Abstract
Considering that oral preparations made with peel green bananas (e.g. flour and extracts) demonstrated healing effects on mucous membranes and skin, this study evaluated the healing and the antimicrobial property of a topical preparation based on extract of Musa sapientum L., Musaceae, (apple banana) in surgically induced wounds in the skin of male Wistar rats, 100 g. The extract was obtained by decoction, the presence of tannins was detected by phytochemical screening and 10% of the extract was incorporated into the carbopol gel (CMS gel). The processes of healing and bacterial isolation were evaluated in the following experimental groups: control (no treatment), treatment with placebo or with the CMS gel. The healing of surgical wounds treated with the CMS gel was faster when compared with the control and placebo groups and the treatment with CMS gel also inhibited the growth of pyogenic bacteria and enterobacteria in the wounds. The results indicate that the extract of Musa sapientum epicarp has healing and antimicrobial properties (in vivo), probably, due to tannins.
Musa sapientum; apple banana; tannin; tissue repair
Evaluation of post-surgical healing in rats using a topical preparation based on extract of Musa sapientum L., Musaceae, epicarp
Priscila B. LinoI; Cleber F. CorrêaII; Márcia E. D. L. ArchondoI; Deise C. A. L. DellovaIII,* * E-mail: leite-dellova@usp.br , Tel. +55 19 3565 4320, Fax +55 19 3565 4117.
ICurso de Farmácia, Universidade do Grande ABC, Av. Industrial, 3.330, Bairro Campestre, 09080-501 Santo André-SP, Brazil
IIUniversidade Católica de Santos, Av. Conselheiro Nébias, 300, Vila Matias, 11015-002 Santos-SP, Brazil
IIIFaculdade de Zootecnia e Engenharia de Alimentos, Departmento de Ciências Básicas, Universidade de São Paulo, Av. Duque de Caxias Norte, 225, Campus da USP, 13635-900 Pirassununga-SP, Brazil
ABSTRACT
Considering that oral preparations made with peel green bananas (e.g. flour and extracts) demonstrated healing effects on mucous membranes and skin, this study evaluated the healing and the antimicrobial property of a topical preparation based on extract of Musa sapientum L., Musaceae, (apple banana) in surgically induced wounds in the skin of male Wistar rats, 100 g. The extract was obtained by decoction, the presence of tannins was detected by phytochemical screening and 10% of the extract was incorporated into the carbopol gel (CMS gel). The processes of healing and bacterial isolation were evaluated in the following experimental groups: control (no treatment), treatment with placebo or with the CMS gel. The healing of surgical wounds treated with the CMS gel was faster when compared with the control and placebo groups and the treatment with CMS gel also inhibited the growth of pyogenic bacteria and enterobacteria in the wounds. The results indicate that the extract of Musa sapientum epicarp has healing and antimicrobial properties (in vivo), probably, due to tannins.
Keywords:Musa sapientum, apple banana, tannin, tissue repair
Introduction
Healing is a complex process of tissue repair, where several cellular events occur synchronously to ensure the integrity of the body (Amorim et al., 2006; Amaral et al., 2006; Mandelbaum et al., 2003; Garros et al., 2006). This process is triggered by tissue damage and its evolution varies according to the anatomic location, skin type, species, strain and metabolism (Amorim et al., 2006; Mandelbaum et al., 2003; Marchionni et al., 2006).
The banana (Musa spp.) originates from Asia, but is widely grown in most countries, including Brazil (Jesus et al., 2004). The consumption of this fruit is great because of their nutritional value; its shell has been studied for treatment of gastrointestinal disorders (diarrhea, gastritis and gastric ulcers) (Best et al., 1984; Dadoo et al., 1995; Goel et al., 1986; Lewis et al., 1999; Pannangpetch et al., 2001) and commonly is used to treat wounds, especially in nipple fissures that arise during breastfeeding (Novak et al., 2003).
According to Santos & Mello (2002), the healing property of the banana peel is related to the presence of tannins in the fruit epicarp and according to Ono et al. (1998), the alcoholic extract of banana has antibacterial activity against Escherichia coli and Staphylococcus aureus.
Thus, the objective of this study was to evaluate the healing and antimicrobial properties of the extract of Musa sapientum L., Musaceae, epicarp (apple banana) in post-surgical wounds in rats.
Material and Methods
Extraction
The fruits of Musa sapientum var. paradisiacal, Musaceae, were collected in Santa Lídia Bananas Climatizadas, located at Av. Brazil, 233, Mauá-SP, and classified as green on the scale of Von Loesecke (1950). After the botanical identification, done by the Universidade Católica de Santos Herbarium (voucher number: UNIS1294), the epicarps were separated, cleaned with sodium hypochlorite 1% for 15 min, washed in running water and cut into small pieces. The extraction was performed by the method of decoction using 250 g of sliced epicarps into 1.8 L of distilled water; after, the plant material was kept in boiling water for 2 h, with the use of a heating pad. Then, the decoction product was filtered and stored at 4 ºC. The extraction process was repeated twice, using the same epicarps for maximum extraction of metabolites from the plant material (Costa, 2000). The decoction allows the extraction of tannins and/or flavonoids, so the phytochemical screening of secondary metabolites was required.
Phytochemical screening for tannins and flavonoids
The phytochemical screening for tannins was carried out by classical reactions as: gelatin 2.5%, ferric chloride 1%, ferric sulphate 1%, iron alum 1%, lead acetate 10%, caffeine 0.1% and potassium cyanide 10%. The presence of flavonoids was performed by Shinoda's test and aluminum chloride 5% reaction (Costa, 2000).
Gel handling
The extract of M. sapientum epicarp 10% was incorporated into the carbopol gel, according to this formulation: aqueous extract of M. sapientum epicarp 10%, carbopol 0.7%, propylene glycol 3%, EDTA 0.1%, methylparaben 0.2%, deionized water q.s 100% and triethanolamine q.s.
The resulting gel, called CMS, was submitted to microbiological testing using: i. trypticase soy agar (48 h of incubation at 35 ºC), tryptose soy broth and lactose broth (24 h of incubation at 35 ºC) to search for bacteria and ii. Sabouraud agar dextrose culture medium (5 d of incubation at 25 ºC) for fungi and yeasts.
Accelerated stability study
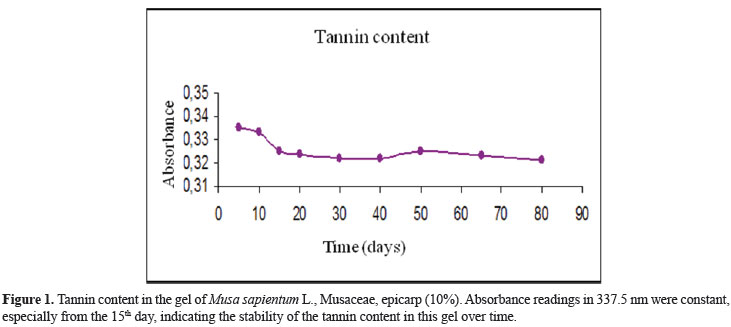
Gel CMS (30 g), stored in polyethylene bottles, were placed in ovens at 25 and 40 ºC for eighty days. Samples of 1 g each were taken in the 24th, 48th e 80th day for organoleptic characterization (color, odor and homogeneity) and to analyze: pH (pH meter Tecnal Tec-2), viscosity (Brookfield RVDV-I+Viscometer) and loss of weight of the formulation (Gehaka AG 200 Analytical Balance). The tannin content was determinate by spectrophotometer (Shimadzu UV-1601) and the absorbance readings were made in the wavelength of highest absorption (337.5 nm) (Anvisa, 2005).
Experimental procedure
A total of thirty rats (male, Wistar, 100 g), raised at the University of Grande ABC Vivarium, were placed in individual polypropylene cages with appropriate supply of water and food. The rats were anesthetized with morphine (2 mg/kg) and tiletamine-zolazepam (Zoletil®, 30 mg/kg) and the cervical dorsal skin was shaved and cleaned (chlorhexidine 1%) to perform an incision (±2 cm). These rats were distributed in three groups with ten animals each: group 1 (control-no treatment), group 2 (treatment with placebo gel, once daily for seven days) and group 3 (treatment with CMS gel, once daily for seven days).
To evaluate the process of healing, the wound was measured (by the same individual) in the 1st, 3rd and 7th postoperative day, using a universal caliper. Immediately after the surgery and on the 7th postoperative day, material from the surgical wound was collected with sterile swabs for bacterial isolation. This material was seeded into Brain Heart Infusion Broth (BHI) agar and incubated at 37 ºC for 24 h. After this period, the material was seeded on blood agar and MacConkey agar and incubated for 24 h at 37 ºC (Gomes et al., 1999). Later, tests were performed to identify the bacteria specific genus and species. This study was approved by the Ethics Committee for Animal Experiments of UniABC (CEUA-UniABC).
Statistics
The size of the wounds is presented as mean values with their standard deviations (±SD). Statistical comparisons between two mean values were made by unpaired t test. When comparing more than two groups, we used the ANOVA test. Differences were considered significant if p<0.05.
Results
The reactions employed in phytochemical screening revealed the presence of tannins and the absence of flavonoids in the extract of Musa sapientum L., Musaceae, epicarp.
The microbiological analysis did not identify pathogenic microorganisms in the formulation of the CMS gel. The stability studies demonstrated that the CMS gel did not lose its initial characteristics and the tannin content (Figure 1 and Table 1).
Figures 2 and 3 show two representative experiments in which the process of wound healing is studied in an animal treated with placebo gel (group 2) or with the gel CMS (group 3), respectively. These figures indicate that in presence of placebo gel in the 3rd postoperative day the wound is open and with signs of inflammation and in the 7th postoperative day the wound has not healed completely. On the other hand, in presence of gel CMS in the 3rd postoperative day the wound do not have signs of inflammation and their edges are close and in the 7th postoperative day the tissue repair is complete.
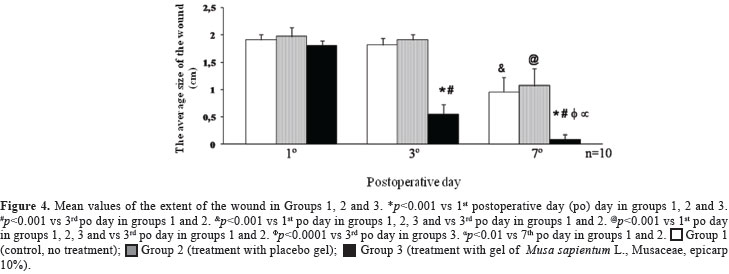
Figure 4 gives the mean values of the wound in the three experimental groups, with ten animals each. In the 1st, 3rd and 7th postoperative day, the average size of the wound in group 1 (control) was 1.91±0.09 cm, 1.8±0.13 cm and 0.95±0.27 cm, respectively; in group 2 (treatment with placebo gel) it was 1.97±0.17 cm, 1.91±0.09 cm and 1.07±0.31 cm, respectively, and complete healing in these two groups was observed only on the 10th postoperative day. In group 3 (treatment with CSM gel), the healing was significantly faster, since on the 1st, 3rd and 7th day, the average size of the wound was 1.8±0.27 cm, 0.54±0.18 cm and 0.08±0.09 cm, respectively. Group 3 presented the smallest average size of the wound on the 3rd and 7th postoperative day (p<0.01) when compared with groups 1 and 2.
Immediately after the incision (1st postoperative day), no bacteria were isolated from any wound. On the 7th postoperative day, the bacteria were isolated from all wounds. The bacteria found are listed in Table 2 according to the percentage of animals that showed each type of bacteria.
Discussion
According to the literature, the anti-inflammatory, antimicrobial and healing activities of plants are related to secondary metabolites: tannins and flavonoids (Ahmad & Beg, 2001; Giovanelli et al., 2000; Lima et al., 2006; Perchellet et al., 1996; Robbers et al., 1997). So, since our results indicate that the tannins are present but the flavanoids are absent in the extract of Musa sapientum L., Musaceae, epicarp, it is probable that the biological activity of the CMS gel found in the present study is related to tannins and not to flavonoids. This result is in accordance with data indicating that tannins are polyphenolic compounds that, in addition to their astringent action, have the ability to precipitate proteins, forming important protective coatings in wound healing, that reduced the volume of secretions and prevented the development of micro-organisms, providing the more efficient tissue regeneration (Costa, 2002).
The data indicate that the extract of M. sapientum epicarp presents a healing property, because a reduction of 70% in the size of the wounds on the 3rd day of treatment with the gel CMS was observed, while the control and the placebo groups showed only 5.5% and 3% of reduction, respectively (Figure 4). On the 7th day of treatment with the CMS gel, the wounds were almost healed (Figures 3 and 4) but in the control and placebo groups, the wounds were reduced only to 50% and 46% of the original size, respectively (Figure 4).
These results are consistent with studies that demonstrate the healing effect of M. sapientum in gastric ulcers (Goel et al., 1986; Lewis et al., 1999; Mohan et al., 2006; Pannangpetch et al., 2001) and other wounds (Agarwal et al., 2009).
In vivo, the CMS gel allowed the growth of bacteria belonging to the normal microbiota of rat skin (Grice et al., 2008), however the CMS gel inhibited the growth of enterobacteria and pyogenic bacteria (from the environment, feet and excreta of animals) since these bacteria were not isolated in group 3, but appeared in the other two groups (Table 3). In vitro the CMS gel did not present antimicrobial activity, since there was no area of inhibition of bacterial growth with the various concentrations tested (50-200 µL, data not shown). It is known that the bacteria can produce an enzyme (tannase) that degrades tannins (Aguilar et al., 1999) and therefore could limit the antimicrobial activity of this compound. So, in the present study, it is possible that the bacteria isolated from skin of rats degraded the tannins in vitro, which did not occur in vivo, because we observed inhibition of growth of some bacteria and of healing activity.
In summary, the present study suggests the CMS gel was produced following adequate quality standards, as shown by the stability tests and microbiological analysis. The results are compatible with the anti-inflammatory, antimicrobial and healing activities of the extract of M. sapientum epicarp related to its content of tannins. These effects of tannins may represent an important share in the healing of skin wounds, with possible clinical use in animals and humans.
Received 20 Apr 2010
Accepted 5 Oct 2010
- Agarwal PK, Singh A, Gaurav K, Goel S, Khanna HD, Goel RK 2009. Evaluation of wound healing activity of extracts of plantain banana (Musa sapientum var. paradisiaca) in rats. Indian J Exp Biol 47: 32-40.
- Aguilar C, Augus C, González G, Favela E 1999. A comparison of methods to determine tannin acyl hydrolase activity. Braz Arch Biol Technol 42: 355-361.
- Ahmad I, Beg AZ 2001.Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol 74: 113-123.
- Amaral MLG, Carneiro LMA, Monte Santo MR, Brito NMB, Brito MVH, Andrade MC 2006. Human milk resulton the healing of wounds produced in rats. Rev Para Med 20: 13-18.
- Amorim E, Matias JEF, Coelho JV, Campos ACL, Stahlke JHJ, Timi JRR, Rocha LCA, Moreira ATR, Rispoli DZ, Ferreira LM 2006. Topic use of aqueous extract of Orbignya phalerata (babassu) in rats: analysis of it's healing effect. Acta Cir Bras 21: 67-76.
- Anvisa 2005. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução-RE no1/2005. Guia para realização de estudos de estabilidade. Diário Oficial da República Federativa do Brasil. Poder Executivo, Brasília, DF, 29 de julho de 2005.
- Best R, Lewis DA, Nasser N 1984. The anti-ulcerogenic activity of the unripe plantain banana (Musa species). Br J Pharmacol 82:107-116.
- Costa AF 2002. Farmacognosia. 6. ed. Lisboa: Fundação Calouste Gulbenkian.
- Costa AF 2000. Farmacognosia: Farmacognosia Experimental. 3. ed. Lisboa: Fundação Calouste Gulbenkian.
- Dadoo RC, Khatri HL, Singla S 1995. Comparative evaluation of gastric secretory response to banana and porridge. Indian J Med Sci 49: 5-8.
- Garros IC, Campos ACL, Tâmbara EM, Tenório SB, Torres OJM, Agulham MA, Araújo ACF, Santis-Isolan PMB, Oliveira RM, Arruda ECM 2006. Extrato de Passiflora edulis na cicatrização de feridas cutâneas em ratos: estudo morfológico e histológico. Acta Cir Bras 21: 55-65.
- Giovannelli L, Testa G, De Filippo C, Cheynier V, Clifford MN, Dolara P 2000. Effect of complex polyphenols and tannins from red wine on DNA oxidative damage of rat colon mucosa in vivo. Eur J Nutr 39: 207-212.
- Goel RK, Gupta S, Shankar R, Sanyal AK 1986. Anti-ulcerogenic effect of banana powder (Musa sapientum var. paradisiaca) and its effect on mucosal resistance. J Ethnopharmacol 18: 33-44.
- Gomes TAT, Campos LC, Trabulsi LR 1999. Diagnóstico bacteriológico. In: Trabulsi LR. Microbiologia. 3. ed. São Paulo: Atheneu, p. 131-141.
- Grice EA, Kong HH, Renaud G, Young AC, Bouffard GG, BlakesleyRW, Wolfsberg TG, Turner ML, Segre JA 2008. A diversity profile of the human skin microbiota. Genome Res 18: 1043-1050.
- Jesus SC, Folegatti MIS, Matsuura FCA, Cardoso RL 2004. Caracterização física e química de frutos de diferentes genótipos de bananeira. Bragantia 63: 315-323.
- Lewis DA, Fields WN, Shaw GP 1999. A natural flavonoid present in unripe plantain banana pulp (Musa sapientum L. var. paradisiaca) protects the gastric mucosa from aspirin-induced erosions. J Ethnopharmacol 65: 283-288.
- Lima RJC, Moreno AJD, Castro SFL, Gonçalves JRS, Oliveira AB, Sasaki JM, Freire PTC 2006. Taninos hidrolisáveis em Bixa orellana L. Quim Nova 29: 507-509.
- Mandelbaum SH, Di Santis EP, Mandelbaum MHS 2003. Cicatrização: conceitos atuais e recursos auxiliares-Parte I. An Bras Dermatol 78: 393-408.
- Marchionni AMT, Pagnoncolli RM, Reis SRA 2006. Influência do meloxicam e da dexametasona no processo inflamatório e no reparo tecidual. Rev Odonto Cienc 21: 22-29.
- Mohan Kumar M, Joshi MC, Prabha T, Dorababu M, Goel RK 2006. Effect of plantain banana on gastric ulceration in NIDDM rats: role of gastric mucosal glycoproteins, cell proliferation, antioxidants and free radicals. Indian J Exp Biol 44: 292-9.
- Novak FR, Almeida JAG, Silva RS 2003. Banana peel: a possible source of infection in the treatment of nipple fissures. J Pediatr 79: 221-226.
- Ono H, Tesaki S, Tanabe S, Watanabe M 1998. 6-Methylsulfinylhexyl isothiocyanate and its homologues as food-originated compounds with antibacterial activity against Escherichia coli and Staphylococcus aureus. Biosci Biotechnol Biochem 62: 363-365.
- Santos SC, Mello JCP 2002. Taninos. In Simões CMO (org.) Farmacognosia: da planta ao medicamento. 6 ed. Florianópolis: Editora da UFSC, p. 615-656.
- Pannangpetch P, Vuttivirojana A, Kularbkaew C, Tesana S, Kongyngyoes B, Kukongviriyapan V 2001. The antiulcerative effect of Thai Musa species in rats. Phytother Res 15: 407-410.
- Perchellet EM, Gali HU, Makkar HPS, Perchellet JP 1996. Ability of tannins extracted from the leaves of various trees and shrubs to inhibit the biomarkers of tumor promotion in mouse skin in vivo. Int J Oncol 9: 801-809.
- Robbers JE, Speedie MK, Tyler VE 1997. Farmacognosia e Farmacobiotecnologia. São Paulo: Editorial Premier.
- Von Loesecke HW 1950. Bananas. New York: Interscience Publishers.
Publication Dates
-
Publication in this collection
01 Apr 2011 -
Date of issue
June 2011
History
-
Received
20 Apr 2010 -
Accepted
05 Oct 2010