ABSTRACT
Objective:
We aimed to investigate a potential association between B-lines and weaning failure.
Methods:
Fifty-seven subjects eligible for ventilation liberation were enrolled. Patients with tracheostomy were excluded. Lung ultrasound assessments of six thoracic zones were performed immediately before and at the exnd of the spontaneous breathing trial. B-predominance was defined as any profile with anterior bilateral B-pattern. Patients were followed up to 48 hours after extubation.
Results:
Thirty-eight individuals were successfully extubated; 11 failed the spontaneous breathing trial and 8 needed reintubation within 48 hours of extubation. At the beginning of the T-piece trial, B-pattern or consolidation was already found at the lower and posterior lung regions in more than half of the individuals and remained non-aerated at the end of the trial. A trend toward loss of lung aeration during spontaneous breathing trials was observed only in the spontaneous breathing trial-failure group (p = 0.07), and there was higher B-predominance at the end of the trial (p = 0.01).
Conclusion:
A loss of lung aeration during the spontaneous breathing trial in non-dependent lung zones was demonstrated in subjects who failed to wean.
Keywords:
Ventilator weaning; Respiration, artificial; Ultrasonography; Respiratory failure; Pulmonary edema
RESUMO
Objetivo:
Investigar potencial associação entre a presença de linhas B e a falha do desmame.
Métodos:
Foram inscritos 57 pacientes elegíveis para liberação da ventilação. Excluíram-se pacientes com traqueostomia. Realizou-se avaliação ultrassonográfica pulmonar de seis zonas torácicas imediatamente antes e após o final da tentativa de respiração espontânea. Definiu-se a predominância de linhas B como qualquer perfil com padrão B bilateral anterior. Os pacientes foram seguidos por 48 horas após a extubação.
Resultados:
Foram extubados com sucesso 38 pacientes; 11 tiveram falha da tentativa de respiração espontânea; e 8 necessitaram de reintubação dentro de 48 horas após extubados. No início da tentativa com peça T, já se observava padrão B ou consolidação nas regiões posterior e inferior dos pulmões em mais de metade dos indivíduos, que permaneceram não aeradas ao final da tentativa. Observou-se certa tendência à perda da aeração pulmonar durante a tentativa de respiração espontânea apenas no grupo com falha da tentativa de respiração espontânea (p = 0,07), assim como maior predominância de padrão B ao final da tentativa (p = 0,01).
Conclusão:
A perda de aeração pulmonar durante a tentativa de respiração espontânea em áreas pulmonares não dependentes foi demonstrada em pacientes que tiveram falha do desmame.
Descritores:
Desmame do respirador; Respiração artificial; Ultrassonografia; Insuficiência respiratória; Edema pulmonar
INTRODUCTION
The weaning process comprises progressive withdrawal from invasive ventilatory support until removal of the endotracheal tube and might represent approximately 42% of the duration of mechanical ventilation (MV).(11 Tobin MJ, Jubran A. Weaning from mechanical ventilation. In: Tobin MJ, editor. Principles and practice of mechanical ventilation. 3rd ed. New York: McGraw-Hill; 2012. p. 1185-220.
2 MacIntyre NR, Cook DJ, Ely EW Jr, Epstein SK, Fink JB, Heffner JE, Hess D, Hubmayer RD, Scheinhorn DJ; American College of Chest Physicians; American Association for Respiratory Care; American College of Critical Care Medicine. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest. 2001;120(6 Suppl):375S-95S.-33 Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29(5):1033-56.) In the intensive care unit (ICU), the use of multiple respiratory indices to dictate the weaning process have been largely supplanted by the more rapid and predictive spontaneous breathing trial (SBT).(44 Macintyre NR. Evidence-based assessments in the ventilator discontinuation process. Respir Care. 2012;57(10):1611-8.
5 Tanios MA, Nevins ML, Hendra KP, Cardinal P, Allan JE, Naumova EN, et al. A randomized, controlled trial of the role of weaning predictors in clinical decision making. Crit Care Med. 2006;34(10):2530-5.-66 Savi A, Teixeira C, Silva JM, Borges LG, Pereira PA, Pinto KB, Gehm F, Moreira FC, Wickert R, Trevisan CB, Maccari JG, Oliveira RP, Vieira SR; Gaúcho Weaning Study Group. Weaning predictors do not predict extubation failure in simple-to-wean patients. J Crit Care. 2012;27(2):221.e1-8.) Better assessments of patients before and during an SBT are of paramount importance to predict weaning failure and to focus treatment that could reduce the time spent on artificial ventilation.
Cardiac dysfunction is a well-established cause of weaning failure, representing 40% of all weaning failures.(77 Teboul JL, Monnet X, Richard C. Weaning failure of cardiac origin: recent advances. Crit Care. 2010;14(2):211.,88 Teboul JL. Weaning-induced cardiac dysfunction: where are we today? Intensive Care Med. 2014;40(8):1069-79.) Switching a patient from positive pressure ventilation to spontaneous breathing reinstitutes negative inspiratory intra-thoracic pressure, thus increasing venous return, central blood volume, and left ventricular afterload. This normal condition, often an effort test for the patient, could decompensate cardiorespiratory function in cases of volume overload and left ventricular systolic or diastolic dysfunction.(99 Perren A, Brochard L. Managing the apparent and hidden difficulties of weaning from mechanical ventilation. Intensive Care Med. 2013;39(11):1885-95.) Spontaneous breathing trial-induced increases in extravascular lung water (EVLW) and B-type natriuretic peptide are reliable alternatives to the pulmonary artery catheter for diagnosing weaning-induced pulmonary edema.(1010 Dres M, Teboul JL, Anguel N, Guerin L, Richard C, Monnet X. Extravascular lung water, B-type natriuretic peptide, and blood volume contraction enable diagnosis of weaning-induced pulmonary edema. Crit Care Med. 2014;42(8):1882-9.)
Lung ultrasound (LUS) is a basic application of critical ultrasound - a loop associating urgent diagnoses with immediate therapeutic decisions.(1111 Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):1.,1212 Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T; International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577-91.) Multiple B-lines are considered the sonographic sign of lung interstitial syndrome.(1111 Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):1.
12 Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T; International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577-91.-1313 Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134(1):117-25.) The so-called B-pattern has been validated to measure EVLW,(1414 Enghard P, Rademacher S, Nee J, Hasper D, Engert U, Jörres A, et al. Simplified lung ultrasound protocol shows excellent prediction of extravascular lung water in ventilated intensive care patients. Crit Care. 2015;19:36.
15 Agricola E, Bove T, Oppizzi M, Marino G, Zangrillo A, Margonato A, et al. "Ultrasound comet-tail images": a marker of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest. 2005;127(5):1690-5.-1616 Volpicelli G, Skurzak S, Boero E, Carpinteri G, Tengattini M, Stefanone V, et al. Lung ultrasound predicts well extravascular lung water but is of limited usefulness in the prediction of wedge pressure. Anesthesiology. 2014;121(2):320-7.) and emergency presentations with shortness of breath, patients with known heart failure and fluid overload in the context of chronic hemodialysis have all been studied with LUS.(1717 Noble VE, Murray AF, Capp R, Sylvia-Reardon MH, Steele DJ, Liteplo A. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis. Time course for resolution. Chest. 2009;135(6):1433-9.) LUS has a demonstrated sensitivity of 97-100% and specificity of 95% for detecting acute pulmonary edema.(1313 Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134(1):117-25.,1818 Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16.) In patients with suspected interstitial syndrome, a negative lung ultrasound examination is superior to conventional chest radiography in ruling out significant interstitial syndrome.(1212 Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T; International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577-91.)
Reasons for failure to wean from MV support are often multifactorial and involve a complex interplay between cardiac and pulmonary dysfunction. A recent review suggests the intensivist might productively use ultrasonography to identify impediments to successful extubation.(1919 Mayo P, Volpicelli G, Lerolle N, Schreiber A, Doelken P, Vieillard-Baron A. Ultrasonography evaluation during the weaning process: the heart, the diaphragm, the pleura and the lung. Intensive Care Med. 2016;42(7):1107-17.) To further investigate the relationship between B-lines and MV-weaning, we report the LUS findings of 57 MV subjects subjected to SBT, immediately before and after the procedure.
METHODS
Nonconsecutive individuals older than 18 years of age who had undergone invasive MV for at least 24 hours were enrolled from a medical-surgical, semi-closed unit in a private hospital that is covered full-time by intensivists. Individuals with a tracheostomy were excluded. The Research Ethics board approved the study and waived the requirement for informed consent. The study is registered as NCT02022839 at ClinicalTrials.gov.
Patients were assessed daily for eligibility for weaning according to improvement of underlying condition that led to acute respiratory failure; alert and able to communicate; adequate gas exchange, as indicated by an arterial pressure of oxygen of at least 60mmHg with an inspired fraction of oxygen < 0.40; no significant respiratory acidosis; rapid shallow breathing index equal to or less than 105 cycles per minute per liter; and vasoactive drugs at low and stable doses (norepinephrine doses lower than 0.12µg per kilogram per minute or dopamine equivalent doses).
Spontaneous breathing trial failure was defined as inability to tolerate a T-piece trial of spontaneous breathing for 30 to 120 minutes, in which case subjects were not extubated. The breathing trial was interrupted if the patient developed signs of respiratory discomfort (respiratory frequency > 35 breaths per minute, arterial oxyhemoglobin saturation < 90%, use of accessory respiratory muscles or paradoxical thoracoabdominal ventilation), tachycardia (heart rate more than 140 beats per minute), hemodynamic instability (systolic blood pressure less than 90mmHg or 20% over basal levels) or change in mental status (drowsiness, coma, anxiety). Extubation failure was defined as the need for reintubation within 48 hours after planned removal of the artificial airway.
Demographic data including age, sex, race, comorbidities, and severity of illness at the time of ICU admission, reason for the initiation of MV, physiological weaning predictors and fluid balance in the 48 hours preceding SBT were recorded. The presence of diastolic or systolic left ventricular dysfunction (the latter condition defined as ejection fraction < 45%) was documented according to formal echocardiogram report dated up to six months prior to admission.
A Siemens Sonoline G50 ultrasound machine and a 3.5-MHz curved array probe were used for all examinations. Patients were scanned while in the supine position. Using a longitudinal view, each intercostal space of upper and lower parts of the anterior, lateral, and posterior regions of the left and right chest wall were carefully examined (Figure 1).
Prevalence of B-pattern and consolidation (C-lines) in 12 zones before spontaneous breathing trial in all 57 individuals. At the beginning of the T-piece trial, B-pattern and/or C-lines were already found at the lower and posterior lung regions in more than half of the individuals and remained non-aerated at the end of the trial.
A - right side; B - left side.
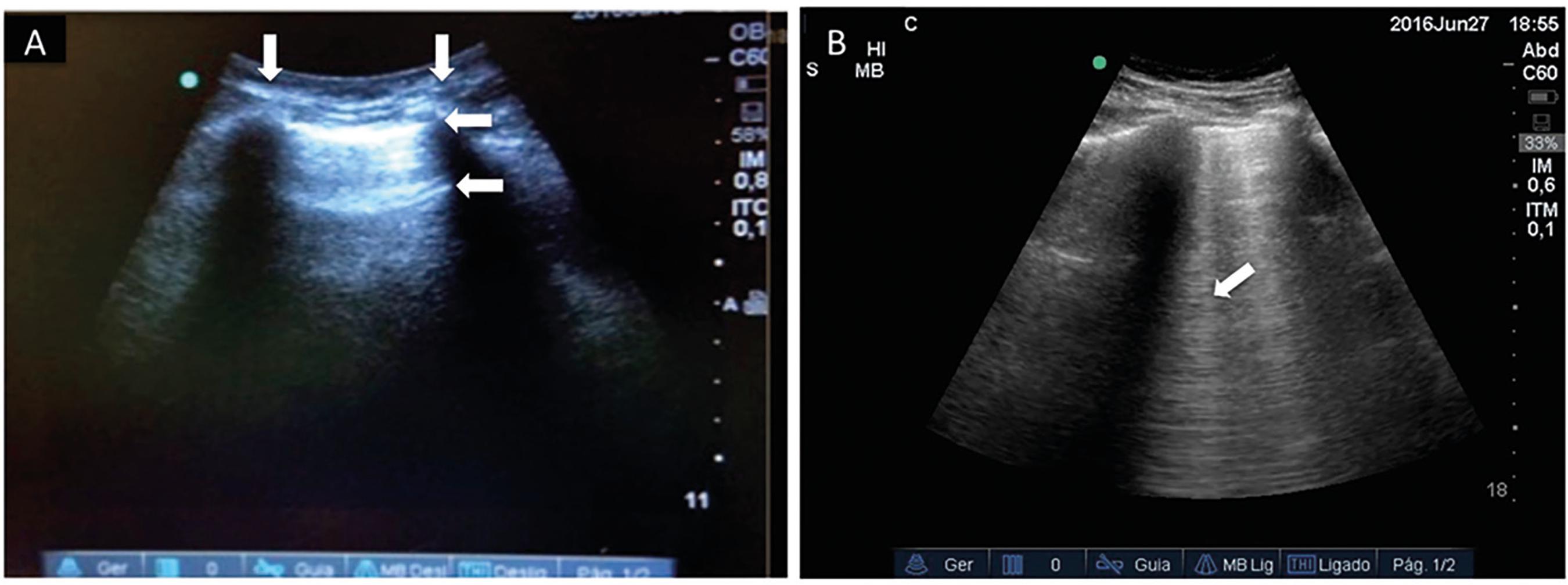
The pleural line, sought between two rib shadows, indicates the pleural layers and generates a permanent landmark known as the bat sign. The pleural line displays sliding of the visceral pleura against the parietal pleura, a movement in rhythm with respiration. The normal lung surface associates lung sliding with horizontal repetitions of the pleural line, called A-lines. These lines indicate physiological or free gas (Figure 2A). B-lines are defined as discrete laser-like vertical hyperechoic reverberation artifacts that arise from the pleural line, extend to the bottom of the screen without fading, move synchronously with lung sliding, and erase A-lines. B-line reflects the coexistence of elements with a major acoustic impedance gradient, such as fluid and air. Fluid at the subpleural interlobular septum surrounded by air-filled alveoli fulfills this condition (Figure 2B).(1111 Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):1.,1212 Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T; International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577-91.,2020 Lichtenstein DA. Ultrasound in the management of thoracic disease. Crit Care Med. 2007;35(5 Suppl):S250-61.,2121 Lichtenstein DA. Lung. In: Lichtenstein DA, editor. General ultrasound in the critically ill. 1. Berlin: Springer; 2002. p. 116.) Three or more B lines in a single view are called a B-pattern. Presence of the B-pattern at two or more regions of the chest bilaterally characterizes interstitial edema.(1212 Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T; International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577-91.) A C-line is a vertical line not originating at the pleural line but inside a consolidated lung tissue or on an irregular lung surface away from the pleural line, leading to ultrasound images similar to liver or splenic tissue. This line corresponds to non-aerated lung tissue such as seen in atelectasis, acute respiratory distress syndrome (ARDS) or pneumonia.(2222 Nalos M, Kot M, McLean AS, Lichtenstein D. Bedside lung ultrasound in the care of the critically ill. Cur Respir Med Rev. 2010;6(4):271-8.) In summary, from A to C-lines, there is a progressive decrease of the air-fluid ratio at the lung parenchyma.
Lung ultrasound is largely based on the interpretation of artifacts created by the interplay of air and fluid in the lung. (A) The ribs on each side of the lung window (vertical arrows) form the bat wings of the "bat" sign, and the hyperechoic pleural line (horizontal arrow at the top) resemble the bat's body. Normal or well-aerated lung tissue leads to the formation of horizontal reverberation artifacts repeated in distance intervals roughly equal to the parietal pleura to the skin distance; these intervals are labeled A-lines (horizontal arrow below pleural line). (B) If the amount of fluid in the lung tissue is increased such as in pulmonary edema, the repetition artifacts multiply and vertical lines appear (B-lines - arrow), erasing A-lines.
Ultrasound evaluations were performed at the following time points: before starting SBT and at its conclusion - either after 30 - 120 minutes, prior to extubation or at the appearance of criteria for SBT interruption. The same trained investigator conducted the LUS assessment at each time point of the study. To avoid expeditious examinations in conditions of overwhelming respiratory distress, immediately before reconnecting the patient to the ventilator, we did not describe patterns of aeration other than the A-line, B-line and C-line; the number of single or confluent B-lines could not be reported.
At the beginning of the T-piece trial, B-pattern and/or C-lines were already found at the lower and posterior lung regions in more than half of the individuals and remained non-aerated at the end of the trial (Figure 1). According to the aforementioned papers,(1313 Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134(1):117-25.,2323 Lichtenstein DA, Mezière GA, Lagoueyte JF, Biderman P, Goldstein I, Gepner A. A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest. 2009;136(4):1014-20.) we postulated that a simplified approach on four chest anterior zones - 1 and 2 on the right and left sides - would be enough for the specific purpose of our study. This concept allowed a dichotomous approach to the lung. Therefore, despite collecting ultrasound data of 12 thoracic regions, only LUS findings on the following four anterior chest zones were analyzed: the intercostal space between the third and fourth ribs, and the intercostal space between the sixth and seventh ribs, to the left and right of the sternum and between the parasternal and midclavicular line. We noted B-predominance as any profile with an anterior bilateral B-pattern, based on previous studies.(1313 Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134(1):117-25.,2323 Lichtenstein DA, Mezière GA, Lagoueyte JF, Biderman P, Goldstein I, Gepner A. A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest. 2009;136(4):1014-20.)
Statistics
The results are expressed as the mean and standard deviation, median and interquartile range, and proportions, as appropriate. The normal distribution of the various parameters was investigated observing the distribution of data and the Shapiro-Wilk test. We used Fisher's exact test to compare proportions. Comparisons among the following three groups were made through one-way analysis of variance (ANOVA) for continuous variables with a normal distribution, and through the Kruskal-Wallis test for variables with a non-normal distribution: patients successfully extubated (successful SBT and extubation group); patients who failed the SBT (SBT failure group); andpatients reintubated within 48 hours (extubation failure group). The sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio and negative likelihood ratio of B-predominance for the prediction of SBT failure and extubation failure were calculated. A p value < 0.05 was considered statistically significant. Statistical analysis was performed with Statistical Package for Social Science (SPSS) version 20.0.
RESULTS
All included individuals were successfully examined, and no dropouts due to poor examination conditions occurred. Forty-six subjects (80.7%) successfully completed the T-piece trial and were immediately extubated; 8 of these subjects required reintubation within 48 hours. The remaining 11 individuals had signs of poor tolerance during SBT and were reconnected to the ventilator. Overall, weaning failure (failed SBT and extubation) occurred in 19 patients (33%). Table 1 shows the baseline characteristics of the cohort according to outcomes. There was a higher prevalence of chronic obstructive pulmonary disease in the SBT failure group (54.5% versus 7.9% and 12.5% in the successful SBT and extubation group and extubation failure group, respectively). Sepsis from any source constituted the main reason for initiating MV in all groups. Thirty-four patients (59.6%) were extubated at the first attempt -, i.e., simple weaning patients.
In the SBT failure group, there was a slightly statistical trend of increasing B-predominance during the T-piece trial (p = 0.07). These subjects also exhibited higher B-predominance compared to the other groups at the end of trial (90% versus to 42.1% and 62.5% in the successful SBT and extubation and in extubation failure groups, respectively; p = 0.01). Although the difference did not reach significance (p = 0.26), the successful SBT and extubation group started the procedure with a lower B-predominance (39.5% compared to 63.6% and 50% in, respectively, the SBT failure and in extubation failure groups) (Table 2).
B-predominance prior to spontaneous breathing trial and at the end of trial according to weaning groups
Table 3 shows the sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio and negative likelihood ratio of B-predominance for the prediction of SBT failure and extubation failure outcomes.
DISCUSSION
We presented an analysis of changes observed in LUS findings before and after SBT; while we acknowledge the low sample size of this study, our results lend credence to the idea that the increments of B-pattern on four anterior chest zones in subjects who failed T-piece trial represent a cardiac disturbance mechanism. Prior to conducting the T-piece trial, however, we were not able to identify individuals who would fail SBT or who would need reintubation within 48 hours.
Rapid changes in the respiratory and cardiac load occurring throughout SBT might manifest with dynamic changes in LUS that are only visible with real-time scanning. At the start of the trial, we could not demonstrate statistically significant differences in B-predominance among groups, conceivably because of type II error. During the trial, the SBT-failure group behaved differently, exhibiting higher increases in LUS B-predominance, similar to the other parameters of lung mechanics, hemodynamic performance and global tissue oxygenation.(2424 Jubran A, Mathru M, Dries D, Tobin MJ. Continuous recordings of mixed venous oxygen saturation during weaning from mechanical ventilation and the ramifications thereof. Am J Respir Crit Care Med. 1998;158(6):1763-9.) The clinical utility of such findings is uncertain because the clinical manifestations of severe respiratory distress were already evident at the moment of its detection.
The initiation of SBT after a period of MV is associated with some loss of lung aeration in critically ill subjects. Using the same LUS score technique as Bouhemad et al. (lower scores = better aeration),(2525 Bouhemad B, Liu ZH, Arbelot C, Zhang M, Ferarri F, Le-Guen M, et al. Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit Care Med. 2010;38(1):84-92.) Soummer et al.(2626 Soummer A, Perbet S, Brisson H, Arbelot C, Constantin JM, Lu Q, Rouby JJ; Lung Ultrasound Study Group. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress*. Crit Care Med. 2012;40(7):2064-72.) showed that progressive lung derecruitment during an SBT identified patients likely to fail extubation. At the end of the SBT, patients with an LUS score of less than 13 had a 9% risk of post-extubation failure (4 of 43), whereas patients with an LUS score of more than 17 had an 85% risk of post-extubation failure (18 of 21). An end SBT LUS score between 13 and 17, seen in 25% of patients, did not allow for an accurate prediction of the extubation outcome. It may not be possible to draw conclusions regarding the risk of failed SBT prior to a T-piece trial.
Our data showed a lack of B-predominance accuracy to predict the need for reintubation within 48 hours. Given our small sample size, it is unclear whether considering the simplified four-region LUS protocol is truly imprecise for such purposes. However, considering that extubation failure might occur due to causes other than imbalances between cardiorespiratory capacity and load (failure to maintain airway patency due to upper airway edema, excessive secretions, inadequate muscle strength, neurological impairment, etc.), the behavior of the LUS findings during SBT might not portend reintubation rates accurately.
The quantification of pulmonary over-hydration was not the main scope of our investigation; however, from a practical point of view, the B-pattern indicates an increase in extravascular lung water with an absolute sensitivity.(2727 Shyamsundar M, Attwood B, Keating L, Walden AP. Clinical review: the role of ultrasound in estimating extra-vascular lung water. Crit Care. 2013;17(5):237.) An association between the absence of B-lines detected by LUS and a low level of wedge pressure (pulmonary artery occlusion pressure) has been reported; nonetheless, B-predominance is observed in a wide range of pulmonary artery occlusion pressure values, precluding firm conclusions for the need of fluid withdrawal.(2323 Lichtenstein DA, Mezière GA, Lagoueyte JF, Biderman P, Goldstein I, Gepner A. A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest. 2009;136(4):1014-20.) Other observational studies demonstrated a better specificity of the finding of B-pattern in detecting elevated EVLW by the trans-pulmonary thermodilution method (PiCCO system).(1515 Agricola E, Bove T, Oppizzi M, Marino G, Zangrillo A, Margonato A, et al. "Ultrasound comet-tail images": a marker of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest. 2005;127(5):1690-5.,1616 Volpicelli G, Skurzak S, Boero E, Carpinteri G, Tengattini M, Stefanone V, et al. Lung ultrasound predicts well extravascular lung water but is of limited usefulness in the prediction of wedge pressure. Anesthesiology. 2014;121(2):320-7.) Enghard et al.(1414 Enghard P, Rademacher S, Nee J, Hasper D, Engert U, Jörres A, et al. Simplified lung ultrasound protocol shows excellent prediction of extravascular lung water in ventilated intensive care patients. Crit Care. 2015;19:36.) applied a four-region LUS protocol and found a good correlation with trans-pulmonary thermodilution measurements. Finally, Dres et al.(1010 Dres M, Teboul JL, Anguel N, Guerin L, Richard C, Monnet X. Extravascular lung water, B-type natriuretic peptide, and blood volume contraction enable diagnosis of weaning-induced pulmonary edema. Crit Care Med. 2014;42(8):1882-9.) reported a link between SBT-induced increases in EVLW and weaning failure of cardiac origin with a specificity of 100%.
The present study is practical, qualitative, and highly reproducible.(1313 Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134(1):117-25.,2323 Lichtenstein DA, Mezière GA, Lagoueyte JF, Biderman P, Goldstein I, Gepner A. A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest. 2009;136(4):1014-20.) Documenting, for instance, the lateral walls, cardiac function, volume of pleural effusion, and vein calipers could provide additional information but would undermine simplicity. In this preliminary approach, the authors did not focus on posterior changes because posterior B-lines might indicate gravitational changes. Reducing scanning to four anterior chest zones is aimed to facilitate the initial assessment of this subset of patients through a simple, rapid and easy-to-perform method. Within 1 minute of LUS examination, researchers were able to acquire valuable information regarding the diagnosis of lung edema. The LUS score as presented(2525 Bouhemad B, Liu ZH, Arbelot C, Zhang M, Ferarri F, Le-Guen M, et al. Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit Care Med. 2010;38(1):84-92.,2626 Soummer A, Perbet S, Brisson H, Arbelot C, Constantin JM, Lu Q, Rouby JJ; Lung Ultrasound Study Group. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress*. Crit Care Med. 2012;40(7):2064-72.) has utility as a research tool, but might be overly complicated for the frontline intensivist to use in a busy ICU. We did not compare different protocols using, for example, an 8-, 12- or even a 28-zone approach, so no final conclusions could be drawn regarding the superiority of these approaches.
Our major limitations are the fact that this study was done at a single center and using a small sample size. Lung ultrasound examinations were performed only during working hours. The choice of a convenient sample and the small sample size also limit the interpretation and generalization of the findings. The overall rate of weaning failure was relatively high (33%). The rate of reintubation following extubation (17.4%) was, however, comparable to rates that have been reported before,(2828 Krinsley JS, Reddy PK, Iqbal A. What is the optimal rate of failed extubation? Crit Care. 2012;16(1):111.) as well as the prevalence of simple-weaning (75%),(11 Tobin MJ, Jubran A. Weaning from mechanical ventilation. In: Tobin MJ, editor. Principles and practice of mechanical ventilation. 3rd ed. New York: McGraw-Hill; 2012. p. 1185-220.
2 MacIntyre NR, Cook DJ, Ely EW Jr, Epstein SK, Fink JB, Heffner JE, Hess D, Hubmayer RD, Scheinhorn DJ; American College of Chest Physicians; American Association for Respiratory Care; American College of Critical Care Medicine. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest. 2001;120(6 Suppl):375S-95S.-33 Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29(5):1033-56.) indicating that our prospective convenience sample had the same expected pre-test probability of SBT failure as any ordinary, medical-surgical ICU population. Like all techniques of ultrasonography, bedside LUS could be operator-dependent; however, a high intra- and inter-observer reproducibility has been reported.(2525 Bouhemad B, Liu ZH, Arbelot C, Zhang M, Ferarri F, Le-Guen M, et al. Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit Care Med. 2010;38(1):84-92.)
CONCLUSION
Our study does not allow for general conclusions, but some important points could be inferred. Scanning of four regions is quite feasible and time-saving, as long as inferior and posterior B-lines might reflect gravitational changes. We speculate that a higher loss of lung aeration during a spontaneous breathing trial suggests weaning-induced cardiovascular dysfunction and increases in extravascular lung water.
The observation that spontaneous breathing trial-failure subjects display more severely deranged lung mechanics than successfully extubated subjects raises the question of whether these derangements might be detectable while patients are receiving full ventilatory support. Usual practice, physiology and well-known causes of weaning failure all support the use of lung ultrasound to identify patients who are at high risk of a failed spontaneous breathing trial. However, we do concede that these data need to be confirmed with an enlarged sample population to reduce the considerable data dispersion affecting the study. Therefore, we designed a multicenter observational study to evaluate whether lung ultrasound findings prior to T-piece trial are able to predict the earliest time that an individual might resume spontaneous breathing.
-
Responsible editor: Jorge Ibrain Figueira Salluh
REFERÊNCIAS
-
1Tobin MJ, Jubran A. Weaning from mechanical ventilation. In: Tobin MJ, editor. Principles and practice of mechanical ventilation. 3rd ed. New York: McGraw-Hill; 2012. p. 1185-220.
-
2MacIntyre NR, Cook DJ, Ely EW Jr, Epstein SK, Fink JB, Heffner JE, Hess D, Hubmayer RD, Scheinhorn DJ; American College of Chest Physicians; American Association for Respiratory Care; American College of Critical Care Medicine. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest. 2001;120(6 Suppl):375S-95S.
-
3Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29(5):1033-56.
-
4Macintyre NR. Evidence-based assessments in the ventilator discontinuation process. Respir Care. 2012;57(10):1611-8.
-
5Tanios MA, Nevins ML, Hendra KP, Cardinal P, Allan JE, Naumova EN, et al. A randomized, controlled trial of the role of weaning predictors in clinical decision making. Crit Care Med. 2006;34(10):2530-5.
-
6Savi A, Teixeira C, Silva JM, Borges LG, Pereira PA, Pinto KB, Gehm F, Moreira FC, Wickert R, Trevisan CB, Maccari JG, Oliveira RP, Vieira SR; Gaúcho Weaning Study Group. Weaning predictors do not predict extubation failure in simple-to-wean patients. J Crit Care. 2012;27(2):221.e1-8.
-
7Teboul JL, Monnet X, Richard C. Weaning failure of cardiac origin: recent advances. Crit Care. 2010;14(2):211.
-
8Teboul JL. Weaning-induced cardiac dysfunction: where are we today? Intensive Care Med. 2014;40(8):1069-79.
-
9Perren A, Brochard L. Managing the apparent and hidden difficulties of weaning from mechanical ventilation. Intensive Care Med. 2013;39(11):1885-95.
-
10Dres M, Teboul JL, Anguel N, Guerin L, Richard C, Monnet X. Extravascular lung water, B-type natriuretic peptide, and blood volume contraction enable diagnosis of weaning-induced pulmonary edema. Crit Care Med. 2014;42(8):1882-9.
-
11Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):1.
-
12Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T; International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577-91.
-
13Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134(1):117-25.
-
14Enghard P, Rademacher S, Nee J, Hasper D, Engert U, Jörres A, et al. Simplified lung ultrasound protocol shows excellent prediction of extravascular lung water in ventilated intensive care patients. Crit Care. 2015;19:36.
-
15Agricola E, Bove T, Oppizzi M, Marino G, Zangrillo A, Margonato A, et al. "Ultrasound comet-tail images": a marker of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest. 2005;127(5):1690-5.
-
16Volpicelli G, Skurzak S, Boero E, Carpinteri G, Tengattini M, Stefanone V, et al. Lung ultrasound predicts well extravascular lung water but is of limited usefulness in the prediction of wedge pressure. Anesthesiology. 2014;121(2):320-7.
-
17Noble VE, Murray AF, Capp R, Sylvia-Reardon MH, Steele DJ, Liteplo A. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis. Time course for resolution. Chest. 2009;135(6):1433-9.
-
18Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16.
-
19Mayo P, Volpicelli G, Lerolle N, Schreiber A, Doelken P, Vieillard-Baron A. Ultrasonography evaluation during the weaning process: the heart, the diaphragm, the pleura and the lung. Intensive Care Med. 2016;42(7):1107-17.
-
20Lichtenstein DA. Ultrasound in the management of thoracic disease. Crit Care Med. 2007;35(5 Suppl):S250-61.
-
21Lichtenstein DA. Lung. In: Lichtenstein DA, editor. General ultrasound in the critically ill. 1. Berlin: Springer; 2002. p. 116.
-
22Nalos M, Kot M, McLean AS, Lichtenstein D. Bedside lung ultrasound in the care of the critically ill. Cur Respir Med Rev. 2010;6(4):271-8.
-
23Lichtenstein DA, Mezière GA, Lagoueyte JF, Biderman P, Goldstein I, Gepner A. A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest. 2009;136(4):1014-20.
-
24Jubran A, Mathru M, Dries D, Tobin MJ. Continuous recordings of mixed venous oxygen saturation during weaning from mechanical ventilation and the ramifications thereof. Am J Respir Crit Care Med. 1998;158(6):1763-9.
-
25Bouhemad B, Liu ZH, Arbelot C, Zhang M, Ferarri F, Le-Guen M, et al. Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit Care Med. 2010;38(1):84-92.
-
26Soummer A, Perbet S, Brisson H, Arbelot C, Constantin JM, Lu Q, Rouby JJ; Lung Ultrasound Study Group. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress*. Crit Care Med. 2012;40(7):2064-72.
-
27Shyamsundar M, Attwood B, Keating L, Walden AP. Clinical review: the role of ultrasound in estimating extra-vascular lung water. Crit Care. 2013;17(5):237.
-
28Krinsley JS, Reddy PK, Iqbal A. What is the optimal rate of failed extubation? Crit Care. 2012;16(1):111.
Publication Dates
-
Publication in this collection
21 Aug 2017 -
Date of issue
Jul-Sep 2017
History
-
Received
24 Dec 2016 -
Accepted
08 Mar 2017