Abstracts
PURPOSE: To evaluate the development of Walker 256 tumor in male Wistar rats treated with tacrolimus using an experimental kidney tumor model. METHODS: 40 male Wistar rats were divided into four groups: Tumor group (TU) (n=10), Tacrolimus-Tumor group (TT) (n=10), Tacrolimus group (TC) (n=10) and Control group (C) (n=10). Treatment with tacrolimus was performed in groups TT and TC. Under anesthesia, the right kidney of each animal of TU and TT was accessed through a supraumbilical incision and inoculated with a 0.1mL solution containing 2x10(6) tumor cells (Walker 256 carcinosarcoma tumor cells). Group TC was treated with a saline solution. All the animals of groups TC and TT were treated with tacrolimus (5mg/kg/day) by gavage for 15 days. TU group animals received saline by gavage for 15 days. On the 15th postoperative day, all animals were submitted to euthanasia and blood sampling for analysis of serum creatinine (Cr) and blood urea nitrogen (BUN). Abdominal gross examination was performed, the right kidney removed and prepared for histological analysis by hematoxylin-eosin staining. The resulting data were submitted to statistical analysis by ANOVA. RESULTS: Statistical significance was found when comparing creatinine level between groups TU, TT and TC -TT group culminated with a marked increased in creatinine levels (Cr=1.013 ± 0.3028 mg/mL), TU group (Cr=0.5670 ± 0.03536 mg/dL) P=0.00256, TC group (Cr =0.711 ± 0.1653 mg/mL) P= 0.02832. Statistical significance was found when comparing BUN levels in TT group (71.32 ± 17.14 mg/mL), compared with TU group (45.83 ± 5.046 mg/dL), P=0.000318. There were no statistically significant differences between groups TT and TC (61.23 ± 9.503 mg/mL) P=0.7242. Histological analysis showed a poor evolution in TT group with multiple foci of hemorrhage and cortical invasion by the Walker tumor. CONCLUSION: The Tacrolimus-treated group developed a more aggressive tumor and a drug-related nephrotoxic effect.
Carcinoma 256, Walker; Tacrolimus; Tacrolimus Binding Proteins; Rats
OBJETIVO: Avaliar as alterações na evolução do carcinosarcoma 256 de Walker, inoculado no rim de ratos Wistar, sob tratamento imunossupressor com o tacrolimus. MÉTODOS: Foram utilizados 40 ratos Wistar, machos divididos em quatro grupos de 10: grupo Tumor (TU), Tumor-Tacrolimus (TT), Tacrolimus (TC) e Controle (C). Os ratos dos grupos TU e TT foram inoculados com 0,1 mL de solução contendo 2x10(6) células do tumor de Walker no parênquima do rim direito. Os dos grupos TC e TT receberam tratamento com tacrolimus na dose de 5mg/kg de peso, via gavagem orogástrica durante 15 dias. Os ratos do grupo TU receberam solução salina isotônica pH 7,2. Ao 15º dia de evolução, todos foram submetidos à eutanásia. Amostras de sangue eram coletadas para dosagem de creatinina (Cr) e uréia (Ur) e posteriormente realizada nefrectomia para avaliação histológica. RESULTADOS: As dosagens séricas de creatinina foram maiores no grupo TT (Cr = 1,013±0,3028 mg/mL), que diferiu significantemente dos grupos TU (Cr=0,5670 ± 0,03536 mg/dL) com p=0,00256 e do TC (Cr=0,711 ± 0,1653 mg/mL) com p=0,02832. As dosagens séricas de uréia foram maiores no grupo TT (71,32 ± 17,14 mg/mL), que diferiu significantemente do grupo TU (45,83 ± 5,046mg/dL) com p=0,000318, mas comparado ao grupo TC (61,23 ± 9,503 mg/mL) não houve diferença significante (p=0,7242). No inventário da cavidade abdominal dos grupos TU e TT, observou-se presença macroscópica de tumor em todos os rins direitos; não foram evidenciadas efusões ascíticas, formação de bridas ou metástases tumorais em outros órgãos ou tecidos adjacentes aos rins direitos. CONCLUSÃO: O tacrolimus exerceu efeito nefrotóxico e induziu exacerbação do crescimento do tumor de Walker 256, quando implantado no rim de ratos Wistar.
Carcinoma 256 de Walker; Tacrolimo; Proteínas de Ligação a Tacrolimo; Ratos
19 - ORIGINAL ARTICLE
EFFECT OF DRUGS
Action of tacrolimus on Wistar rat kidneys implanted with Walker 256 carcinosarcoma1 1 Research performed at Research Medical Institute, University Evangelic Hospital/ Evangelic Parana Faculty, Curitiba-PR, Brazil.
Estudo da ação do tacrolimus em rins de ratos Wistar implantados com carcinossarcoma de Walker
Cristiano Machado InácioI; Ulrich Andréas DietzII; Osvaldo MalafaiaIII; Jurandir Marcondes Ribas FilhoII; Paulo Afonso Nunes NassifII; Nicolau Gregori CzeczkoII; Carmen Australia Paredes MarcondesII
IMaster in Sciences, Principles of Surgery Post-Graduation Program, University Evangelic Hospital/Evangelic Faculty of Parana, Curitiba-PR, Brazil
IIPhD, Associate Professor, University Evangelic Hospital/Evangelic Faculty of Parana, Curitiba-PR, Brazil
IIICoordinator of Principles of Surgery, Post-Graduation Program and Full Professor of Surgery, University Evangelic Hospital/Evangelic Faculty of Parana, Curitiba-PR, Brazil
Correspondence Correspondence: Cristiano Machado Inácio Al. Augusto Stellfeld, 1980 80730-150 Curitiba - PR Brazil Phone: (55 41)3240-5488 ipem@evangelico.org.br
ABSTRACT
PURPOSE: To evaluate the development of Walker 256 tumor in male Wistar rats treated with tacrolimus using an experimental kidney tumor model.
METHODS: 40 male Wistar rats were divided into four groups: Tumor group (TU) (n=10), Tacrolimus-Tumor group (TT) (n=10), Tacrolimus group (TC) (n=10) and Control group (C) (n=10). Treatment with tacrolimus was performed in groups TT and TC. Under anesthesia, the right kidney of each animal of TU and TT was accessed through a supraumbilical incision and inoculated with a 0.1mL solution containing 2x106 tumor cells (Walker 256 carcinosarcoma tumor cells). Group TC was treated with a saline solution. All the animals of groups TC and TT were treated with tacrolimus (5mg/kg/day) by gavage for 15 days. TU group animals received saline by gavage for 15 days. On the 15th postoperative day, all animals were submitted to euthanasia and blood sampling for analysis of serum creatinine (Cr) and blood urea nitrogen (BUN). Abdominal gross examination was performed, the right kidney removed and prepared for histological analysis by hematoxylin-eosin staining. The resulting data were submitted to statistical analysis by ANOVA.
RESULTS: Statistical significance was found when comparing creatinine level between groups TU, TT and TC -TT group culminated with a marked increased in creatinine levels (Cr=1.013 ± 0.3028 mg/mL), TU group (Cr=0.5670 ± 0.03536 mg/dL) P=0.00256, TC group (Cr =0.711 ± 0.1653 mg/mL) P= 0.02832. Statistical significance was found when comparing BUN levels in TT group (71.32 ± 17.14 mg/mL), compared with TU group (45.83 ± 5.046 mg/dL), P=0.000318. There were no statistically significant differences between groups TT and TC (61.23 ± 9.503 mg/mL) P=0.7242. Histological analysis showed a poor evolution in TT group with multiple foci of hemorrhage and cortical invasion by the Walker tumor.
CONCLUSION: The Tacrolimus-treated group developed a more aggressive tumor and a drug-related nephrotoxic effect.
Key words: Carcinoma 256, Walker. Tacrolimus. Tacrolimus Binding Proteins. Rats.
RESUMO
OBJETIVO: Avaliar as alterações na evolução do carcinosarcoma 256 de Walker, inoculado no rim de ratos Wistar, sob tratamento imunossupressor com o tacrolimus.
MÉTODOS: Foram utilizados 40 ratos Wistar, machos divididos em quatro grupos de 10: grupo Tumor (TU), Tumor-Tacrolimus (TT), Tacrolimus (TC) e Controle (C). Os ratos dos grupos TU e TT foram inoculados com 0,1 mL de solução contendo 2x106 células do tumor de Walker no parênquima do rim direito. Os dos grupos TC e TT receberam tratamento com tacrolimus na dose de 5mg/kg de peso, via gavagem orogástrica durante 15 dias. Os ratos do grupo TU receberam solução salina isotônica pH 7,2. Ao 15º dia de evolução, todos foram submetidos à eutanásia. Amostras de sangue eram coletadas para dosagem de creatinina (Cr) e uréia (Ur) e posteriormente realizada nefrectomia para avaliação histológica.
RESULTADOS: As dosagens séricas de creatinina foram maiores no grupo TT (Cr = 1,013±0,3028 mg/mL), que diferiu significantemente dos grupos TU (Cr=0,5670 ± 0,03536 mg/dL) com p=0,00256 e do TC (Cr=0,711 ± 0,1653 mg/mL) com p=0,02832. As dosagens séricas de uréia foram maiores no grupo TT (71,32 ± 17,14 mg/mL), que diferiu significantemente do grupo TU (45,83 ± 5,046mg/dL) com p=0,000318, mas comparado ao grupo TC (61,23 ± 9,503 mg/mL) não houve diferença significante (p=0,7242). No inventário da cavidade abdominal dos grupos TU e TT, observou-se presença macroscópica de tumor em todos os rins direitos; não foram evidenciadas efusões ascíticas, formação de bridas ou metástases tumorais em outros órgãos ou tecidos adjacentes aos rins direitos.
CONCLUSÃO: O tacrolimus exerceu efeito nefrotóxico e induziu exacerbação do crescimento do tumor de Walker 256, quando implantado no rim de ratos Wistar.
Descritores: Carcinoma 256 de Walker. Tacrolimo. Proteínas de Ligação a Tacrolimo. Ratos.
Introduction
Malignancies are the third cause of death in renal transplant recipients and account for 12% of deaths, surpassed only by cardiovascular and infectious causes1. Those malignancies are related to the recipient's more advanced age, use of immunosuppressants and viral infections.
During the Second World War, it was found that transplants involving genetically similar tissues or organs could lead to rejection, especially when skin grafts were used.
The number of renal transplants in Brazil went from 3,332 cases in 2004 to 3,362 procedures in 2005. Today, Brazil is the third country in the world in absolute numbers, behind the United States and China only. The rise in immunosuppressive drug use to prevent rejection from the recipient organism is currently a fact. The most frequently used drugs for this purpose are tacrolimus - also known as FK 506 - and mycophenolate mofetil.
The occurrence of malignancies in transplant recipients is high and often implies a somber prognosis. In transplanted patients, kidney tumors occur more frequently than the expected rate for this type of tumor2. Surgical treatment is restricted due to the risk of graft loss. The recommendation of immunosuppressive drug withdrawal in the event of kidney tumor in the transplanted graft is not always feasible, since the progression to kidney failure is rapid with the withdrawal of the drugs1,3,4.
New lines of research involving drugs that act on the immune system have been proposed in the literature for tumor control in a situation of immunosuppression. The drug tacrolimus has been targeted in numerous clinical and experimental trials due to its immunosuppressive properties. However, its action in the presence of tumors is scarcely known4.
Kidney implantation of a Walker tumor (carcinosarcoma 256) has a rapid and aggressive course, and is the experimental model of choice to simulate tumors and in the evaluation of the effect of immunomodulating drugs. The use of Walker tumors in studies related to tumor biology is extensive, and the tumor can be inoculated in several types of tissue5. Kidney implantation is used in research involving immunosuppression and kidney transplants. Such procedure carries a high level of accuracy for the evaluation of immunomodulating drugs and their action on tumor development6.
The objective of this study was to evaluate, through histological and renal function assessment, the course of Walker 256 carcinosarcomas inoculated in the right kidney of Wistar rats under immunosuppressive treatment with tacrolimus.
Methods
This study was conducted at the Institute for Medical Research of the University Evangelic Hospital/ Evangelic Parana Faculty as part of the stricto sensu activities of the Graduate Program in Principles of Surgery, and was approved by the Research Ethics Committee of the Sociedade Evangélica Beneficente of Curitiba, Brazil.
Forty Wistar rats aged between 120 and 140 days, weighing 265.34 ± 23.73 g, were used. The rats were divided into four groups of five animals each and identified with picric acid (Chart 1).
The rats were kept in a specific environment with controlled temperature and humidity and automatically regulated 12-hour light/dark cycles, and were fed a specific ration for the species (Nuvilab®, Nuvital) as well as water ad libitum.
Experimental design
a) Assessments
The following assessments were undertaken: 1) serum creatinine and urea in all rats of groups TC, TT, TU and C on day 15; 2) tacrolimus levels in group TT; 3) histological evaluation of both kidneys of each rat in the experimental groups, and one kidney of each control group rat on the 15th day of evolution.
b) Implantation of Walker 256 tumor cells and maintenance of the tumor line
Rats intraperitoneally injected with the Walker 256 tumor type 2 were provided by Professor Rui Curi, ICB - USP, São Paulo, Brazil.
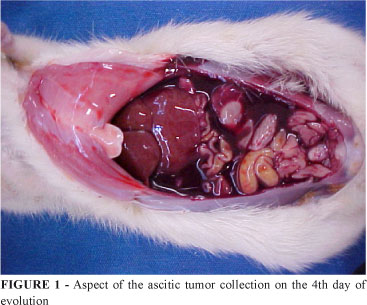
Every four days, the ascitic effusion produced by the tumor was collected through an extensive laparotomy and aspiration by syringe and needle. Subsequently, the animal underwent lethal ether inhalation. The rat's waste and all the material used in the procedure were disposed of as laboratory waste of high biological hazard and immediately destroyed by incineration.
The sample obtained was submitted to tumor cell count and diluted so as to contain 2x107 cells/mL of the Walker 256 tumor, and 1 mL was injected into another animal. After four days, this procedure was repeated, thus maintaining the cell line identified as a "tumor seed" (Figure 1).
c) Obtaining the inoculum for the experiment
On the day of tumor implantation, the rat bearing the "tumor seed" was anesthetized as previously described and 10 mL isotonic saline, pH 7.2, were injected into the abdominal cavity. Subsequently, the abdomen was opened and the fluid extracted through a Pasteur pipette. A 2-mL aliquot was taken for bacterial and fungal sterility testing.
Subsequently, cell count was carried out in a Neubauer counting chamber (Figure 2), and 0.1 mL of that suspension, containing 3x105 cells/mL, was injected into the right kidney of the rats which were the subjects of this study6.
d) Inoculation of Walker tumor 256 in the kidney of rats
The inoculated rats (groups TU, TT) were separated on the previous day and fur-marked with picric acid. They were weighed and submitted to extensive abdominal shaving. On the following day, they were anesthetized with 10% chloral hydrate at the dose of 300 mg/kg intraperitoneally.
Subsequently, sterile drapes were placed and the abdominal cavity was opened (a median laparotomy of approximately 3 cm). The bowel loops were pushed aside, and thus the right kidney was located - where 0.1mL of the tumor suspension described above was inoculated.
Inoculation was standardized with a 1-mL sterilized syringe and hypodermic needle with a piece of latex in order to restrain the trajectory and direction of inoculation so as to be done always in the medullary region of the lower pole of the kidney. The inoculation of 2x106 tumor cells was then carried out, in a slow fashion to prevent intra-abdominal extravasation of the inoculum (Figure 3). The contralateral kidney, by the same procedure, was injected with only 0.1mL of the medium of the tumor cell suspension and isotonic saline, pH 7.2.
The procedure was similar for group TC, yet no inoculation of tumor suspension was conducted; both kidneys were inoculated with 0.1 mL isotonic saline, pH 7.2.
After the inoculations, the bowel loops were replaced and the surgical wound was closed on two planes with continuous 3-0 mononylon suture.
The rats were then caged in a controlled environment as previously described and mantained there for 15 days. The following clinical parameters were recorded daily on a protocol: piloerection, occurrence of blepharitis, the animal's deambulation pattern in its box, presence of feces and weighing on an electronic scale every five days.
e) Tacrolimus treatment
The product was used as oral FK 506 (Tacrolimus). The dose administered was 5 mg/kg of body weight via orogastric gavage7,8.
Specimen collection and laboratory tests
On the 15th day of evolution, all rats in the study underwent anesthesia with 10% chloral hydrate and intracardiac puncture was performed for blood sampling and euthanasia.
Following confirmation of death, extensive opening of the abdominal cavity was undertaken, as well as an inventory and resection of the kidneys, which were subsequently immersed in bottles with formalin and identified.
In the inventory, the aim was to note the tumor take rate, i.e., the percentage of tumors induced, the presence of ascitic effusion, adhesion formation or tumor metastases. For the control group, blood and kidney sampling followed the same methodology.
Serum urea and creatinine levels were measured in a Cobas Mira automated biochemical equipment - COBAS System - MIRA "S", with specific reagents and standards.
Monitoring tacrolimus levels
The IMx tacrolimus II assay (Abbot csc 0800-11-90-99)9, based on the microparticle enzyme immunoassay methodology (MEIA), was used in this study.
Prior to the initiation of the automated IMx sequence, a manual pretreatment step was performed, in which the whole blood specimen was extracted with a precipitation reagent and centrifuged. The supernatant was decanted to the sample well and IMx tacrolimus II reagents, along with the sample, were added to the reaction vessel (RV). The probe/electrode assembly dispensed the microparticles coated with anti-tacrolimus murine monoclonal antibodies and the tacrolimus-alkaline phosphatase conjugate into the incubation well of the reaction vessel. Tacrolimus and the conjugate competed to bind to the antibody-coated microparticles, composing an "antibody-antigen" complex and an "antibody-antigen-alkaline phosphatase" complex. An aliquot containing the "antibody-antigen" and "antibody-antigen-alkaline phosphatase" complexes bound to the microparticles was transferred to the glass fiber matrix. The microparticles bound irreversibly to the matrix, which was washed in order to remove unbound materials. The 4-methylumbelliferyl phosphate substrate was added to the matrix, and the fluorescent product was measured by the optical assembly of the equipment. Positive and negative controls were employed, and photocolorimetric readings interpolated according to the IMx software assay implant module/TDM, version 4.0. The results were expressed in ng/mL.
Histopathology
After fixation in 10% formalin, the samples were referred for routine staining and mounting by the hematoxylin-eosin technique.
The histological slides were examined under light microscopy according to the criteria by Hard10, who considered the description of the neoplasia infiltration as medullary and/or cortical, the characterization of the neoplastic cells, the determination of the mitotic index and the characterization of the inflammatory infiltration.
Statistical analysis
The statistical method ANOVA was applied and the significance level of P was set at 5%. For comparisons between results of animals within a group at different time points, the nonparametric Wilcoxon method was used, and between different groups, the Tukey-Kramer test. The 95% confidence intervals were also calculated.
Results
Maximum reference values of < 48.16 mg/mL for urea and < 0.60 mg/mL for creatinine were etsblished.
The biochemical test levels of the Control group for urea and creatinine were, respectively: means, 44.8 and 0.576; standard deviations, 6.058 and 0.03627, confidence intervals (95%), 40.48 to 48.16 and 0.55 to 0.6019 (Table 1).
Gross examination, biochemistry of the inoculated groups and tacrolimus dosing
During the 15 days of the experiment, no deaths occurred of any rat; however, signs of prostration and piloerection were observed in the animals of groups TU and TT. In the abdominal cavity inventory, the presence of the tumor was detected in all right kidneys; ascitic effusions, adhesion formation or tumor metastases were not found in other organs or tissues adjacent to the right kidneys.
Serum creatinine levels were higher in group TT (1.013 ± 0.3028 mg/mL), which differed significantly from groups TU (0.5670 ± 0.03536 mg/dL) with P=0.00256 and TC (0.711 ± 0.1653 mg/mL) with P=0.02832 (Figure 4).
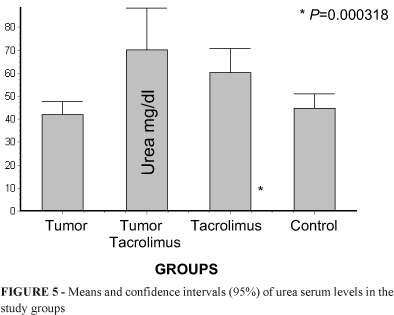
Urea serum levels were higher in group TT (71.32 ± 17.14 mg/mL), which differed significantly from group T (45.83 ± 5.046 mg/dL) with P=0.000318, while no significant difference was found when compared with group TC (61.23 ± 9.503 mg/mL), with P= 0.7242 (Figure 5).
Tacrolimus dosing was conducted only in the rats of groups TC and TT, which showed values of 11.29 ± 3.22 ng/mL and 10.58 ± 4.29 ng/mL (P= 1.4462), respectively.
Histopathology
Specimens from the animals in groups C and TC were histologically similar and showed no histological alterations.
Only blood vessel congestion was observed in the left kidney specimens of the animals in groups TU and TT, all inoculated with isotonic saline.
In the right kidneys of groups TU and TT, inoculated with the Walker tumor, the following histological differences were found: the neoplasms in the specimens of group TT were larger, affecting a more extensive area of the organ; the foci of tumor necrosis and areas of hemorrhage were also larger and inflammatory infiltration was a little more pronounced. Inflammation was similar for these two groups, as was the mitotic index of the neoplastic cells. The principal differences of the specimens between these groups are described in Chart 2.
Discussion
Clinical11 and experimental12 studies with tacrolimus have demostrated its advantages over other immunomodulating drugs such as cyclosporine concerning the rejection of transplanted organs. Many studies have reported on tacrolimus as a promising therapy in cases of failure with other immunomodulating drugs13,14,15. After a few years of tacrolimus use, an increase was observed in the development of malignancies, pointing to its withdrawal or replacement16. Few experimental studies have evaluated its actual effect and impact on tumor progression.
In the present study, the dose of tacrolimus was 5 mg/kg of body weight, since that dose promotes greater lymphocyte inhibition and immunosuppression. Barten et al.8, in 2005, proved through flow cytometry, proliferating cell nuclear antigen (PCNA) and CD25 lymphocyte receptor expression that the maximal dose for lymphocyte inhibition in rats was 5 mg /kg.
Immunosuppressive therapy is a risk factor for increased tumor incidence or progression in a transplanted organ recipient17. Growth factor beta 1 (TGFbeta1) is associated with tumor invasion and aggressiveness.
Maluccio18, in a study of rats with renal cell carcinoma that were treated with tacrolimus at the dose of 2 and 4 mg/kg, noted an increase in pulmonary metastases in the treated animals, which showed a worsening evolution with higher doses of tacrolimus and greater TGFbeta1 production. In the present study, the implantation of carcinosarcomas in kidneys of rats under immunosuppression showed an aggravation in the clinical course and tumor aggressiveness comparable to that in the abovementioned study.
Everett et al.19, in a study involving rats with the Walker tumor treated with hydrocortisone and cyclophosphamide, demonstrated a tendency to metastases when those drugs were used. In the present study, the action of tacrolimus was comparable, since it revealed a tendency to increased tumor aggressiveness.
In most studies described in the literature, tacrolimus accelerates tumor growth as found in the present study; there are, however, cases of tumor regression with tacrolimus. Taguchi et al.20 reported total regression of pleural metastases with the use of tacrolimus in a thymoma patient.
A study conducted by Niwa21 with rats submitted to induced skin tumors found that topical application of tacrolimus accelerated carcinogenesis when compared with other drugs for topical application. Shinozuka22, in an experimental study of liver tumors, found a worsening picture with the use of tacrolimus. In the present study, similar results were found with the implantation of Walker tumors, the same being true for other authors.
Rettori et al.23 remarked that Walker tumors, when experimentally implanted, may trigger a number of water-electrolyte alterations such as sodium retention and reduced creatinine clearance. In the present study, those findings were observed in the groups inoculated with this tumor and in the tacrolimus-treated tumor groups.
The Walker tumor causes metabolic alterations in the lymphocytes and macrophages of rats; these changes could compromise the animal's immune response, metabolism and renal function. The present study confirmed those findings, since animals in groups TU and TT exhibited a decline in renal function indices.
Conclusion
Immunosuppressive treatment with tacrolimus in the presence of a Walker tumor in murine kidney induced exacerbated tumor growth and nephrotoxicity with elevation of serum urea and creatinine levels.
Received: August 12, 2009
Review: October 19, 2009
Accepted: November 16, 2009
Conflict of interest: none
Financial source: none
How to cite this article
Inácio CM, Dietz UA, Malafaia O, Ribas Filho JM, Nassif PAN, Czeczko NG, Marcondes CAP. Action of tacrolimus on Wistar rat kidneys implanted with Walker 256. Acta Cir Bras. [serial on the Internet] 2010 Jan-Feb;25(1). Available from URL: http://www.scielo.br/acb
- 1. Dantal J, Pohanka E. Malignancies in kidney transplantation: an unmet medical need. Nephrol Dial Transplant. 2007;22(suppl. 1):4-10.
- 2. Ianhez LE, Paula FJ. Tumores malignos no pós-transplante renal. J Bras Nefrol. 1999;21(4):161-6.
- 3. Mazzali M. Morbidade e mortalidade em transplante renal no Brasil. Int Braz J Urol. 2004;29(suppl 2):7-14.
- 4. Silva SL, Silva SF, Cavalcante RO, Mota RS, Carvalho RA, Moraes MO, Campos HH, Moraes ME. Mycophenolate mofetil attenuates Walker's tumor growth when used alone, but the effect is lost when associated with cyclosporine. Transplant Proc. 2004;36(4):1004-6.
- 5. Alves APNN, Guedes RC, Costa-Lotufo LV, Moraes MEA, Pessoa CO, Ferreira FVA, Moraes MO. Modelo experimental de tumor na cavidade oral de ratos com carcinossarcoma de Walker 256. Acta Cir Bras. 2004;19(4):406-14.
- 6. Silva LFG, Soares FSD, Anselmo JNN, Fé DMM, Cavalcante JLBG, Moraes MO, Vasconcelos PRL. Modelo de tumor experimental em rim de ratos. Acta Cir Bras. 2002;17(1):62-6.
- 7. Nicoluzzi JE, Marmamnillo WC, Repka JCD. Transplante simultâneo de pâncreas-rim em portador de Diabetes Mellitus tipo 1 com insuficiência renal crônica. Experiência inicial do Hospital Angelina Caron. Arq Bras Endocrinol Metab. 2003;3:243-7.
- 8. Barten MJ, Shipkova M, Bartsch P, Dhein S, Streit F, Tarnok A, Armstrong VW, Mohr FW, Oellerich M, Gummert JF. Mycophenolic acid interaction with cyclosporine and tacrolimus in vitro and in vivo: evaluation of additive effects on rat blood lymphocyte function. Ther Drug Monit. 2005;27(2):123-31.
- 9. Winkler M, Wonigeit K, Undre N, Ringe B, Oldhafer K, Christians U, Pichlmayr R. Comparison of plasma vs whole blood as matrix for FK 506 drug level monitoring. Transplant Proc. 1995;27(1):822-5.
- 10. Hard GC. Pathology of tumours in laboratory animals. Tumours of the rat. Tumours of the kidney, kidney pelvis and ureter. IARC Sci Publ. 1990;99:301-44.
- 11. Van Buren D, Payne J, Geevarghese S, MacDonell R, Chapman W, Wright JK, Helderman JH, Richie R, Pinson CW. Impact of sandimmune, neoral, and prograf on rejection incidence and renal function in primary liver transplant recipients. Transplant Proc. 1998;30(5):1830-2.
- 12. Fujino Y, Kawamura T, Hullett DA, Sollinger HW. Evaluation of cyclosporine, mycophenolate mofetil, and Brequinar sodium combination therapy on hamster-to-rat cardiac xenotransplantation. Transplantation. 1994;57(1):41-6.
- 13. Greg A, Robert C. Tacrolimus versus cyclosporin for immunosuppression in kidney transplantation: meta-analysis of randomised trials. BMJ. 1999;318:1104-7.
- 14. Fung JJ. Tacrolimus and transplantation: a decade in review. Transplantation. 2004;77(9 Suppl):S41-3.
- 15. Christians U, Jacobsen W, Benet LZ, Lampen A. Mechanisms of clinically relevant drug interactions associated with tacrolimus. Clin Pharmacokinet. 2002;41(11):813-51.
- 16. Gutierrez-Dalmau A, Campistol JM. The role of proliferation signal inhibitors in post-transplant malignancies. Nephrol Dial Transplant. 2007;22(suppl.1):11-6.
- 17. Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905-13.
- 18. Maluccio M, Sharma V, Lagman M, Vyas S, Yang H, Suthanthiran M. Tacrolimus enhances transforming growth factor-beta1 expression and promotes tumor progression. Source Transplant. 2003;76(3):597-602.
- 19. Everett V, Sugarbaker MD, Alfred M, Cohen MD, Alfred S, Ketcham MD. Facilitated metastatic distribution of the Walker 256 tumor in sprague-dawley rats with hydrocortisone and/or cyclophosphamide. J Surg Oncol. 1970;2(3):277-89.
- 20. Taguchi T, Suehiro T, Toru K, Ogami N, Takata H, Hashimoto K. Pleural dissemination of thymoma showing tumor regression after combined corticosteroid and tacrolimus therapy. Eur J Intern Med. 2006;17(8):575-7.
- 21. Niwa Y, Terashima T, Sumi H. Topical application of the immunosuppressant tacrolimus accelerates carcinogenesis in mouse skin. Br J Dermatol. 2003;149(5):960-7.
- 22. Shinozuka H, Warty VS, Masuhara M, Murase N, Iwatsuki S. Effect of FK 506 on experimental liver carcinogenesis. Transplant Proc. 1991;23(6):3197-9.
- 23. Rettori O, Vieira MAN, Gontijo JA. Re-assessment of kidney hydrossaline disfunction in rats bearing Walker-256 tumor. Ren Fail. 2000;22(6):769-84.
Publication Dates
-
Publication in this collection
04 Feb 2010 -
Date of issue
Feb 2010
History
-
Accepted
16 Nov 2009 -
Reviewed
19 Oct 2009 -
Received
12 Aug 2009