Abstracts
PURPOSE: Reactive oxygen species (ROS) inactivation was studied to determine alterations in the pancreatic capillary blood flow (PCBF) during caerulein-induced pancreatitis in rats. METHODS: A laser-Doppler flowmeter to measure PCBF and N-t-Butyl-Phenylnitrone (PBN) compound to inactivate ROS were used. Forty rats were divided in groups: 1) control; 2) caerulein; 3) PBN; 4) caerulein+PBN. Serum biochemistry and histopathological analyses were performed. RESULTS: PCBF measured a mean of 109.08 ± 14.54%, 68.24 ± 10.47%, 102.18 ± 10.23% and 87.73 ± 18.72% in groups 1, 2, 3 and 4, respectively. PCBF in groups 2 and 4 decreased 31.75 ± 16.79% and 12.26 ± 15.24%, respectively. Serum amylase was 1323.70 ± 239.10 U/l, 2184.60 ± 700.46 U/l, 1379.80 ± 265.72 U/l and 1622.10 ± 314.60 U/l in groups 1, 2, 3 and 4, respectively. There was a significant difference in the PCBF and serum amylase when compared groups 2 and 4. Cytoplasmatic vacuolation was present in groups 2 and 4. Otherwise, no qualitative changes were seen. CONCLUSION: ROS inactivation improves PCBF and minimizes the serum amylase increase during caerulein-induced pancreatitis. ROS effect may be one of the leading causative events in this model of acute pancreatitis.
Blood flow; Caerulein; Laser-Doppler; Oxygen radicals; Pancreatitis; Spin-trapping nitrone
OBJETIVO: A inativação de radicais livres (RL) foi estudada para determinar as alterações do fluxo capilar pancreático (FCP) na pancreatite aguda induzida por ceruleína em ratos. MÉTODOS: Um laser-Doppler fluxímetro determinou o FCP e o composto N-t-Butyl-Phenylnitrone (PBN), para inativar os RL, foi utilizado. Quarenta ratos foram divididos em 4 grupos: 1) controle; 2)ceruleína; 3) PBN; 4)ceruleína+PBN. Dosagens bioquímicas e análise histopatológica foram realizadas. RESULTADOS: O FCP foi em média 109.08 ± 14.54%, 68.24 ± 10.47%, 102.18 ± 10.23% e 87.73 ± 18.72% nos grupos 1, 2, 3 and 4, respectivamente. O FCP nos grupos 2 e 4 diminuíram em média 31.75 ± 16.79% e 12.26 ± 15.24%, respectivamente. A média da amilase sérica foi de 1323,70 ± 239.10 U/l, 2184,60 ± 700,46 U/l, 1379,80 ± 265,72 U/l e 1622,10 ± 314,60 U/l nos grupos 1, 2, 3 e 4, respectivamente. Observou-se diferença significante no FCP e na amilase sérica quando comparados os grupos 2 e 4. Vacuolização citoplasmática estava presente nos grupos 3 e 4. Não foram observadas outras alterações qualitativas. CONCLUSÃO: A inativação de RL melhorou o FCP e minimizou a elevação da amilase sérica na pancreatite aguda induzida por ceruleína. A presença de RL parece ser um evento precoce neste modelo de pancreatite aguda experimental.
Fluxo sanguíneo; Ceruleína; Laser-Doppler; Radicais de oxigênio
ARTIGO ORIGINAL
Reactive oxygen species inactivation improves pancreatic capillary blood flow in caerulein-induced pancreatitis in rats
A inativação de radicais livres melhora o fluxo capilar pancreático em pancreatite aguda induzida por ceruleína em ratos
Roberto Ferreira Meirelles Jr.I; Reginaldo CenevivaII; José Liberato Ferreira CabocloIII; Michael M. EisenbergIV
IPós-graduando da área de Clínica Cirúrgica do Departamento de Cirurgia e Anatomia da FMRP-USP e Prof. Assistente do Departamento de Cirurgia da FAMERP- S.P.
IIProfessor Titular do Departamento de Cirurgia e Anatomia da FMRP-USP
IIIProfessor Titular do Departamento de Cirurgia da FAMERP- S.P.
IVProfessor Adjunto do Departamento de Cirurgia da Cornell University Medical College, NY, EUA
Correspondence Correspondence to Dr. Roberto Ferreira Meirelles Junior Faculdade de Medicina de São José do Rio Preto, S.P. Departamento de Cirurgia Av. Brigadeiro Faria Lima, 5544 Cep; 15090-000 São José do Rio Preto, S.P. e-mail: rmeirelles.hbse@famerp.br
ABSTRACT
PURPOSE: Reactive oxygen species (ROS) inactivation was studied to determine alterations in the pancreatic capillary blood flow (PCBF) during caerulein-induced pancreatitis in rats.
METHODS: A laser-Doppler flowmeter to measure PCBF and N-t-Butyl-Phenylnitrone (PBN) compound to inactivate ROS were used. Forty rats were divided in groups: 1) control; 2) caerulein; 3) PBN; 4) caerulein+PBN. Serum biochemistry and histopathological analyses were performed.
RESULTS: PCBF measured a mean of 109.08 ± 14.54%, 68.24 ± 10.47%, 102.18 ± 10.23% and 87.73 ± 18.72% in groups 1, 2, 3 and 4, respectively. PCBF in groups 2 and 4 decreased 31.75 ± 16.79% and 12.26 ± 15.24%, respectively. Serum amylase was 1323.70 ± 239.10 U/l, 2184.60 ± 700.46 U/l, 1379.80 ± 265.72 U/l and 1622.10 ± 314.60 U/l in groups 1, 2, 3 and 4, respectively. There was a significant difference in the PCBF and serum amylase when compared groups 2 and 4. Cytoplasmatic vacuolation was present in groups 2 and 4. Otherwise, no qualitative changes were seen.
CONCLUSION: ROS inactivation improves PCBF and minimizes the serum amylase increase during caerulein-induced pancreatitis. ROS effect may be one of the leading causative events in this model of acute pancreatitis.
Key Words: Blood flow. Caerulein. Laser-Doppler. Oxygen radicals. Pancreatitis. Spin-trapping nitrone.
RESUMO
OBJETIVO: A inativação de radicais livres (RL) foi estudada para determinar as alterações do fluxo capilar pancreático (FCP) na pancreatite aguda induzida por ceruleína em ratos.
MÉTODOS: Um laser-Doppler fluxímetro determinou o FCP e o composto N-t-Butyl-Phenylnitrone (PBN), para inativar os RL, foi utilizado. Quarenta ratos foram divididos em 4 grupos: 1) controle; 2)ceruleína; 3) PBN; 4)ceruleína+PBN. Dosagens bioquímicas e análise histopatológica foram realizadas.
RESULTADOS: O FCP foi em média 109.08 ± 14.54%, 68.24 ± 10.47%, 102.18 ± 10.23% e 87.73 ± 18.72% nos grupos 1, 2, 3 and 4, respectivamente. O FCP nos grupos 2 e 4 diminuíram em média 31.75 ± 16.79% e 12.26 ± 15.24%, respectivamente. A média da amilase sérica foi de 1323,70 ± 239.10 U/l, 2184,60 ± 700,46 U/l, 1379,80 ± 265,72 U/l e 1622,10 ± 314,60 U/l nos grupos 1, 2, 3 e 4, respectivamente. Observou-se diferença significante no FCP e na amilase sérica quando comparados os grupos 2 e 4. Vacuolização citoplasmática estava presente nos grupos 3 e 4. Não foram observadas outras alterações qualitativas.
CONCLUSÃO: A inativação de RL melhorou o FCP e minimizou a elevação da amilase sérica na pancreatite aguda induzida por ceruleína. A presença de RL parece ser um evento precoce neste modelo de pancreatite aguda experimental.
Descritores: Fluxo sanguíneo. Ceruleína. Laser-Doppler. Radicais de oxigênio.
INTRODUCTION
The pathogenesis of septic shock, adult respiratory distress syndrome, acute renal failure, and participation in the ischemia-reperfusion organs like myocardial infarction, stroke, and organ transplantation seem to be involved with the production of reactive oxygen species (ROS)1,2,3. The increase in capillary permeability and vascular reactivity has been attributed to ROS generation4. The generation of ROS appears to play a central role in the pathogenesis5 of ischemic, alcoholic and gallstone pancreatitis animal models. In the caerulein-induced pancreatitis model, formation of ROS has also been reported 6.
Recently the N-tert-phenyl-buthyl-nitrone (PBN), a spin-trapping nitrone, has been used to determine the presence of ROS7. The ROS effects can be analyzed when spin-trapping nitrone is employed once the nitrone compound reacts covalently with ROS creating a relatively stable radical 8.
The purpose of this experiment was to study pancreatic capillary blood flow (PCBF) changes, using a laser-Doppler flowmeter, to determine whether ROS inactivation by a spin-trapping nitrone (PBN) changes the PCBF during caerulein-induced pancreatitis.
METHODS
Surgical preparation: Forty Sprague-Dawley male rats weighing between 290 and 448 g were used. All rats were starved for 18 hours prior to the experiment, except for water ad libitum. A single subcutaneous injection of 25% urethan anesthetic (1.75 g of urethane/ 1000 g body weight; Urethane, Sigma, St. Louis, MO) was used. The body temperature during the experiment was kept between 36.4 -36.6 C using a thermo controller (made by Béla Kurucz, E.E., Maglód, Hungary). An arterial and venous line was obtained via the right iliac artery and left iliac vein that were isolated and cannulated with heparinized PE-50 polyethylene tubing. The abdominal wall was opened by a mid-line incision extending from the xiphoid to the suprapubic region. The pancreas was isolated and two gauze sponges were placed between the posterior abdominal wall and the pancreas. The laser-Doppler probe was placed on the anterior surface of the body of the pancreas.
Measurement of PCBF, Blood pressure (BP) and heart rate (HR):PCBF
measurement was performed with a laser-Doppler Capillary Perfusion Monitor (model LD- 6000, Medpacific Corp., Seattle, W A)9. The laser-Doppler flowmeter was connected to a computer (IBM PS/2 model 50Z, Armonk, NY) equipped with an appropriate software package (LD-6000 Data Collection; written by Howard Amols, Ph.D., Columbia University, New York, NY) that collected, recorded and stored six data points per second of PCBF.
BP and HR were monitored throughout the experiment (Weco VT -1, Winston Electronics Co., Millbrae, CA) via the right iliac artery.
After a 20 minutes stability period, the baseline of the PCBF, BP and HR was determined during the next 10 minutes; the means were considered 100%. PCBF was measured continuously over 120 minutes with recordings of the mean and standard deviation taken every 5 minutes. BP and HR were recorded every 5 minutes throughout the experiment.
Pancreatitis model and spin-trapping nitrone solution: Acute pancreatitis was induced using 5 X 10-6 g/ 1000 g body weight/ h of caerulein (Sigma, St. Louis, MO) i.v. infusion10. This infusion began immediately after the baseline measurements.
The PBN (Sigma, St. Louis, MO) compound was used in a dose of 150 mg/ 1000 g body weight. Special precautions were taken during handling the PBN, since it is inactivated by light and air. The PBN was diluted in dimethylsulfate (DMS; Sigma, St. Louis, MO).
Experimental protocol: The animals were divided in four groups of ten. All groups received 0.9% sodium chloride intravenously (0.083 ml/ 1000 g body weight/ min.; Syringe infusion Pump 22, Harvard Apparatus, South Natick, MA) to compensate for insensible losses. This infusion began when the laser-Doppler probe was placed on the pancreas.
Group 1: Animals in the control group received DMS 20 minutes before baseline and 0.9% sodium chloride i.v. after baseline.
Group 2: Animals in the caerulein-induced pancreatitis received DMS 20 minutes before baseline and caerulein solution after baseline.
Group 3: Animals in the PBN group received PBN solution ip 20 minutes before baseline and 0.9% sodium chloride i.v. after baseline.
Group 4: Animals in the caerulein-induced pancreatitis plus PBN group received PBN solution i.p. 20 minutes before baseline and caerulein solution after baseline.
Blood collection, biopsy and analysis: At the end of the experiment arterial blood samples were taken to determine gases (288 Blood Gas System, Ciba-Corning Diagnostics Corp., Medfield, MA) at the Blood Gas Laboratory, Lenox Hill Hospital, New York, NY. Venous blood samples were taken to determine serum amylase, glucose, calcium, sodium, potassium and chloride (Kodak Ektachem 700 XR Analyzer, Eastman Kodak, Rochester, NY) at Lenox Hill Hospital Laboratory, New York, NY.
Pancreatic biopsies were taken from the pancreatic tissue underlying the laser-Doppler probe.
Histophatological analysis: The pancreatic biopsies from forty rats were fixed in Bouin's solution, paraffin embedded and sectioned at 4 microns and then stained with hematoxilin phloxin safran stain. The slides were examined randomly and blindly by two pathologists using a Optiphot Labpot Nikon microscope (Yokohama, Japan). The slides were screened for vacuolation, piknosis and ballooning degeneration. The vacuolation was characterized by the presence of micro and macrovacuolation of the cytoplasm that was normal in color and the granules were distinct. Piknosis and ballooning degeneration was characterized by small foci of piknosis of the nuclei and distention of the cytoplasm becoming pale pink in color with loss of granules.
Statistical analysis: Statistical analysis was performed using PC Statistical Software , (Human Systems Dynamics, Northridge, CA). The results are described as the mean ± standard deviation. Student's t-test was employed to make comparison between group means. P values less than 0.05 were considered significant. All PCBF, BP and HR results were expressed in percentage.
RESULTS
The PCBF measured a mean of 109.08 ± 14.54%, 68.24 ± 10.47%, 102.18 ± 10.23% and 87.73 ± 18.72% in groups 1, 2, 3 and 4, respectively. The PCBF measurement did not change significantly (p>0.05) in groups 1 and 3 throughout the experiment. The PCBF decreased over time a mean of 31.75 ± 16.79% and 12.26 ± 15.24% in groups 2 and 4, respectively. These PCBF decreases were statistically (p<0.05) significant after 20 minutes following baseline for group 2 when compared with group 1. The PCBF increased significantly (p<0.05) in group 4 compared with group 3 during the first 20 minutes following baseline, and there was no statistical (p>0.05) difference until 60 minutes when a significant (p<0.05) decrease was seen. There was a significant (p<0.05) increase in the PCBF when compared groups 2 and 4, up to 105 minutes following baseline. No statistical (p>0.05) difference was seen in the PCBF between groups 1 and 3. (Fig.1).
The BP measured a mean of 93.11 ± 9.85%, 108.81 ± 16.43%, 94.37 ± 12.19%, 97.78 ± 17.86% in groups 1, 2, 3 and 4, respectively. The BP increased (p<0.05) throughout the experiment only in group 2 and it was significant (p<0.05) when compared with group 1.
The HR measured a mean of 102.88 ± 14.80%, 121.42 ± 12.26%, 106.88 ± 10.28%, 100.66 ± 11.24% in groups 1, 2, 3 and 4, respectively. The HR increased significantly (p<0.05) throughout the experiment only in group 2 and it was significant (p<0.05) when compared with groups 1 and 4.
The serum amylase was 1323.70 ± 239.10 U/l, 2184.60 ± 700.46 U/l, 1379.80 ± 265.72 U/l and 1622.10 ± 314.60 U/l in groups 1, 2, 3 and 4 respectively. There was significant increase (p<0.01) when comparing groups 1 and 3 with groups 2 and 4, respectively. There was a significant (p<0.05) decrease in the serum amylase when compared groups 2 and 4 (Table I).
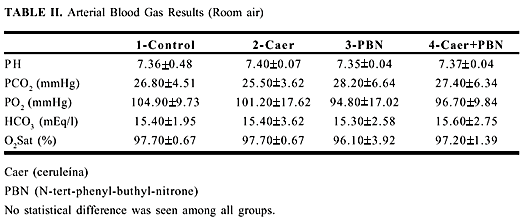
The serum glucose, sodium, potassium and chloride results are summarized table I. Results of arterial gases are summarized in table II.
No statistical difference was seen among all groups.
The histopathological study reviewed no qualitative changes in 52.50% of all slides. Vacuolation of the cytoplasm in the acinar cells was found in 3 and 7 slides in groups 2 and 4, respectively. These lesions were isolated in some cases, and multifocal in others. Small foci of piknosis and ballooning degenaration were seen in 6, 1, 3 and 1 slide(s) in groups 1, 2, 3 and 4, respectively. No qualitative difference was seen among groups 1 and 2 when compared with groups 3 and 4, respectively.
DISCUSSION
Overall, experimental11 and clinical12 studies have found that during acute pancreatitis there is a decrease in the blood flow to the pancreas. In our experiment, the PCBF decreased significantly a mean of 31% after 20 minutes of caerulein infusion. It leads us to assume that the PCBF impairment may allow the pancreas to become subject and susceptible to ischemia in this model of pancreatitis. Whether ischemia is a cause or an effect during the course of acute pancreatitis is still controversial. Nevertheless, the important relationship between pancreatic blood flow and the complications following acute pancreatitis should not be underestimated12. Ischemia seems to play a key role in the transition from pancreatic edema to necrosis and improvement of capillary perfusion has been shown to be an efficient therapeutic tool13,14. Ischemia can serve as an important co-factor to potentiate pancreatitis and convert an incipient insult to the pancreas into a frank pancreatitis15. During caerulein-induced pancreatitis, sympathetic excitation induced by water-immersion precipitated hemorrhagic pancreatItIs16 and phenylephrine exacerbated the acute pancreatitis17. Moreover, ischemia has been related with ROS generation18.
The term ROS defines independent chemical species with one or more unpaired electrons19. The unpaired electron determines the instability and reactivity of this species. The reaction of a radical with another molecule forms a new radical leading to a perpetuating chain process. The ROS reactions affect proteins, lipids and nucleic acids20. These reactions are influenced by presence and concentration of oxygen, availability of transition metals, level of reductants and antioxidants21. When a radical process spreads within a cell, low molecular weight antioxidants (primary damage defense) may interfere with the chain reaction by donating H-atoms to radicals. This results in "reconstitution" of the original radical site. After it becomes a radical itself, the antioxidant may be stable enough to slow the chain process down. So it can await being "healed" by H-atoms derived from metabolism. Vitamin C and E, gluthatione (GSH) and H-atoms from NAD(P)H may be involved in the primary damage control22. A secondary damage defense is put forth by GSH-peroxidase, catalase, superoxide dismutase, DT -diaphorase and/or chelators. The final step of defense involves repair processes through lipid degradation/membrane repair enzymes (phospholipases, peroxidases, some transferases and reductases), protein disposal or repair enzymes (proteases, GSSG-reductase) and DNA degradation repair enzymes (exonuclease III, endonucleases III and IV, glycosylases, polymerases). The principal sources of ROS in vivo are phagocytes, mitochondrial electron transport system, microsomal electron transport system, solubleoxidase enzymes, autooxidation of endogenous or exogenous substrates and transition metals20. Potentially all aerobic cells are capable of producing ROS. ROS have been implicated in the pathogenesis of many conditions23 such as septic shock, adult respiratory distress syndrome and acute renal failure. Participation in the ischemia-reperfusion organs like myocardial infarction, stroke and organ transplantation seems to be involved with free-radical production1,2,3. During ischemic, alcoholic and gallstone pancreatitis animal models, the generation of ROS appears to play a central role in its pathogenesis5. In the caerulein-induced pancreatitis model, formation of ROS has also been reported6.
The N-tert-phenyl-buthyl-nitrone (PBN), a spin-trapping nitrone, was used to determine the presence of ROS during caerulein-induced pancreatitis. A spin-trapping nitrone is a compound that reacts covalently with ROS creating relatively stable radicals8. The PBN bioavaibility in vivo was demonstrated in various mouse organs7 and the compound arrives at the sites of ROS generation in a short period of time4. No signs of behavioral, parenchymal or local damage were seen during PBN administration over a 7-day period in rats24.
The PCBF improved during the first 105 minutes following the baseline in group 4 compared with group 2. The overall improvement was a mean of 28.56% in the PCBF when a single PBN dose was added to the caerulein-induced pancreatitis model. Furthermore, the suppression of ROS shows that there was an increase in the PCBF in the first 20 minutes of caerulein infusion when compared group 3 and 4. Also the serum amylase increase in group 2 was statistically reduced in the presence of the PBN. These findings are in accord with reports of gluthatione depletion25, decrease of superoxide dismutase activity in the pancreatic tissue and elevation malondialdehyde concentration26 (a by-product of lipid peroxidation) seen during caerulein-induced pancreatitis. During the effort to produce and secret pancreatic enzymes by caerulein hyperstimulation, a disturb in the oxidant-antioxidant balance may occur in the pancreatic cell. Since the presence of polymorfonucleares were not seen in our pathological findings, the mitochondrial system and the microsomal electron transport system are most likely be involved in the ROS generation. The result is a leaking of ROS from these sites leading to lipid peroxidation and consequent membrane cell damage. This hypothesis agrees with marked changes in the Golgi complex and mitochondrias described during caerulein-induced pancreatitis10. The increase in capillary permeability and vascular reactivity has been attributed to ROS generation4,27. Similar morphological alterations were found in the hepatic sinusoids28,29 suggesting that caerulein-induced pancreatitis is associated with extrapancreatic microvascular damage. In addition, acute lung injury30 and hepatic impairment31 associated with caerulein-induced pancreatitis appears in part to be mediated by ROS. Therefore, the edema formation and PCBF decrease, at least in part, may be attributed to ROS generation.
In conclusion, our findings suggest that ROS generation has a early onset and may be related with PCBF decrease during caerulein-induced pancreatitis model. The ROS inactivation by PBN improves PCBF and minimizes the serum amylase increase during caerulein-induced pancreatitis. The ROS inactivation may be helpful both in improving the PCBF and decreasing the local and systemic complication following caerulein-induced acute pancreatitis.
- 1. Cross CE, Halliwell B, Borish ET, et al. Oxygen radicals and human disease. Ann Intern Med 1987; 107: 526-45.
- 2. Zimmeflllann JJ. Oxyradical species and their relationship to pathophysiology in pediatric critical care illness. Crit Care Clin 1988; 4: 645-60.
- 3. Sznajder JI, Fraiman A, Hall JB, Sanders W, et al. Increased hydrogen peroxide in the expired breath of patients with acute hypoxemic respiratory failure. Chest 1989; 96: 606-12.
- 4. McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 1989; 312: 159-63.
- 5. Sanfey H, Sarr MG, Bulkley, Cameron JL. Oxygen-derived free radicals and acute pancreatitis: a review. Acta Physiol Scand 1986, 548: 109-18.
- 6. Niederau C, Niederau M, Borchard F, et al. Effects of antioxidants and free radicals scavengers in three different models of acute pancreatitis. Pancreas 1992; 7(4): 486-96.
- 7. Novelli PG. Oxygen radicals in experimental shock: effects of spin-trapping nitrones in ameliorating shock pathophysiology. Crit Care Med 1992; 20(4): 499-507.
- 8. Finkelstein E, Rosen GM, Rauckman EJ. Spin trapping of superoxide and hydroxyl radical: Practical aspects. Arch Biochem Biophys 1980; 200: 1-16.
- 9. Dib JA, Cooper-Vastola SA, Meirelles Jr. RF, et al. Acute effects of ethanol and ethanol plus furosemide on pancreatic capillary blood flow in rats. Am J Surg 1993; 166: 18-23.
- 10. Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Path Anat and Histol 1977; 373: 97 -117.
- 11. Klar E, Endrich B, Messmer K. Microcirculation of the pancreas. A quantitative study of physiology and changes in pancreatitis. Int J Microcir: Clin Exp 1990; 9: 85-101.
- 12. Clavien P-A, Hauser H, Meyer P, Rohner A. Value of contrast-enhanced computerized tomography in the early diagnosis and prognosis of acute pancreatitis. A prospective study of 202 patients. Am J Surg 1988; 155: 457-66.
- 13. Klar E. Etiology and pathogenesis of acute pancreatitis. Helv Chir Acta 1992; 59(1): 7-16.
- 14. Klar E, Messmer K, Warshaw AL, Herfarth C. Pancreatic ischemia in experimental acute pancreatitis: mechanism, significance and therapy. Br J Surg 1990; 77(11): 1205-10.
- 15. Prinz RA. Mechanisms of acute pancreatitis. Intern Journal of Pancreatology 1991; 9: 31-8.
- 16. Yamaguchi H, Kimura T, Nawata H. Does stress play a role in the development of severe pancreatitis in rats? Gastroenterology 1990; 98(6): 682-8.
- 17. Klar E, Rattner DW, Compton C, Stanford G, Chemow B, Warshaw AL. Adverse effect of therapeutic vasoconstrictors in experimental acute pancreatitis. Annals of Surg 1991; 214(2): 168-74.
- 18. Halliwell B, Gutteridge JMC. Role of free radicals and catalytic metal ions in human disease: an overview. Meth Enzym 1990; 186: 1-85.
- 19. Keher JP. Free radicals as mediators of tissue injury and disease. Crit Rev T oxicol 1993 ; 23(1): 21-48.
- 20. Dianzani MU. Free radicals in physiology and pathology. Boll Soc It Biol Sper 1992; 68(8-9):491-511.
- 21. VanLente F. Free radicals. Analyt Chem 1993; 65(12): 374R-77R.
- 22. Niki E, Yamamoto K, Takahashi M. Role of iron, ascorbic acid and tocopherol in the oxidation of lipids. In: Ando W, Morooka Y, eds. The role of oxygen in chemistry and biology. Studies in organic chemistry. Elsevier, Amsterdam 1988; 509-14.
- 23. Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med 1992; 119(6): 598-620.
- 24. Novelli GP, Angiolini P, Tani R, Consales G, Bordi L. Phenyl-t-butyl-nitrone is active against traumatic shock in rats. Free Rad Res Comms 1985; 1(5): 321-27.
- 25. Neuschwander-Tetri BA, Ferrell LD, Sukhabote RJ, Grendell JH. Glutathione monoethyl ester ameliorates caerulein-induced pancreatitis in the mouse. J Clin Invest 1992; 89: 109-16.
- 26. Dabrowski A, Gabryelewicz A, Wereszcynska-Siemiatkowska U, Chyczewski L. Oxygen- derived free radicals in cerulein-induced acute pancreatitis. Scand J Gastroenterol 1988; 23: 1245-9.
- 27. Del Maestro RF, Bj6rk J, Arfors KE. Increase in microvascular permeability induced by enzymatically generated free radicals. Microvascular Research 1981; 22: 255-70.
- 28. Kelly MD, McEntee GP, Mcgeeney KF, Fitzpatrick JM. Microvasculature of the pancreas, liver, and kidney in cerulein-induced pancreatitis. Arch Surg 1993; 128: 293-95.
- 29. Delaney C, McEntee G, Cotell D, Mcgeeney K, Fitzpatrick JM. The effects of caerulein-induced pancreatitis on the hepatic microvasculature. Br Jour Surg 1990; 77(3): 294-6.
- 30. Guice KS, Oldham KT, Caty MG, Johnson KJ, Ward P A. Neutrophil-dependent, Oxigen- radical mediated lung injury associated with acute pancreatitis. Ann Surg 1989; 210(6): 740-47.
- 31. Dabrowski A, Andrzejewska A. Role of oxygen radicals in hepatocellular impairment in cerulein-induced acute pancreatitis. Materia Medica Polona 1992; 3(83): 147-50.
Publication Dates
-
Publication in this collection
29 Apr 2004 -
Date of issue
2003