visitors in detail. A. Syrphidae (fly), B. Ptiloglossa sp. (large-bee), Melipona quinquefasciata (small-bee), D. Curculionidae beetle and E. Inflorescence with a Bombus cf. morio (large-sized bee) visiting the flower. Illustrated by Pedro Lorenzo.
Pollination and pollen tubes
Flowers of C. americana were visited by bees, flies, and beetles (Fig. 2, Tab. 1). Due to visitor behaviour (contact with sexual organs), mobility, and flight ranges observed in the field, large-sized bees (larger than Apis mellifera) were considered the main pollinators (for more details see AR Rech et al. unpubl. res.). The pollinator visitation probability was highest in the Southern populations (Tab. 2). Fruit set under natural pollination and visitation rate of main pollinators were positively correlated (R2 = 0.49, P1,8 = 0.02, Tab. 2). On the other hand, the relationship between autogamy and pollinator availability was negative and significant only when the two Southern edge populations were not considered (R2 = 0.18, P1,8 = 0.61 for all populations and R2 = 0.65, P1,8 = 0.02 for the restricted analysis). Similarly, the fruit set under natural pollination only negatively correlated to the level of autogamy when the same two populations were not considered (r = -0.29, P8 = 0.40 for all populations and r = -0.78, P6 = 0.02 for the restricted analysis). The two populations from the Southern edge had similar natural pollination fruit sets as the other populations in Central Brazil, but autogamy levels were like the populations from the North. Since the two Southern edge populations face a special biogeographical scenario, we ran the analysis with and without these populations and compared the results (see Materials and methods, and Discussion).
Flower visitors registered in the flowers of Curatella americana in the ten studied populations in Brazil. Curculionid Beetles were also observed but not quantified as the stay for the entire observation period without moving from the observed flower.
Average proportion of fruit for each of the ten studied populations of Curatella americana in Brazil. Autogamy refers to bagged inflorescences with no pollinator access while natural pollination is the fruit set in exposed flowers and cross-pollination (cross) is the supplemented hand cross-pollination treatment. Single flower visitation (SFV) refers to the mean number of visits by large-sized bees to a single flower in each population per day. Population numbers follow Figure 1.
The amount of pollen on stigmas increased from 52 pollen grains at the beginning to 141 pollen grains per stigma at the end of the anthesis in bagged flowers (T8 = -4.96, p< 0.001), indicating the potential for spontaneous self-pollination in all populations. Nevertheless, the level of autogamy varied considerably among populations and individuals (Tab. 2). These differences in fruit set can be directly related to the way pollen tubes grew in the different treatments. Higher amounts of pollen tubes grew regularly and continuously through the styles of cross-pollinated flowers. Pollen tubes in self-pollinated flowers grew irregularly (strong callose reactions) and the difference between treatments (self- and cross-pollination) was clearly more contrasting in the Southern region than in the North (Fig. 3A-D). Most of the pollen tubes in self-pollinated flowers stopped growing in the first two thirds of the style. Therefore, we only saw pollen tubes in the self-pollination treatment reaching the ovules 24 hours after hand pollination in flowers from the Northern region (Fig. 3E-F).
Pollen tube growth in flowers of Curatella americana after different pollination tests. A and B - Cross and self-pollination in one population from Southern Cerrado region (Cuiabá). C - Self-pollination in one population from the Northern region (Boa Vista). D - Self-incompatiblity reaction (black arrow) in one self-pollinated flower from Southern Cerrado region (Manso). E - pollen tube growing through the base of the style and (F) reaching the ovule (white arrow) at the base of ovary in one population from the Northern Cerrado region (Boa Vista).
Morphometrics
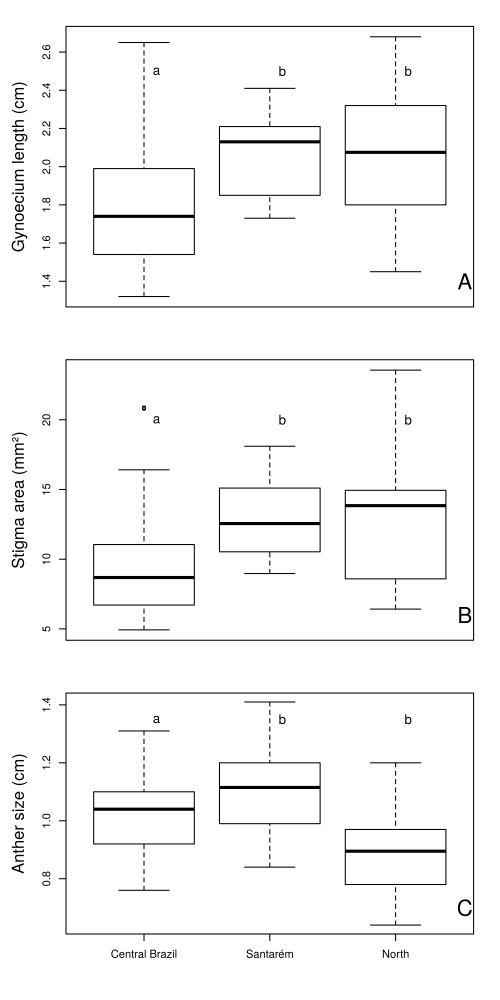
The size (measured as centroid size) of petals, sepals, and leaves did not differ between regions, however, the linear lengths of anthers and gynoecium, and stigma area, varied significantly (Tab. 3 Fig. 4). For floral structures (petal and sepal), the null model including only individual size variation regardless of population and region was as likely as the more complex models. For leaves, the best model included differences among populations, but only the model with regions did not perform well, indicating a more idiosyncratic population-level variation in size instead of a geographical pattern. The number of stamens did not vary among regions (Tab. 3). Anthers were larger in the Southern region, while stigma area was larger in the North. Santarém was similar to Southern populations regarding anther size, and to the Northern populations regarding stigma area (Tab. 3, Fig. 4).
Analysis of Variance (ANOVA) for counted and linearly measured floral traits of Curatella americana from ten populations of three disjunct regions of Brazilian savanna (Southern Cerrado, Santarém and Northern Cerrado).
Comparison (ANOVA) of floral traits of Curatella americana from different regions in the Brazilian Cerrado (Neotropical savanna).
On the other hand, there were regional differences in shape for all traits considered, even though the proportion of variation among regions varied widely among structures (Tab 4). Although for the ovary, region was the main correlate of shape variation, for the other structures it had a minor role (Tab. 4 , Fig. 5). Population-level variation in shape within regions had a minor contribution to all structures, whereas individual variation was always high. Given the major role of regions in ovary shape variation, a clear geographic gradient was observed (Fig. 4), where northern populations presented the two stigmas close together, while in southern populations stigmas were further apart. Santarém, geographically located between populations from the other regions, had an intermediate ovary shape (Fig. 4 and Fig. S2 in supplementary material). For the other structures (leaves, petals, and sepals), no clear patterns of geographic variation were present (Fig. S2 in supplementary material).
Nested Procrustes ANOVA for shape of floral and leaf traits of C. americana in the three geographical regions studied (Southern Cerrado, Santarém, and Northern Cerrado). DF: Degree of freedom, MS: Mean Square Sum, p: p value.
Principal component analysis (PCA) using geometric morphometric coordinates for ovary shape of Curatella americana. Each dot is an individual measure projected according to the first two axes of the PCA. Colours indicate regions: orange is Santarém, red is Northern Cerrado and blue is Southern Cerrado. Different shapes indicate different populations within each region.
Discussion
Pollinator availability varies greatly for the Neotropical tree C. americana throughout its wide distribution range. The availability of pollinators (represented by bees larger than A. mellifera, such as species of Bombus, Centris, Epicharis and Xylocopa) had a direct effect on the level of natural pollination, and a more complex relationship with autogamy. We also found that C. americana populations at edge areas (Northern region and populations 9, 10 at the Southern limit of Cerrado) had higher levels of autogamy. We expected smaller flowers and lower herkogamy in more self-pollinated populations and, as predicted, these populations had larger stigma area, smaller anthers, and less herkogamy. Meanwhile, other structures such as petals and sepals did not vary in size according to the regions studied. Interpretations of these results are discussed in the following sections.
Floral biology, pollination, and pollen tubes
The mass-flowering pattern of C. americana was already reported for another Neotropical Dilleniaceae species - Davilla kunthii (Rech et al. 2011Rech AR, Manente-Balestieri FCL, Absy ML. 2011. Reproductive biology of Davilla kunthii A. St-Hil.(Dilleniaceae) in Central Amazonia. Acta Botanica Brasilica 25: 487-496. ). Apart from flower colour (white in C. americana and yellow in D. kunthii) and the closing sepals of D. kunthii, both species have similar floral biology, pollination, and mixed mating systems (Rech et al. 2011Rech AR, Manente-Balestieri FCL, Absy ML. 2011. Reproductive biology of Davilla kunthii A. St-Hil.(Dilleniaceae) in Central Amazonia. Acta Botanica Brasilica 25: 487-496. ). The open flower morphology with exposed anthers makes it easy for visitors to contact anthers and stigmas in a single visit, however, many flower visitors hardly moved between individuals, making them unlikely to be significant pollen vectors (Ollerton et al. 2007Ollerton J, Killick A, Lamborn E, Watts S, Whiston M. 2007. Multiple meanings and modes: on the many ways to be a generalist flower. Taxon 56: 717-728. ). Pollen as the only reward and the white UV absorbing pattern found in the petals, indicates a flower that is attractive to bees (Lunau et al. 2011Lunau K, Papiorek S, Eltz T, Sazima M. 2011 Avoidance of achromatic colours by bees provides a private niche for hummingbirds. Journal of Experimental Biology 214: 1607-1612. ), which was clearly confirmed by visitation frequency (AR Rech et al. unpubl. res.).
Some populations presented very low levels of natural fruit production, as well as low availability of effective pollinators. We interpreted the negative relationship between fruit set under natural pollination and autogamy as an assurance strategy selecting autogamy in areas with low pollinator availability, herein, the Northern region and populations at the Southern edge of the Brazilian Cerrado (Baker 1967Baker HG. 1967. Support for Baker’s law-as a rule. Evolution 21: 853-856.; Cheptou 2012Cheptou P-O. 2012. Clarifying Baker’s law. Annals of Botany 109: 633-641. ). Two populations at the Southern edge of the C. americana distribution (Jatai and Caldas Novas) presented natural fruit sets that were equal to the populations in Central Brazil, but the levels of autogamy were similar to the populations in the North. Palynological evidence shows that the Southern edge of Brazilian savannas presented very dry, treeless environments from 18,000 to 6,000 bp (Salgado-Labouriau et al. 1997Salgado-Labouriau ML, Casseti V, Ferraz-Vicentini KR, et al. 1997. Late Quaternary vegetational and climatic changes in cerrado and palm swamp from Central Brazil. Palaeogeography, Palaeoclimatology, Palaeoecology 128: 215-226. ). Only from 5,000 bp onwards did trees, such as C. americana, colonize the area. Thus, the level of autogamy in the area may still be a consequence of the colonization process. Indeed, genetic data suggests a recent range expansion for C. americana in Brazilian savannas (Canuto 2011Canuto JZ. 2011. Filogeografia de Curatella americana L. (Dilleniaceae): uma espécie arbórea das savanas da Amazônia e Brasil Central. MSc Thesis, Instituto Nacional de Pesquisas da Amazônia, Manaus.). Also, changes in mating systems may be flexible, as seen in artificial selection experiments (Levin 2012Levin DA. 2012. Mating system shifts on the trailing edge. Annals of Botany 109: 613-620.). Furthermore, two generations of strong induced directional selection were enough to elevate the autogamy level from 4 % to 56 % in Phlox drummondii (Bixby & Levin 1996Bixby PJ, Levin DA. 1996. Response to selection on autogamy in Phlox. Evolution 50: 892-899. ). Therefore, past climate and colonization events related to expansion of Neotropical Savannas could underpin the distribution and consequently the contemporary autogamy rate found in C. americana (Canuto 2011Canuto JZ. 2011. Filogeografia de Curatella americana L. (Dilleniaceae): uma espécie arbórea das savanas da Amazônia e Brasil Central. MSc Thesis, Instituto Nacional de Pesquisas da Amazônia, Manaus.; AR Rech et al. unpublished res.).
Curatella americana has a lifespan of over 200 years (Costa 2013Costa CP. 2013. Padrões da distribuição de plantas arbóreo-arbustivas em meso-escala no pantanal de Mato Grosso. PhD Thesis, Universidade de Brasília, Brasília.), with a slower process of population replacement when compared to short-lived herbs such as P. drummondii. Therefore, if there is an ecological filter preventing colonization by animal-pollination dependent individuals, the establishment of a predominantly autogamous population could be very quick, as is the case for some invasive plant species (Ollerton et al. 2012Ollerton J, Watts S, Connerty S. 2012. Pollination ecology of the invasive tree tobacco Nicotiana glauca: comparisons across native and non-native ranges. Journal of Pollination Ecology 9: 85-95.). Nonetheless, recovery of higher rates of self-incompatibility when population size increases and pollinators come back into the environment can be slower, specially for long lived species like C. americana. It is important to consider that populations are normally not exclusively self-pollinated, so the unfavourable effects of a pure strategy may be reduced in mixed mating systems (Goodwillie et al. 2005Goodwillie C, Kalisz S, Eckert CG. 2005. The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence. Annual Review of Ecology, Evolution, and Systematics 36: 47-79. ). We also cannot rule out the possible effect of inbreeding depression caused by selfing at edge populations, which leads to less visitation and should be the focus of future genetic studies.
Morphology
Most of the traits studied here had a latitudinal gradient of variation. Moreover, individuals from Santarém frequently presented intermediate shape and size compared to Northern and Southern populations and were more similar to one region or another depending on the structure considered. This seems to be related to a similar palaeoecological history as the Northern region, and a pollinator availability similar to the one found in the Southern region. All the savannas studied herein, currently within isolated regions, were probably connected in the past and the Northern region was probably the first to disconnect from the others (Mayle & Power 2008Mayle FE, Power MJ. 2008. Impact of a drier Early-Mid-Holocene climate upon Amazonian forests. Philosophical Transactions of the Royal Society B: Biological Sciences 363: 1829. ).
At the regional level, floral and vegetative traits varied similarly but with different intensities. The synchronized variation in floral and vegetative traits in plants with generalized pollination systems (open flower morphology and many unrelated species of pollinators) is one of the expectations to “Berg’s rule” (Berg 1960Berg RL. 1960. The ecological significance of correlation pleiades. Evolution 14: 171-180. ; Fenster 1991Fenster CB. 1991. Selection on floral morphology by hummingbirds. Biotropica 23: 98-101. ; Conner & Sterling 1995Conner JK, Sterling A. 1995. Testing hypotheses of functional relationships: a comparative survey of correlation patterns among floral traits in five insect-pollinated plants. American Journal of Botany 82: 1399-1406.; Armbruster et al. 1999Armbruster WS, Stilio VSD, Tuxill JD, Flores TC, Runk JLV. 1999. Covariance and decoupling of floral and vegetative traits in nine Neotropical plants: a re-evaluation of Berg’s correlation-pleiades concept. American Journal of Botany 86: 39-55.). As floral traits derive ultimately from the vegetative ones, decoupled patterns of variation are not expected unless clearly different regimes of selection drive the evolution of different groups of traits in different ways (Berg 1960Berg RL. 1960. The ecological significance of correlation pleiades. Evolution 14: 171-180. ; Armbruster et al. 1999Armbruster WS, Stilio VSD, Tuxill JD, Flores TC, Runk JLV. 1999. Covariance and decoupling of floral and vegetative traits in nine Neotropical plants: a re-evaluation of Berg’s correlation-pleiades concept. American Journal of Botany 86: 39-55.). However, the variation pattern found in the ovary structure is much clearer than in the other structures. Therefore, we interpreted the progressive segregation of stigmas from north to south as a strategy selected to increase genetic diversity by touching different pollinators or pollinators’ body parts during visits.
However, contrary to the expectation, not all traits varied in the same direction as an integrated unit or “pleiades” (Berg 1960Berg RL. 1960. The ecological significance of correlation pleiades. Evolution 14: 171-180. ). Stigma area was larger and there was less herkogamy in the North. These two traits may be related to female reproductive insurance, as both increase the chance a flower will be self-pollinated (see review in Levin 2012Levin DA. 2012. Mating system shifts on the trailing edge. Annals of Botany 109: 613-620.). The same pattern was also reported in Nicotiana glauca after it was introduced to parts of the world where there were no suitable pollinators present, reinforcing the role pollinators have in mediating the phenotypic variation of flowers (Ollerton et al. 2012Ollerton J, Watts S, Connerty S. 2012. Pollination ecology of the invasive tree tobacco Nicotiana glauca: comparisons across native and non-native ranges. Journal of Pollination Ecology 9: 85-95.). On the other hand, anthers were larger in the Southern populations. Considering that anther size and pollen production are positively correlated (Harder & Thompson 1989Harder LD, Thomson JD. 1989. Evolutionary options for maximizing pollen dispersal of animal-pollinated plants. American Naturalist 133: 323-344.; Philipp et al. 1990Philipp M, Böcher J, Mattsson O, Woodell RJ, 1990. A quantitative approach to the sexual reproductive biology and population structure in some arctic flowering plants: Dryas integrifolia, Silene acaulis and Ranunculus nivalis. Meddelelser om Grønland: Bioscience 34: 3-60.), and pollen removal can be related to seed set (Broyles & Wyatt 1990Broyles SB, Wyatt R. 1990. Paternity analysis in a natural population of Asclepias exaltata: Multiple paternity, functional gender, and the “pollen-donation hypothesis”. Evolution 44: 1454-1468. ), there seems to be a pressure on the male component (pollen production) of fitness in areas with higher levels of cross-pollination (Lloyd 1984Lloyd DG. 1984. Gender allocations in outcrossing cosexual plants. In: Dirzo R, Sarukhán J. (eds.) Perspectives on plant population ecology. Mass, Sinauer Associates Inc. p. 277-300.).
Finally, we showed that floral structures did not have an integrated pattern of variation across regions and populations. Conversely, different flower traits may respond independently to the pressures imposed by pollinator availability and the importance of autogamy in the different populations (Rosas-Guerrero et al. 2010Rosas-Guerrero V, Quesada M, Armbruster WS. 2010. Influence of pollination specialization and breeding system on floral integration and phenotypic variation in Ipomoea. Evolution 65: 350-64. ; Opedal et al. 2016Opedal ØH, Albertsen E, Armbruster WS, Pérez-Barrales R, Falahati-Anbaran M, Pélabon C. 2016. Evolutionary consequences of ecological factors: pollinator reliability predicts mating-system traits of a perennial plant. Ecology Letters 19: 1486-1495. ). These divergent responses fulfil the function of the flower by maximizing reproduction, with flexibility to adapt to different scenarios.
Conclusions
Trees have longer lives, and, therefore, slower individual substitution rates than most herbs and smaller woody plants. However, herein we found a similar pattern of variation in the mating systems and flower morphology of a tropical tree, as previously demonstrated for herb and a vine species, indicating colonization filters and pollinator availability as important drivers of flower morphology and mating system (Opedal et al. 2016Opedal ØH, Albertsen E, Armbruster WS, Pérez-Barrales R, Falahati-Anbaran M, Pélabon C. 2016. Evolutionary consequences of ecological factors: pollinator reliability predicts mating-system traits of a perennial plant. Ecology Letters 19: 1486-1495. ). The results discussed here re-emphasize mating systems as a property of populations and not of species (Whitehead et al. 2018Whitehead MR, Lanfear R, Mitchell RJ, Karron JD. 2018. Plant mating systems often vary widely among populations. Frontiers Ecology and Evolution. 6: 38.). Even though, in general, longer-lived organisms seem to have higher outcrossing rates, pollinator availability may also help explain local reproductive strategies and interpopulation variations in mating system.
Acknowledgements
This work is in homage of Prof. Dr. Antonio Carlos Webber, for his retirement after a career dedicated to teaching and researching pollination in the Amazon region. ARR thanks FAPESP (Proc. 2009/54491-0), CNPq, and CAPES (PDSE) for scholarships, FAEPEX and Santander Universities for financial support for research. LRJ was supported by PhD and PostDoc scholarships by FAPESP (#09/54806-0 and 14/16082-9). JO thanks FAPESP for support as a Visiting Researcher (Proc. 2013/14442-5). MS thanks CNPq for the research grant (303084/2011-1).
References
- Adams DC, Otarola-Castillo E. 2013. Geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods in Ecology and Evolution 4: 393-399.
- Armbruster WS, Mulder CPH, Baldwin BG, Kaliz S, Wessa B, Nute H. 2002. Comparative analysis of late floral development and mating-system evolution in tribe Collinsieae (Scrophulariaceae s.l). American Journal of Botany 89: 37-49.
- Armbruster WS, Stilio VSD, Tuxill JD, Flores TC, Runk JLV. 1999. Covariance and decoupling of floral and vegetative traits in nine Neotropical plants: a re-evaluation of Berg’s correlation-pleiades concept. American Journal of Botany 86: 39-55.
- Baker HG. 1967. Support for Baker’s law-as a rule. Evolution 21: 853-856.
- Baker HG, Stebbins GL. 1965. The genetics of colonizing species: proceedings of the first international union of biological sciences symposia on general biology. 1st. edn. New York, Academic Press Inc.
- Barrett SCH. 2013. The evolution of plant reproductive systems: how often are transitions irreversible? Proceedings of the Royal Society B: Biological Sciences 280: 20130913. doi: 10.1098/rspb.2013.0913
» https://doi.org/10.1098/rspb.2013.0913 - Barrett SCH, Morgan MT, Husband BC. 1989. The dissolution of a complex genetic polymorphism: the evolution of self-fertilization in tristylous Eichhornia paniculata (Pontederiaceae). Evolution 43: 1398-1416.
- Berg RL. 1960. The ecological significance of correlation pleiades. Evolution 14: 171-180.
- Bixby PJ, Levin DA. 1996. Response to selection on autogamy in Phlox Evolution 50: 892-899.
- Bookstein FL. 1991. Morphometric tools for landmark data: geometry and biology. Cambridge, Cambridge University Press.
- Broyles SB, Wyatt R. 1990. Paternity analysis in a natural population of Asclepias exaltata: Multiple paternity, functional gender, and the “pollen-donation hypothesis”. Evolution 44: 1454-1468.
- Brys R, Jacquemyn H. 2011. Variation in the functioning of autonomous self-pollination, pollinator services and floral traits in three Centaurium species. Annals of Botany 107: 917-925.
- Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. 2nd. edn. New York, Springer.
- Cabanne GS, Santos FR, Miyaki CY. 2007. Phylogeography of Xiphorhynchus fuscus (Passeriformes, Dendrocolaptidae): vicariance and recent demographic expansion in southern Atlantic forest. Biological Journal of the Linnean Society 91: 73-84.
- Canuto JZ. 2011. Filogeografia de Curatella americana L. (Dilleniaceae): uma espécie arbórea das savanas da Amazônia e Brasil Central. MSc Thesis, Instituto Nacional de Pesquisas da Amazônia, Manaus.
- Cárdenas ML, Gosling WD, Sherlock SC, Poole I, Pennington RT, Mothes P. 2011. The response of vegetation on the Andean flank in western Amazonia to pleistocene climate change. Science 331: 1055-1058.
- Cheptou P-O. 2012. Clarifying Baker’s law. Annals of Botany 109: 633-641.
- Conner JK, Sterling A. 1995. Testing hypotheses of functional relationships: a comparative survey of correlation patterns among floral traits in five insect-pollinated plants. American Journal of Botany 82: 1399-1406.
- Costa CP. 2013. Padrões da distribuição de plantas arbóreo-arbustivas em meso-escala no pantanal de Mato Grosso. PhD Thesis, Universidade de Brasília, Brasília.
- Dafni A, Kevan PG, Husband BC. 2005. Practical pollination biology. Cambrigde, Enviroquest Ltd.
- Dalsgaard B, Magård E, Fjeldså J. 2011. Specialization in plant-hummingbird networks is associated with species richness, contemporary precipitation and quaternary climate-change velocity. PLOS ONE 6: e25891. https://doi.org/10.1371/journal.pone.0025891
» https://doi.org/10.1371/journal.pone.0025891 - Dalsgaard B, Trøjelsgaard K, González AMM. 2013. Historical climate-change influences modularity and nestedness of pollination networks. Ecography 36: 1331-1340.
- Dart SR, Samis KE, Austen E, Eckert CG. 2012. Broad geographic covariation between floral traits and the mating system in Camissoniopsis cheiranthifolia (Onagraceae): multiple stable mixed mating systems across the species’ range? Annals of Botany 109: 599-611.
- Darwin C. 1876. The effects of cross and self fertilisation in the vegetable kingdom. London, John Murray.
- Dudley LS, Mazer SJ, Galusky P. 2007. The joint evolution of mating system, floral traits and life history in Clarkia (Onagraceae): genetic constraints vs. independent evolution. Journal of Evolutionary Biology 20: 2200-2218.
- Fenster CB. 1991. Selection on floral morphology by hummingbirds. Biotropica 23: 98-101.
- Gómez JM. 2002. Generalización en las interacciones entre plantas y polinizadores. Revista Chilena de Historia Natural 75: 105-115.
- Gómez JM, Munoz-Pajares AJ, Abdelaziz M, Lorite J, Perfectti F. 2014. Evolution of pollination niches and floral divergence in the generalist plant Erysimum mediohispanicum Annals of Botany 113: 237-249.
- Goodwillie C, Kalisz S, Eckert CG. 2005. The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence. Annual Review of Ecology, Evolution, and Systematics 36: 47-79.
- Grazziotin FG, Monzel M, Echeverrigarauy S, Bonato SL. 2006. Phylogeography of the Bothrops jararaca complex (Serpentes: Viperidae): past fragmentation and island colonization in the Brazilian Atlantic Forest. Molecular Ecology 15: 3969-3982.
- Griffin PC, Willi Y. 2014. Evolutionary shifts to self-fertilisation restricted to geographic range margins in North American Arabidopsis lyrata Ecology Letters 17: 484-490.
- Harder LD, Thomson JD. 1989. Evolutionary options for maximizing pollen dispersal of animal-pollinated plants. American Naturalist 133: 323-344.
- Hargreaves AL, Eckert CG. 2013. Evolution of dispersal and mating systems along geographic gradients: implications for shifting ranges. Functional Ecology 28: 5-21.
- Herlihy CR, Eckert CG. 2002. Genetic cost of reproductive assurance in a self-fertilizing plant. Nature 416, 320-323.
- Herrera CM. 2005. Plant generalization on pollinators: species property or local phenomenon? American Journal of Botany 92: 13-20.
- Ivey CT, Carr DE. 2012. Tests for the joint evolution of mating system and drought escape in Mimulus Annals of Botany 109: 583-598.
- Kalisz S, Vogler DW. 2003. Benefits of autonomous selfing under unpredictable pollinator environments. Ecology 84: 2928-2942.
- Kissling WD, Dormann CF, Groeneveld J, et al 2012. Towards novel approaches to modelling biotic interactions in multispecies assemblages at large spatial extents. Journal of Biogeography 39: 2163-2178.
- Levin DA. 2012. Mating system shifts on the trailing edge. Annals of Botany 109: 613-620.
- Lloyd DG. 1984. Gender allocations in outcrossing cosexual plants. In: Dirzo R, Sarukhán J. (eds.) Perspectives on plant population ecology. Mass, Sinauer Associates Inc. p. 277-300.
- Lloyd DG. 1992. Self- and cross-fertilization in plants. II. The selection of self-fertilization. International Journal of Plant Sciences 153: 370-380.
- Lunau K, Papiorek S, Eltz T, Sazima M. 2011 Avoidance of achromatic colours by bees provides a private niche for hummingbirds. Journal of Experimental Biology 214: 1607-1612.
- Martin FW. 1959. Staining and observing pollen tubes in the style by means of fluorescence. Stain Technology 34: 125-128.
- Martin NH, Willis JH. 2007. Ecological divergence associated with mating system causes nearly complete reproductive isolation between sympatric Mimulus species. Evolution 61: 68-82
- Mayle FE, Power MJ. 2008. Impact of a drier Early-Mid-Holocene climate upon Amazonian forests. Philosophical Transactions of the Royal Society B: Biological Sciences 363: 1829.
- Mazer SJ, Paz H, Bell MD. 2004. Life history, floral development, and mating system in Clarkia xantiana (Onagraceae): do floral and whole-plant rates of development evolve independently? American Journal of Botany 91: 2041-2050.
- Moeller DA, Runquist RDB, Moe AM, et al 2017. Global biogeography of mating system variation in seed plants. Ecology Letters 20: 375-384.
- Moeller DA. 2006. Geographic structure of pollinator communities, reproductive assurance, and the evolution of self-pollination. Ecology 87: 1510-1522.
- Ollerton J, Killick A, Lamborn E, Watts S, Whiston M. 2007. Multiple meanings and modes: on the many ways to be a generalist flower. Taxon 56: 717-728.
- Ollerton J, Watts S, Connerty S. 2012. Pollination ecology of the invasive tree tobacco Nicotiana glauca: comparisons across native and non-native ranges. Journal of Pollination Ecology 9: 85-95.
- Opedal ØH, Albertsen E, Armbruster WS, Pérez-Barrales R, Falahati-Anbaran M, Pélabon C. 2016. Evolutionary consequences of ecological factors: pollinator reliability predicts mating-system traits of a perennial plant. Ecology Letters 19: 1486-1495.
- Ornduff R. 1969. Reproductive biology in relation to systematics. Taxon 18: 121-133.
- Philipp M, Böcher J, Mattsson O, Woodell RJ, 1990. A quantitative approach to the sexual reproductive biology and population structure in some arctic flowering plants: Dryas integrifolia, Silene acaulis and Ranunculus nivalis Meddelelser om Grønland: Bioscience 34: 3-60.
- R Development Core Team 2014. R: A language and environment for statistical computing. Vienna, R Foundation for Statistical Computing.
- Ratter JA, Bridgewater S, Ribeiro JF. 2003. Analysis of the floristic composition of the Brazilian cerrado vegetation III: comparison of the woody vegetation of 376 areas. Edinburgh Journal of Botany 60: 57-109.
- Rech AR, Manente-Balestieri FCL, Absy ML. 2011. Reproductive biology of Davilla kunthii A. St-Hil.(Dilleniaceae) in Central Amazonia. Acta Botanica Brasilica 25: 487-496.
- Rohlf FJ. 2010. TpsDig. Stony Brook/ New York, Department of Ecology and Evolution, State University of New York at Stony Brook.
- Rosas-Guerrero V, Aguilar R, Martén-Rodríguez S, et al 2014. A quantitative review of pollination syndromes: do floral traits predict effective pollinators? Ecology Letters 17: 388-400.
- Rosas-Guerrero V, Quesada M, Armbruster WS. 2010. Influence of pollination specialization and breeding system on floral integration and phenotypic variation in Ipomoea. Evolution 65: 350-64.
- Salgado-Labouriau ML, Casseti V, Ferraz-Vicentini KR, et al 1997. Late Quaternary vegetational and climatic changes in cerrado and palm swamp from Central Brazil. Palaeogeography, Palaeoclimatology, Palaeoecology 128: 215-226.
- Sandel B, Arge L, Dalsgaard B, et al 2011. The influence of Late Quaternary climate-change velocity on species endemism. Science 334: 660-664.
- Stebbins GL. 1974. Flowering plants: evolution above the species level. Cambridge, Harvard University Press.
- Svenning JC, Skov F. 2007. Ice age legacies in the geographical distribution of tree species richness in Europe. Global Ecology and Biogeography 16: 234-245.
- Thompson JN. 2005. The geographic mosaic of coevolution. Chicago, University of Chicago Press.
- Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. 1996. Generalization in pollination systems, and why it matters. Ecology 77: 1043-1060.
- Whitehead MR, Lanfear R, Mitchell RJ, Karron JD. 2018. Plant mating systems often vary widely among populations. Frontiers Ecology and Evolution. 6: 38.
- Wyatt R. 1988. Phylogenetic aspects of the evolution of self-pollination. In: Gottlieb LD, Jain SK. (eds.) Plant evolutionary biology. London, Springer p. 109-131
Publication Dates
-
Publication in this collection
Jul-Sep 2018
History
-
Received
21 June 2018 -
Accepted
02 July 2018