Abstract
PURPOSE:
To evaluate a new perfusate solution to be used for ex vivo lung perfusion.
METHODS:
Randomized experimental study using lungs from rejected brain-dead donors harvested and submitted to 1 hour of ex vivo lung perfusion (EVLP) using mainstream solution or the alternative.
RESULTS:
From 16 lungs blocs tested, we found no difference on weight after EVLP: Steen group (SG) = 1,097±526g; Alternative Perfusion Solution (APS) = 743±248g, p=0.163. Edema formation, assessed by Wet/dry weigh ratio, was statistically higher on the Alternative Perfusion Solution group (APS = 3.63 ± 1.26; SG = 2.06 ± 0.28; p = 0.009). No difference on PaO2 after EVLP (SG = 498±37.53mmHg; APS = 521±55.43mmHg, p=0.348, nor on histological analyses: pulmonary injury score: SG = 4.38±1.51; APS = 4.50±1.77, p=0.881; apoptotic cells count after perfusion: SG = 2.4 ± 2.0 cells/mm2; APS = 4.8 ± 6.9 cells/mm2; p = 0.361).
CONCLUSION:
The ex vivo lung perfusion using the alternative perfusion solution showed no functional or histological differences, except for a higher edema formation, from the EVLP using Steen Solution(r) on lungs from rejected brain-dead donors.
Organ Preservation Solutions; Lung Injury; Lung Transplantation
Introduction
Around the world, many patients with end-stage lung diseases are included on the waiting lists for lung transplantation as their best chance of treatment11. Christie JD, Edwards LB, Kucheryavaya AY, Aurora P, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report--2010. J Heart Lung Transplant. 2010 Oct;29(10):1104-18. doi: 10.1016/j.healun.2010.08.004.
https://doi.org/10.1016/j.healun.2010.08...
. It is know that the adequate donors for lung transplantation is scarce and this shortage of suitable organs is the key factor for high mortality rates on these waiting lists22. Costa da Silva F Jr, Afonso JE Jr, Pêgo-Fernandes PM, Caramori ML, Jatene FB. São Paulo lung transplantation waiting list: patient characteristics and predictors of death. Transplant Proc. 2009 Apr;41(3):927-31. doi: 10.1016/j.transproceed.2009.01.048.
https://doi.org/10.1016/j.transproceed.2...
. One of major initiatives to improve organ usage was the development of ex vivo lung perfusion (EVLP) by Steen et al.1717. Steen S, Ingemansson R, Eriksson L, Pierre L, Algotsson L, Wierup P, Liao Q, Eyjolfsson A, Gustafsson R, Sjöberg T. First human transplantation of a nonacceptable donor lung after reconditioning ex vivo. Ann Thorac Surg. 2007;83(6):2191-4. PMID: 17532422. that could be used not only to test the organs, but also recover some of the initially rejected lungs for the transplant.
The EVLP system originally described by Steen et al.1717. Steen S, Ingemansson R, Eriksson L, Pierre L, Algotsson L, Wierup P, Liao Q, Eyjolfsson A, Gustafsson R, Sjöberg T. First human transplantation of a nonacceptable donor lung after reconditioning ex vivo. Ann Thorac Surg. 2007;83(6):2191-4. PMID: 17532422. consists of a transparent rectangular box to support the lung block (with inlets for connecting the cardiopulmonary bypass circuit tubes), a centrifugal pump, a heat exchanger, and a membrane oxygenator, as well as a pressure transducer, a flow meter, and a thermometer. They used as perfusate 1,500 mL of Steen Solution mixed with a variable amount of packed red blood cells, in order to reach a hematocrit level of approximately 15%. A gas mixture (of nitrogen, oxygen, and carbon dioxide) is used to "deoxygenate" the perfusate through the gas exchange membrane, gas flow being adjusted so that the gas concentration in the perfusate is similar to that in the venous blood44. Wierup P, Haraldsson A, Nilsson F, Pierre L, Scherstén H, Silverborn M, Sjöberg T, Westfeldt U, Steen S. Ex vivo evaluation of nonacceptable donor lungs. Ann Thorac Surg. 2006 Feb;81(2):460-6. PMID: 16427831..
The Toronto group implanted some modifications like the rounded box, the modified cannula for the atrium drainage and probably the most important modification, the use of the Steen solution without the red blood cells, in other words, they recommend a cellular perfusate55. Cypel M, Yeung JC, Hirayama S, Rubacha M, Fischer S, Anraku M, Sato M, Harwood S, Pierre A, Waddell TK, de Perrot M, Liu M, Keshavjee S. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008 Dec;27(12):1319-25. doi: 10.1016/j.healun.2008.09.003.
https://doi.org/10.1016/j.healun.2008.09...
.
However, the necessity to import the Steen solution, as well the final price could be limitations to the reproduction and propagation of EVLP in development countries. A Brazilian pharmacological company has developed a new solution to be used as perfusate for the EVLP in substitution of the Steen solution. Besides the fact that this new solution have similar composition of the Steen Solution (extracellular solution with electrolytes and enriched with albumin), this new solution was not tested, therefore cannot be used.
The aim of this study was to evaluate the alternate perfusate solution to perform EVLP against the standard Steen solution.
Methods
This study was approved by Ethics Committee (CAPPESQ 0309/10) and supported by the Transplant Center from Sao Paulo State, Department of Health, Organ Procurement Agency from HC-FMUSP and the Organ Procurement Agency from Santa Casa de Misericórdia de São Paulo Hospital.
We conducted a randomized experimental study using lungs retrieved from brain-dead donors who were rejected by pulmonary transplantation teams based on criteria previously defined by the International Society for Heart and Lung Transplantation66. Orens JB, Boehler A, de Perrot M, Estenne M, Glanville AR, Keshavjee S, Kotloff R, Morton J, Studer SM, Van Raemdonck D, Waddel T, Snell GI. A review of lung transplant donor acceptability criteria. Pulmonary Council, International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2003 Nov;22(11):1183-200. PMID: 14585380..
The harvest technique was the same as the one used in clinical practice, pulmonary artery trunk cannulation and perfusion with 50-75 mL/kg of preservation solution at 4ºC.
After the harvesting, all 16 lung blocs were transported, weighted and stored at 4ºC for 10 hours and randomized in a control group (Steen Solution) and a test group (test solution) each group with eight organs. After 10h, the lungs were again weighted and then, using an EVLP, both groups were tested.
The EVLP consisted in the reperfusion and ventilation of the lungs as described forward. We inserted the lung bloc in to a rigid, transparent support (XVIVO chamber). The pulmonary artery had a cannula with a build-in catheter to enable continuous pulmonary artery pressure attached to its trunk. An endotracheal tube was inserted in the trachea and secured circumferentially with an umbilical tape. An extracorporeal circuit with a heater, membrane oxygenator, reservoir and centrifugal pump, was primed with 1.5L of Steen Solution or test solution. The left atrium was kept open, and the perfusate was drained from the chamber to the reservoir of the circuit. A digital thermometer inserted at the pulmonary veins took temperature. The membrane oxygenator was connected to a gas mixer that received a CO2/NO2 blend (CO2 - 7% and NO2 - 93%) and oxygen. So lung oxygenation could be tested, the mixer was set to deoxygenate the solution before going in the pulmonary artery, as it was venous blood. The pH was adjusted using tromethamol to be similar to physiological. After ventilation was started, the membrane oxygen was turned off. This way, all the oxygen registered analyses is from the lung ventilation. Figure 1.
Ex Vivo system. Extracorporeal tube kit, centrifugal pump, reservoir, membrane oxygenator, gas mixer and Xvivo chamber with lung bloc.
Reperfusion has been started with a 100mL/min inflow at 25ºC and gradually the lungs were warmed up and the inflow was increased up to 40% of the cardiac output, but keeping pulmonary artery pressure below 20mmHg as shown in Table 1.
After a 60-minute perfusion, a fluid sample from the pulmonary artery and from the pulmonary vein was taken for gases analyses. The perfusion was stopped, new biopsy was made and the lungs were weighted. The left lung was used to assess the formation of pulmonary edema using the wet/dry ratio method. By this method, we can measure how much water there is and assess the edema. So, left lung was weighed after EVLP and maintained in an incubator at 60ºC for 24 hours (to dehydrate) and weighed again to calculate the wet/dry weight ratio.
The lung tissue samples were fixed in 10% buffered formalin for 24 hours, embedded in paraffin, sectioned in 5-mm thickness and stained with hematoxylin and eosin. We perform semiquantitative scoring with experienced lung pathologist using histology parameters already described77. Mariani AW, Medeiros IL, Pêgo-Fernandes PM, Fernandes FG, Unterpertinguer Fdo V, Fernandes LM, Cardoso PF, Canzian M, Jatene FB. Cold ischemia or topical-ECMO for lung preservation: a randomized experimental study. Sao Paulo Med J. 2014;132(1):28-35. doi: 10.1590/1516-3180.2014.1321594.
https://doi.org/10.1590/1516-3180.2014.1...
. The severity of these findings was determined using a 4-grade scale: absent=0; minimal=1; moderate=2; and intense=3. The sum of each parameter resulted in the Lung Injury Score (LIS), with a value of 0 to 66.
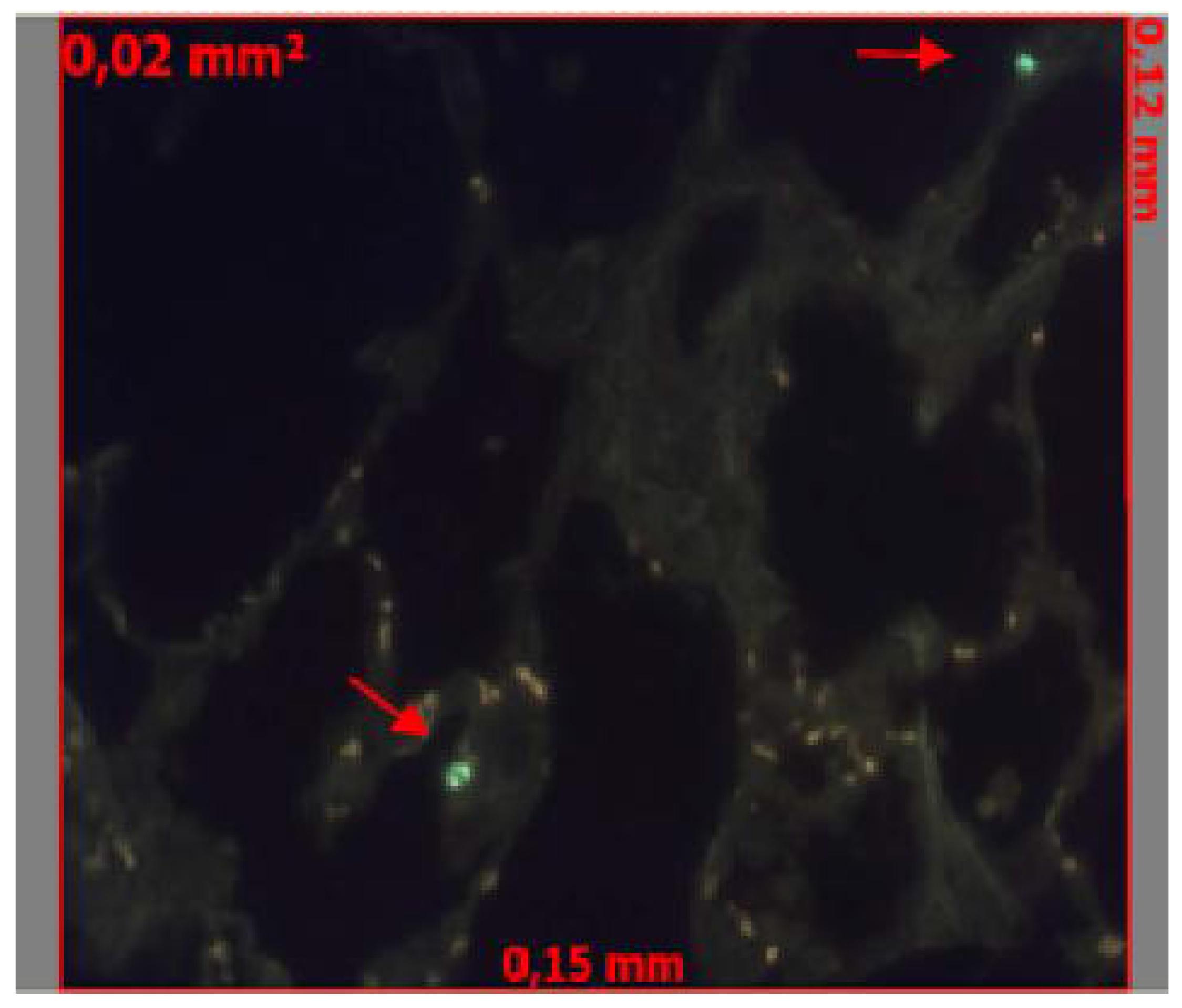
We assessed apoptosis with the TUNEL method, using the In Situ Cell Death Detection Kit (Roche, Basel, Switzerland), this technique is based on the enzymatic ability of TdT to catalyze a template-independent addition of deoxyribonucleotide triphosphate to the 3'-OH ends of double- or single-stranded DNA (Figure 2). To localize apoptotic cells, we randomly selected specimens of lung tissue photographed under the fluorescent light microscope at high magnification. Apoptotic cells appear in bright green. Counts were obtained from 5 randomly chosen fields per slide, representing a total area of 0.1 mm2. An independent, blinded examiner performed the cell counting.
TUNEL reaction. Apoptotic cells identification (red arrows) using AxionVision Program (x400).
We conceived this study as non-inferiority, the required sample size based on previously published experimental studies involving the comparison of lung preservation solutions in experimental models of lung transplantation in dogs and pigs would be too large. In this case, the sample size was determined based on the goal of achieving sufficient power (type II error, 0.1) to reject a null hypothesis when the magnitude of effect is very small and therefore clinically insignificant. This criterion would result in a sample, precluding the use of a resource-intensive method that involves the harvesting of human lungs. For this reason, it was established a convenient sample size of 16 cases.
We applied the Normality tests Kolmogorov-Smirnov and Shapiro-Wilk. For normally distributed data comparison, the Student's samples t-test was performed. We used Mann-Whitney test for variables that were not normally distributed. For qualitative variables in 2x2 tables we used the chi-square or Fisher's exact. The results were expressed as mean and standard error of the mean or median and interquartile range for variables that were not normally distributed. We performed the statistical analyses using SPSS 18.0 software (SPSS Inc, Chicago, IL) with a confidence interval of 95% and a significance level of 0.05.
Results
We evaluated 16 pairs of human lungs from April/2009 to September/2010.
Donor demographic characteristics are reported in Table 2. The main cause of lungs refuse was poor pulmonary function, found in 14 cases. Other two cases were refused by pneumonia and incompatibility of size. The mean PaO2 was 203mmHg (FiO2 100% and PEEP 5cmH2O).
No difference was found on weight variation between the groups at the three times studied: a) before ischemic time: Steen Group (SG) = 1.026±451g vs Test Group (TG) = 745±282g, p=0.180; b) before ex vivo perfusion: SG = 998±391g vs TG = 738±316g, p=0.184; c) after ex vivoperfusion: SG = 1.097±526 vs TG = 743±248g, p=0.163. The ratio between left lungs' wet weight and dry weight after reconditioning was greater national solution group (APS = 3.63 ± 1.26; STEEN = 2.06 ± 0.28; p = 0.009 ).
The PaO2 values were similar in both groups before harvesting (SG = 206±119.25mmHg vs TG = 200±58.20mmHg, p=0.906) and after lung perfusion (SG = 498±37.53mmHg vs TG = 521±55.43mmHg, p=0.348). Both groups showed great improvement of the lung function after EVLP (Figure 3).
After a 60 minute perfusion, SG´s pulmonary vascular resistance was 788±367.23 dyna.s.cm-5 vs 1,026±1,112.53 dyna.s.cm-5 in the TG, p=0.575. The mean pulmonary compliance at the end of the experiment was 46.75±21 mL/cmH2O in the SG and 49.74±26 mL/cmH2O in the TG. The airway pressure after the perfusion was also similar in both groups, 17.25±3.81 cmH2O in SG and 18.14±4.91 cmH2O in TG, p=0.698.
The histological analyses has shown that there weren't difference between the groups when used a pulmonary injury score at the three times studied: a) before ischemic time: SG = 3.75±2.82 vs TS = 4.00±2.51, p=0.854; b) before perfusion: SG = 4.50±2.14 vs TS = 4.13±1.64, p=0.7 and c) after perfusion: SG = 4.38±1.51 vs TS = 4.50±1.77, p=0.881.The number of apoptotic cells using the tunnel technique was not significantly higher in either group at the 3 times as well: a) before ischemic time SG = 8.1±13.2 cells/mm2 vs TS = 14.2±25.2 cells/mm2, p=0.576; b) before perfusion: SG = 3.4±3.5 cells/mm2 vs TS = 5.8±7 cells/mm2, p=0.361; c) after perfusion SG = 2.4 ± 2.0 cells/mm2, TG = 4.8 ± 6.9 cells/mm2; p = 0.361).
Discussion
The mechanisms behind EVLP recovering effect are not fully understood88. Carnevale R, Biondi-Zoccai G, Peruzzi M, De Falco E, Chimenti I, Venuta F, Anile M, Diso D, Cavarretta E, Marullo AG, Sartini P, Pignatelli P, Violi F, Frati G. New insights into the steen solution properties: breakthrough in antioxidant effects via NOX2 downregulation. Oxid Med Cell Longev. 2014;2014:242180. doi: 10.1155/2014/242180.
https://doi.org/10.1155/2014/242180...
some of them are better inspection of the lungs; secretions and clots removal; better ventilation, with atelectasis removal; hyperosmolar and anti-inflammatory properties of Steen solution.
The Steen Solution is the main reconditioning solution used both in clinical and experimental fields. Our main goal to develop a national EVLP perfusate solution is to spread the technique free from importation and expense issues.
One of the main reason pointed as donated lung refusal is the PaO2 level below 300mmHg22. Costa da Silva F Jr, Afonso JE Jr, Pêgo-Fernandes PM, Caramori ML, Jatene FB. São Paulo lung transplantation waiting list: patient characteristics and predictors of death. Transplant Proc. 2009 Apr;41(3):927-31. doi: 10.1016/j.transproceed.2009.01.048.
https://doi.org/10.1016/j.transproceed.2...
. In our study, the PaO2at the harvesting was, in average, 200mmHg, close to other studies99. Pêgo-Fernandes PM, Medeiros IL, Mariani AW, Fernandes FG, Unterpertinger FV, Samano MN, Werebe EC, Jatene FB. Ex vivo lung perfusion: initial Brazilian experience. J Bras Pneumol. 2009;35(11):1107-11. PMID: 20011846.
10. Wierup P, Haraldsson A, Nilsson F, Pierre L, Scherstén H, Silverborn M, Sjöberg T, Westfeldt U, Steen S. Ex vivo evaluation of nonacceptable donor lungs. Ann Thorac Surg. 2006;81:460-6. PMID: 16427831.
11. Pêgo-Fernandes PM, Mariani AW, Medeiros IL, Pereira AEA, Fernandes FG, Valle Unterpertinger Fd, Canzian M, Jatene FB. Ex vivo lung evaluation and reconditioning. Rev Bras Cir Cardiovasc. 2010;25(4):441-6. PMID: 21340372.
12. Pêgo-Fernandes PM, Mariani AW, Medeiros IL, Pereira AE, Fernandes FG, Valle Unterpertinger Fd, Canzian M, Jatene FB. Ex vivo lung perfusion: early report of Brazilian experience. Transplant Proc. 2010;42:440-3. doi: 10.1016/j.transproceed.2010.01.015.
https://doi.org/10.1016/j.transproceed.2...
- 1313. Medeiros IL, Pêgo-Fernandes PM, Mariani AW, Fernandes FG, Unterpertinger FV, Canzian M, Jatene FB. Histologic and functional evaluation of lungs reconditioned by ex vivo lung perfusion. J Heart Lung Transplant. 2012;31(3):305-9. doi: 10.1016/j.healun.2011.10.005.
https://doi.org/10.1016/j.healun.2011.10...
. These previous studies demonstrated that, after reconditioning with Steen Solution, mean PaO2 reach levels between 380 and 500, similar to our study, even when the national reconditioning solution was used. Even the greater time in mechanical ventilation noticed in our study about five to seven days, was not significant, our the PaO2 after reconditioning was greater than the reached by Wierup et al.44. Wierup P, Haraldsson A, Nilsson F, Pierre L, Scherstén H, Silverborn M, Sjöberg T, Westfeldt U, Steen S. Ex vivo evaluation of nonacceptable donor lungs. Ann Thorac Surg. 2006 Feb;81(2):460-6. PMID: 16427831.and Egan et al.1414. Egan TM, Haithcock JA, Nicotra WA, Koukoulis G, Inokawa H, Sevala M, Molina PL, Funkhouser WK, Mattice BJ. Ex vivo evaluation of human lungs for transplant suitability. Ann Thorac Surg. 2006;81:1205-13. PMID: 16564244., where donors stood, in average, on mechanical ventilation for two days.
One important benefit from the EVLP with the Steen solution is the lung swelling reduction due to the solution's high osmolarity, set by albumin and dextran levels. Furthermore, dextran has antithrombotic proprieties and coats the endothelial surface of the pulmonary circulation and the tubes of the circuit. In fact, the pulmonary edema is typically present in the process of ischemia-reperfusion factor due to increased vascular permeability and breakage of alveolo-capillary barrier. The more or less water retention in the lung tissue is directly related to graft dysfunction or viability, reflected in the quality of preservation1515. De Perrot M, Keshavjee S. Lung preservation. Semin Thorac Cardiovasc Surg. 2004;16(4):300-8. PMID: 16564244..
In the present study, the domestic manufactured reconditioning solution was less efficient in preventing the formation of edema in the lungs, measured by the ratio of the wet weight (after a 60 minutes perfusion) and dry weight. However, this event does not seem to have done significant loss to the gas exchange, since, as discussed above, oxygenation capacity (measured by PaO2) was good in all reconditioned lungs, regardless of the type of solution used. Furthermore, the weight of the lung blocks recorded in three times throughout the experiment - straight after capture, after 10 hours of cold ischemia, and after 60 minutes of reconditioning - did not change significantly.
We decide to study the hemodynamic behavior on EVLP since it could be an important marker of lung function. Cypel et al.1616. Cypel M, Yeung JC, Hirayama S, Rubacha M, Fischer S, Anraku M, Sato M, Harwood S, Pierre A, Waddell TK, de Perrot M, Liu M, Keshavjee S. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27(12):1319-25. doi: 10.1016/j.healun.2008.09.003.
https://doi.org/10.1016/j.healun.2008.09...
standardized for EVLP a maximum flow rate of approximately 40% of the estimated cardiac output, which allowed the time increasing of organ perfusion due to decreased of edema formation. Low flow protects the pulmonary microvasculature against mechanical injury of the endothelium, maintaining the integrity of the alveolar-capillary barrier and preventing effusion of fluid from blood vessels. In the present study we measured hemodynamic and functional parameters, such as vascular resistance and pulmonary compliance. Actually, the resistance of a vessel is dependent, among others, the intensity of the flow passing through it. Therefore, in this study, we adopted the recommended flow for Cypel et al.1616. Cypel M, Yeung JC, Hirayama S, Rubacha M, Fischer S, Anraku M, Sato M, Harwood S, Pierre A, Waddell TK, de Perrot M, Liu M, Keshavjee S. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27(12):1319-25. doi: 10.1016/j.healun.2008.09.003.
https://doi.org/10.1016/j.healun.2008.09...
to ens ure the maintenance of pulmonary artery pressure between 10 and 15 mmHg. Hence, no difference was observed in pulmonary vascular resistance values between the groups treated with Steen Solution(r) and the APS.
The hypothermia that lungs are subjected in the harvesting and in the cold ischemia preservation period, cause a generalized contraction of the pulmonary microvasculature, which may abruptly increase the vascular resistance, favoring the accumulation of microemboli in the capillaries. The normothermia has been used in the ex vivo perfusion system from the beginning of its planning by Steen et al.1717. Steen S, Ingemansson R, Eriksson L, Pierre L, Algotsson L, Wierup P, Liao Q, Eyjolfsson A, Gustafsson R, Sjöberg T. First human transplantation of a nonacceptable donor lung after reconditioning ex vivo. Ann Thorac Surg. 2007;83(6):2191-4. PMID: 17532422.. In normal body temperature conditions, the vessels dilate, thus favoring the flow of reconditioning solution and the removal of clots and microemboli as well as reducing endothelial injury and pulmonary vascular resistance. In fact, it was shown that lungs kept for 12 hours in cold ischemia and perfused for 12 hours with normothermic solution had a better performance after transplantation then lungs only kept in cold ischemia for 24 horas1818. Cypel M, Rubacha M, Yeung J, Hirayama S, Torbicki K, Madonik M, Fischer S, Hwang D, Pierre A, Waddell TK, de Perrot M, Liu M, Keshavjee S. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am J Transplant. 2009;9:1-8. doi: 10.1111/j.1600-6143.2009.02775.x.
https://doi.org/10.1111/j.1600-6143.2009...
. Since our goal as compare two perfusate solutions, we decide to perform a 1-hour reperfusion with progressive warming of the lung, however longer periods of EVLP could lead to better results of reconditioning.
Decreased lung compliance can also be related to hypothermia, since the cold tissue becomes rigid and offer much resistance to insufflations. Furthermore, the gradual heating of the lung by the normothermic solution circulation provides to the lung tissue conditions closer to the normal physiological state, allowing a more appropriate manner circulation. In this study, lung compliance was similar in all lungs after the 60 minute period of reconditioning both with Steen Solution(r) as with APS, reaching close values to those reported in other studies using the same technique1919. Fischer S, Cassivi SD, Xavier AM, Cardella JA, Cutz E, Edwards V, Liu M, Keshavjee S. Cell death in human lung transplantation: apoptosis induction in human lungs during ischemia and after transplantation. Ann Surg. 2000;231(3):424-31. PMID: 10714636.. The normal lung compliance is also reflected in the values of airway pressure recorded at the end of the reperfusion period, since one of the factors that can cause increased airway pressure is precisely a decrease in lung compliance2020. Barrett KE, Barman SM, Boitano S, Brooks HL. Fisiologia Médica de Ganong. 24ed. Artmed; 2013.. Actually, all tested lungs, either with Solution(r) Steen, or with the APS, exhibited values of airway pressure within the normal range (15-25 cmH2O).
Histological changes of lung tissue are well characterized in the ischemia-reperfusion process and provide important information on the degree of tissue injury2121. De Perrot M, Liu M, Waddell TK, Keshafjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167:490-511. PMID: 12588712.. Considering the semi quantitative evaluation method that we used, the lung injury in the studied lungs was low (four points on average in a 39 points maximum scale) and remained stable throughout the experiment, which was consistent with the physiological parameter both the Steen Solution group and for the APS group.
Cold ischemia may also trigger some series of events that promote the activation of inflammatory mediators2222. Emaminia A, Lapar DJ, Zhao Y, Steidle JF, Harris DA, Laubach VE, Linden J, Kron IL, Lau CL. Adenosine A2A agonist improves lung function during ex vivo lung perfusion. Ann Thorac Surg. 2011;92(5):1840-6. doi: 10.1016/j.athoracsur.2011.06.062.
https://doi.org/10.1016/j.athoracsur.201...
, cellular edema99. Pêgo-Fernandes PM, Medeiros IL, Mariani AW, Fernandes FG, Unterpertinger FV, Samano MN, Werebe EC, Jatene FB. Ex vivo lung perfusion: initial Brazilian experience. J Bras Pneumol. 2009;35(11):1107-11. PMID: 20011846. and cell death induction, either as necrosis, or apoptosis, both associated with impaired pulmonary function after reperfusion2323. Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24(10):1454-9. PMID: 16210116. , 2424. Arcasoy SM, Fisher A, Hachem RR, Scavuzzo M, Ware LB. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part V: predictors and outcomes. J Heart Lung Transplant. 2005;24(10):1483-8. PMID: 16210119.. Two studies have found that apoptosis is time dependent, increasing after 30, 60 and 120 minutes after the transplant (17%, 22% and 35%, respectively). However, there was no change in the number of apoptotic cells during cold ischemia for 1 to 5 hours or during the preimplantation warm ischemia. Moreover, a positive relationship between the type of cell death and graft function was established1919. Fischer S, Cassivi SD, Xavier AM, Cardella JA, Cutz E, Edwards V, Liu M, Keshavjee S. Cell death in human lung transplantation: apoptosis induction in human lungs during ischemia and after transplantation. Ann Surg. 2000;231(3):424-31. PMID: 10714636. , 2525. Fischer S, Maclean AA, Liu M, Cardella JA, Slutsky AS, Suga M, Moreira JF, Keshavjee S. Dynamic changes in apoptotic and necrotic cell death correlate with severity of ischemia-reperfusion injury in lung transplantation. Am J Respir Crit Care Med. 2000;162:1932-9. PMID: 11069837.. Our results are in accordance with these studies, since the number of apoptotic cells was small and there was no significant difference among times or groups studied.
Conclusion
The alternate perfusate solution was similar to the Steen Solution in all evaluated parameters, except for a higher edema formation.
Acknowledgements
To Transplant Center from Sao Paulo State, Department of Health, Organ Procurement Agency from HC-FMUSP and the Organ Procurement Agency from Santa Casa of Misericórdia de São Paulo Hospital.
References
-
1Christie JD, Edwards LB, Kucheryavaya AY, Aurora P, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report--2010. J Heart Lung Transplant. 2010 Oct;29(10):1104-18. doi: 10.1016/j.healun.2010.08.004.
» https://doi.org/10.1016/j.healun.2010.08.004 -
2Costa da Silva F Jr, Afonso JE Jr, Pêgo-Fernandes PM, Caramori ML, Jatene FB. São Paulo lung transplantation waiting list: patient characteristics and predictors of death. Transplant Proc. 2009 Apr;41(3):927-31. doi: 10.1016/j.transproceed.2009.01.048.
» https://doi.org/10.1016/j.transproceed.2009.01.048 -
3Ingemansson R, Eyjolfsson A, Mared L, Pierre L, Algotsson L, Ekmehag B, Gustafsson R, Johnsson P, Koul B, Lindstedt S, Lührs C, Sjöberg T, Steen S. Clinical transplantation of initially rejected donor lungs after reconditioning ex vivo. Ann Thorac Surg. 2009 Jan;87(1):255-60. doi: 10.1016/j.athoracsur.2008.09.049.
» https://doi.org/10.1016/j.athoracsur.2008.09.049 -
4Wierup P, Haraldsson A, Nilsson F, Pierre L, Scherstén H, Silverborn M, Sjöberg T, Westfeldt U, Steen S. Ex vivo evaluation of nonacceptable donor lungs. Ann Thorac Surg. 2006 Feb;81(2):460-6. PMID: 16427831.
-
5Cypel M, Yeung JC, Hirayama S, Rubacha M, Fischer S, Anraku M, Sato M, Harwood S, Pierre A, Waddell TK, de Perrot M, Liu M, Keshavjee S. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008 Dec;27(12):1319-25. doi: 10.1016/j.healun.2008.09.003.
» https://doi.org/10.1016/j.healun.2008.09.003 -
6Orens JB, Boehler A, de Perrot M, Estenne M, Glanville AR, Keshavjee S, Kotloff R, Morton J, Studer SM, Van Raemdonck D, Waddel T, Snell GI. A review of lung transplant donor acceptability criteria. Pulmonary Council, International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2003 Nov;22(11):1183-200. PMID: 14585380.
-
7Mariani AW, Medeiros IL, Pêgo-Fernandes PM, Fernandes FG, Unterpertinguer Fdo V, Fernandes LM, Cardoso PF, Canzian M, Jatene FB. Cold ischemia or topical-ECMO for lung preservation: a randomized experimental study. Sao Paulo Med J. 2014;132(1):28-35. doi: 10.1590/1516-3180.2014.1321594.
» https://doi.org/10.1590/1516-3180.2014.1321594 -
8Carnevale R, Biondi-Zoccai G, Peruzzi M, De Falco E, Chimenti I, Venuta F, Anile M, Diso D, Cavarretta E, Marullo AG, Sartini P, Pignatelli P, Violi F, Frati G. New insights into the steen solution properties: breakthrough in antioxidant effects via NOX2 downregulation. Oxid Med Cell Longev. 2014;2014:242180. doi: 10.1155/2014/242180.
» https://doi.org/10.1155/2014/242180 -
9Pêgo-Fernandes PM, Medeiros IL, Mariani AW, Fernandes FG, Unterpertinger FV, Samano MN, Werebe EC, Jatene FB. Ex vivo lung perfusion: initial Brazilian experience. J Bras Pneumol. 2009;35(11):1107-11. PMID: 20011846.
-
10Wierup P, Haraldsson A, Nilsson F, Pierre L, Scherstén H, Silverborn M, Sjöberg T, Westfeldt U, Steen S. Ex vivo evaluation of nonacceptable donor lungs. Ann Thorac Surg. 2006;81:460-6. PMID: 16427831.
-
11Pêgo-Fernandes PM, Mariani AW, Medeiros IL, Pereira AEA, Fernandes FG, Valle Unterpertinger Fd, Canzian M, Jatene FB. Ex vivo lung evaluation and reconditioning. Rev Bras Cir Cardiovasc. 2010;25(4):441-6. PMID: 21340372.
-
12Pêgo-Fernandes PM, Mariani AW, Medeiros IL, Pereira AE, Fernandes FG, Valle Unterpertinger Fd, Canzian M, Jatene FB. Ex vivo lung perfusion: early report of Brazilian experience. Transplant Proc. 2010;42:440-3. doi: 10.1016/j.transproceed.2010.01.015.
» https://doi.org/10.1016/j.transproceed.2010.01.015 -
13Medeiros IL, Pêgo-Fernandes PM, Mariani AW, Fernandes FG, Unterpertinger FV, Canzian M, Jatene FB. Histologic and functional evaluation of lungs reconditioned by ex vivo lung perfusion. J Heart Lung Transplant. 2012;31(3):305-9. doi: 10.1016/j.healun.2011.10.005.
» https://doi.org/10.1016/j.healun.2011.10.005 -
14Egan TM, Haithcock JA, Nicotra WA, Koukoulis G, Inokawa H, Sevala M, Molina PL, Funkhouser WK, Mattice BJ. Ex vivo evaluation of human lungs for transplant suitability. Ann Thorac Surg. 2006;81:1205-13. PMID: 16564244.
-
15De Perrot M, Keshavjee S. Lung preservation. Semin Thorac Cardiovasc Surg. 2004;16(4):300-8. PMID: 16564244.
-
16Cypel M, Yeung JC, Hirayama S, Rubacha M, Fischer S, Anraku M, Sato M, Harwood S, Pierre A, Waddell TK, de Perrot M, Liu M, Keshavjee S. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27(12):1319-25. doi: 10.1016/j.healun.2008.09.003.
» https://doi.org/10.1016/j.healun.2008.09.003 -
17Steen S, Ingemansson R, Eriksson L, Pierre L, Algotsson L, Wierup P, Liao Q, Eyjolfsson A, Gustafsson R, Sjöberg T. First human transplantation of a nonacceptable donor lung after reconditioning ex vivo. Ann Thorac Surg. 2007;83(6):2191-4. PMID: 17532422.
-
18Cypel M, Rubacha M, Yeung J, Hirayama S, Torbicki K, Madonik M, Fischer S, Hwang D, Pierre A, Waddell TK, de Perrot M, Liu M, Keshavjee S. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am J Transplant. 2009;9:1-8. doi: 10.1111/j.1600-6143.2009.02775.x.
» https://doi.org/10.1111/j.1600-6143.2009.02775.x -
19Fischer S, Cassivi SD, Xavier AM, Cardella JA, Cutz E, Edwards V, Liu M, Keshavjee S. Cell death in human lung transplantation: apoptosis induction in human lungs during ischemia and after transplantation. Ann Surg. 2000;231(3):424-31. PMID: 10714636.
-
20Barrett KE, Barman SM, Boitano S, Brooks HL. Fisiologia Médica de Ganong. 24ed. Artmed; 2013.
-
21De Perrot M, Liu M, Waddell TK, Keshafjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167:490-511. PMID: 12588712.
-
22Emaminia A, Lapar DJ, Zhao Y, Steidle JF, Harris DA, Laubach VE, Linden J, Kron IL, Lau CL. Adenosine A2A agonist improves lung function during ex vivo lung perfusion. Ann Thorac Surg. 2011;92(5):1840-6. doi: 10.1016/j.athoracsur.2011.06.062.
» https://doi.org/10.1016/j.athoracsur.2011.06.062 -
23Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24(10):1454-9. PMID: 16210116.
-
24Arcasoy SM, Fisher A, Hachem RR, Scavuzzo M, Ware LB. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part V: predictors and outcomes. J Heart Lung Transplant. 2005;24(10):1483-8. PMID: 16210119.
-
25Fischer S, Maclean AA, Liu M, Cardella JA, Slutsky AS, Suga M, Moreira JF, Keshavjee S. Dynamic changes in apoptotic and necrotic cell death correlate with severity of ischemia-reperfusion injury in lung transplantation. Am J Respir Crit Care Med. 2000;162:1932-9. PMID: 11069837.
-
Financial sources: São Paulo Research Foundation (FAPESP) (Grant 2010/52222-9), Braile Biomédica and Farmoterápica.
-
1
Research performed at Experimental Surgery Department, Heart Institute (InCor), Clinics Hospital (HC), School of Medicine, Sao Paulo University (USP), LIM 61. Part of PhD degree thesis, Postgraduate Program in Thoracic and Cardiovascular Surgery, USP. Tutor: Paulo Manuel Pêgo Fernandes.
Publication Dates
-
Publication in this collection
May 2015
History
-
Received
23 Jan 2015 -
Reviewed
25 Mar 2015 -
Accepted
24 Apr 2015