Abstract
In this research, a novel organic photocatalyst of 10-(Perylene-3-yl-10H-Phenoxazine (PHP) has been synthesized successfully from perylene and phenoxazine via Buchwald-Hartwig C-N coupling. The chemical structure of catalyst was determined via proton nuclear magnetic resonance (1H-NMR) spectrum and optical properties were investigated through UV-Vis spectroscopy. The PHP has been used as the reducing photoredox catalyst for organocatalyzed atom transfer radical polymerization (ATRP) under UV irradiation. The well controlled molecular weight of polymers based on methyl methacrylate monomers have been obtained with monomer conversion up to 77.61% and low polydispersity index under 1.5.
Keywords:
methacrylate monomer; phenoxazine; organic photocatalyst; atom transfer radical polymerization
1. Introduction
The polymerization for synthetic polymers according to metal catalysts by the ATRP mechanism is considered as the advanced process in the polymer industry[11 Zetterlund, P. B., Kagawa, Y., & Okubo, M. (2008). Controlled/Living radical polymerization in dispersed systems. Chemical Reviews, 108(9), 3747-3794. http://dx.doi.org/10.1021/cr800242x. PMid:18729519.
http://dx.doi.org/10.1021/cr800242x...
2 Moad, G., Rizzardo, E., & Thang, S. H. (2008). Radical addition-fragmentation chemistry in polymer synthesis. Polymer, 49(5), 1079-1131. http://dx.doi.org/10.1016/j.polymer.2007.11.020.
http://dx.doi.org/10.1016/j.polymer.2007...
-33 Keddie, D. J., Carlos, G. S., Moad, G., Rizzardo, E., & Thang, S. H. (2011). Switchable Reversible Addition–Fragmentation Chain Transfer (RAFT) polymerization in aqueous solution, N, N-Dimethylacrylamide. Macromolecules, 44(17), 6738-6745. http://dx.doi.org/10.1021/ma200760q.
http://dx.doi.org/10.1021/ma200760q...
]. The use of transition metal during synthetic procedure gives many advantages including controlled the molecular weight and polydispesity index of obtained polymer as well as controlled the end-groups of obtained polymers but the obtained polymeric products always remain the trace of transition metals[44 Matyjaszewski, K., & Xia, J. (2001). Atom transfer radical Polymerization. Chemical Reviews, 101(9), 2921-2990. http://dx.doi.org/10.1021/cr940534g. PMid:11749397.
http://dx.doi.org/10.1021/cr940534g...
,55 Matyjaszewski, K. (2012). Atom Transfer Radical Polymerization (ATRP): Current status and future perspectives. Macromolecules, 45(10), 4015-4039. http://dx.doi.org/10.1021/ma3001719.
http://dx.doi.org/10.1021/ma3001719...
]. This elimination of metals catalyst caused tremendous damage in the subsequent use of polymer products in bio-medicine, opto-electronic application. To overcome this issue, the organic photocatalyst (O-ATRP/metal-free ATRP) has been developed for synthesis of the metal-free polymer via controlled radical polymerization[66 Shanmugam, S., Xu, J., & Boyer, C. (2017). Photocontrolled living polymerization systems with reversible deactivations through electron and energy transfer. Macromolecular Rapid Communications, 38(13), 1700143. http://dx.doi.org/10.1002/marc.201700143. PMid:28556363.
http://dx.doi.org/10.1002/marc.201700143...
] which gradually replace the traditional ATRP-based transition metal catalysts[66 Shanmugam, S., Xu, J., & Boyer, C. (2017). Photocontrolled living polymerization systems with reversible deactivations through electron and energy transfer. Macromolecular Rapid Communications, 38(13), 1700143. http://dx.doi.org/10.1002/marc.201700143. PMid:28556363.
http://dx.doi.org/10.1002/marc.201700143...
7 Miyake, G. M., & Theriot, J. C. (2014). Perylene as an organic photocatalyst for the radical polymerization of functionalized vinyl monomers through oxidative quenching with alkyl bromides and visible light. Macromolecules, 47(23), 8255-8261. http://dx.doi.org/10.1021/ma502044f.
http://dx.doi.org/10.1021/ma502044f...
-88 Theriot, J. C., Lim, C. H., Yang, H., Ryan, M. D., Musgrave, C. B., & Miyake, G. M. (2016). Organocatalyzed atom transfer radical polymerization driven by visible light. Science, 352(6289), 1082-1086. http://dx.doi.org/10.1126/science.aaf3935. PMid:27033549.
http://dx.doi.org/10.1126/science.aaf393...
]. There have been many studies on the field of O-ATRP polymerization using organic photocatalyst[77 Miyake, G. M., & Theriot, J. C. (2014). Perylene as an organic photocatalyst for the radical polymerization of functionalized vinyl monomers through oxidative quenching with alkyl bromides and visible light. Macromolecules, 47(23), 8255-8261. http://dx.doi.org/10.1021/ma502044f.
http://dx.doi.org/10.1021/ma502044f...
,88 Theriot, J. C., Lim, C. H., Yang, H., Ryan, M. D., Musgrave, C. B., & Miyake, G. M. (2016). Organocatalyzed atom transfer radical polymerization driven by visible light. Science, 352(6289), 1082-1086. http://dx.doi.org/10.1126/science.aaf3935. PMid:27033549.
http://dx.doi.org/10.1126/science.aaf393...
] and light for the catalyst activation process[99 Corrigan, N., Yeow, J., Judzewitsch, P., Xu, J., & Boyer, C. (2019). Seeing the light: advancing materials chemistry through Photopolymerization. Angewandte Chemie International Edition, 58(16), 5170-5189. http://dx.doi.org/10.1002/anie.201805473. PMid:30066456.
http://dx.doi.org/10.1002/anie.201805473...
]. Matyjaszewski and colleagues have used phenoloxazine and phenolthiazine as organic photocatalysts for the controlled polymerization of acrylonitrile[1010 Pintauer, T., Zhou, P., & Matyjaszewski, K. (2002). General method for determination of the activation, deactivation, and initiation rate constants in transition metal-catalyzed atom transfer radical processes. Journal of the American Chemical Society, 124(28), 8196-8197. http://dx.doi.org/10.1021/ja0265097. PMid:12105893.
http://dx.doi.org/10.1021/ja0265097...
]. Miyake and colleagues used organic catalysts (perylene, diaryl dihydrophenazines) for ATRP of MMA monomer under visible light[1111 Dadashi‐Silab, S., Pan, X., & Matyjaszewski, K. (2017). Phenyl Benzo [b] Phenothiazine as a visible light Photoredox Catalyst for metal‐free atom transfer radical Polymerization. Chemistry (Weinheim an der Bergstrasse, Germany), 23(25), 5972-5977. http://dx.doi.org/10.1002/chem.201605574. PMid:28009492.
http://dx.doi.org/10.1002/chem.201605574...
]. Further, Cheng and colleagues used fluorescein as an organic catalyst to control polymerization of MMA[1212 Corrigan, N., Shanmugam, S., Xu, J., & Boyer, C. (2016). Photocatalysis in organic and polymer synthesis. Chemical Society Reviews, 45(22), 6165-6212. http://dx.doi.org/10.1039/C6CS00185H. PMid:27819094.
http://dx.doi.org/10.1039/C6CS00185H...
]. In addition, the metal-free ATRP has been applied for modification/functionalization of polymer surfaces which enhanced the reactivity of polymers[1313 Matyjaszewski, K., & Tsarevsky, N. V. (2014). Macromolecular engineering by atom transfer radical polymerization. Journal of the American Chemical Society, 136(18), 6513-6533. http://dx.doi.org/10.1021/ja408069v. PMid:24758377.
http://dx.doi.org/10.1021/ja408069v...
]. It is clearly that a number of phenoxazine derivatives have been developed as visible light absorbing as organic photoredox catalysts (PCs) with excited state reduction potentials. The phenoxazine derivatives have been modified through extending the conjugation on the phenoxazine core via installation of biphenyl core substituents[1414 McCarthy, B., & Miyake, G. M. (2018). Organocatalyzed atom transfer radical polymerization catalyzed by core modified N-Aryl Phenoxazines Performed under Air. ACS Macro Letters, 7(8), 1016-1021. http://dx.doi.org/10.1021/acsmacrolett.8b00497. PMid:31827976.
http://dx.doi.org/10.1021/acsmacrolett.8...
,1515 McCarthy, B. G., Pearson, R. M., Lim, C. H., Sartor, S. M., Damrauer, N. H., & Miyake, G. M. (2017). Structure-Property relationships for Tailoring Phenoxazines as reducing Photoredox Catalysts. Journal of the American Chemical Society, 140(15), 5088-5101. http://dx.doi.org/10.1021/jacs.7b12074. PMid:29513533.
http://dx.doi.org/10.1021/jacs.7b12074...
]. In addition, the perylene was the first organic photoredox catalysts for O-ATRP using visible light-absorbing, but it is less efficient compared to these other PC families[77 Miyake, G. M., & Theriot, J. C. (2014). Perylene as an organic photocatalyst for the radical polymerization of functionalized vinyl monomers through oxidative quenching with alkyl bromides and visible light. Macromolecules, 47(23), 8255-8261. http://dx.doi.org/10.1021/ma502044f.
http://dx.doi.org/10.1021/ma502044f...
]. However, the combination of phenoxazine with perylene as a photocatalyst 10-(Perylene-3-yl-10H-Phenoxazine) have not been reported which can be proposed to enhance the excited state reduction potentials of PC that would be efficient for O-ATRP process.
In this research, the novel organic photocatalyst 10-(Perylene-3-yl-10H-Phenoxazine) (PHP) have been synthesized and applied for the polymerization of MMA monomer as the first time. The structure of PHP has been characterized via FTIR and 1H NMR spectroscopy. In addition, the optical properties of PHP catalyst have been evaluated via UV-Vis spectroscopy. We also investigated the efficiency of PHP organic photocatalyst for O-ATRP process of MMA monomer.
2. Materials and Methods
2.1 Materials
Perylene (99%), Phenoxazine (99%), Pd(OAc)2 (99%) were purchased by Sigma Aldrich. NBS (99%), P(t-Bu)3 (98%), NaOt-Bu (97%), MMA (99%) were purchased by Merck. Phenyl 2-bromo-2-methylpropanoate (C10H11BrO2) was synthesized at our lab. All the solvents were purchased from Fisher Chemicals.
2.2 Characterization
1H-NMR spectra were recorded in deuterated chloroform (CDCl3) with tetramethylsilane as an internal reference, on a Bruker Avance 500 MHz. UV-vis spectrum of polymer samples was recorded at the key laboratory, Department of Materials Technology - Ho Chi Minh City University of Technology, on Shimadzu UV-Vis 2450 of Shimadzu Sciencetific at room temperature (25 °C) with a range of 300 nm to 800 nm, scanning speed of 200 nm/min. The spectra of GPC were recorded at the key laboratory, Department of Materials Technology - Ho Chi Minh City University of Technology, on Polymer PL-GPC 50.
2.3 Synthesis of 1-Bromoperylene
Perylene (600 mg, 2.378 mmol) and 10 ml anhydrous DMF were added into 50 ml two – necked flask at 0 °C. Aluminum foil was thoroughly wrapped around to cover the reaction vial, blocking out light. In the dark, N-bromosuccinimide (NBS) (423.25 mg, 2.378 mmol) in 5 ml anhydrous DMF was slowly added to this solution using dropping funnel, and stirred for 4 h at 0°C and 20 h at room temperature. Following, the reaction was terminated with a 2M HCl solution, extracted with 100 ml CHCl3 and dried with anhydrous K2SO4. The product was precipitated in cold n-hexane and dried under vacuum to give a yellow powder (590.71 mg; 75%).
1H NMR (500 MHz, CDCl3), δ (ppm): 8.23 (d, 1H), 8.20 (d, 1H), 8.15 (d, 1H), 8.07 (d, 1H), 7.99 (d, 1H), 7.76 (d, 1H), 7.71 (d, 2H), 7.58 (t, 1H), 7.49 (t, 2H).
2.4 Synthesis of 10- (Perylene-3-yl-10H-Phenoxazine (PHP)
A literature procedure was adapted for this synthesis. A 50 ml storage flask was charged with magnetic stir bar, flamed under vacuum and back-filled with nitrogen three times. The flask was then charged with phenoxazine (183.21 mg), NaOtBu (144.15 mg), Pd(OAc)2 catalyst (4.49 mg), P(tBu)3 (8.09 mg) and dry toluene (45 ml). The flask was evacuated and back-filled three times with nitrogen before 3-bromoperylene (331.21 mg) was added. The flask was then placed in an oil bath at 110 oC under while stirring for 24 hours. The flask was then cooled to room temperature and solution was diluted with CHCl3, washed with water, brine, dried with K2CO3 and purified using column chromatography (3.3% EtOAc/hexane). The product was dried under reduced pressure to yield 78% of a red orange solid.
1H NMR (500 MHz, CDCl3), δ (ppm): 8.34 – 7.48 (m, 11H), 6.73 (d, 2H), 6.64 (t, 2H), 6.53 (t, 2H), 5.89 (d, 2H).
2.5 Synthesis of initiator of phenyl 2-bromo-2-methylpropanoate (PhBMP-initiator).
A 50 ml storage flask was charged with magnetic stir bar, flamed under vacuum and back-filled with nitrogen three times. 10 mg (0.106 mmol) of phenol was added to anhydrous THF (10 ml) at 0 oC under nitrogen. Then, 21.45 mg (0.116 mmol) of 2-bromo-2-methylpropanoyl chloride in 10 ml of THF was dropwise added to the mixture reaction at 0 oC. The mixture was continuously stirred at room temperature for 4 h. After completion of the reaction, 10 mL of distilled water was added to the reaction mixture, which was extracted with dichloromethane. The organic layer was washed with 10% solution of Na2S2O3 and 10% solution of KOH, dried over anhydrous K2CO3. The product was purified via column chromatography using eluent of EtOAc/hexane (50/50) to obtained the pure product of phenyl 2-bromo-2-methylpropanoate as colorless liquid (Yield: 97%, Rf = 0.7).
1H NMR (500 MHz, CDCl3), δ (ppm): 7.31 (t, 3H), 7.520 (d, 2H), 1.98 (d, 6H).
2.6 Measure UV-vis of PHP and Perylene at different concentrations
10-(Perylene-3-yl-10H-Phenoxazine (PHP) and perylene were analyzed by UV-vis to compare the spectral absorption in soluble form in THF solvent. Perylene and PHP were dissolved in THF at different concentrations of 50, 40, 30, 20, 10 (μM) as measured by Shimadzu UV-Vis 2450 at room temperature range of 200 nm to 800 nm, 50 nm/min.
2.7 General Synthesis of Polymers
PMMA was synthesized via UV light-induced metal-free ATRP using the PhBMP-initiator and PHP as organic photocatalyst. In a typical experiment, 11.43 mg (47 μmol) of PhBMP initiator was placed in a 25 mL flask, to the solution 1 mL of degassed THF was added by a syringe. The solution was stirred until it became homogeneous solution. Then, MMA monomer (0.5 mL, 4.7 mmol) and PHP (2.1 mg, 4.7 μmol) was added separately. The mixture was degassed by three freeze-pump-thaw cycles. The solution was continuously stirred until it became homogeneous and placed in a UV-box (wavelength of 365 nm) for 24 h at room temperature. Finally, the resulted polymer solution was precipitated in cold methanol, followed drying under vacuum to give the desired product.
3. Results and Discussion
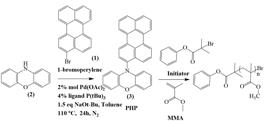
The synthesis of organic photocatalyst 10- (Perylene-3-yl-10H-Phenoxazine (PHP) was illustrated in Scheme 1. 1-bromo perylene was synthesized through bromination of electrophilic substitution using NBS in DMF, and the yield of reaction was obtained as 95%. Then 1-bromo pyrelene was reacted with phenothiazine via Buchwald-Hartwig C-N coupling in the presence of Pd(OAc)2 and P(t-Bu)3 as catalyst and ligand, respectively (Yield: 78%).
The chemical structure of PHP was analyzed via 1H NMR spectrum in Figure 1. The data showed that 1H NMR spectrum of PHP exhibited fully characteristic peaks of PHP including peaks of phenoloxazine and perylene. The peaks from 7.48 ppm to 8.34 ppm are corresponding to the protons of perylene. The peaks from 5.89 ppm to 6.73 ppm which assigned to the protons of phenoloxazine ring. Based on the characteristics peaks are reasonable their integration, the obtained product has been concluded that PHP catalyst was synthesized successfully.
Based on the UV-vis spectrum of PHP catalyst (Figure 2), we recognized that that the PHP absorbs the wavelength from 200 – 500 nm while the pyrene absorbs the wavelength from 200 – 450 nm. The UV-vis spectrum of 10-(Perylene-3-yl-10H-Phenoxazine (PHP) curves exhibited two distinct absorption peaks at 250 and 450 nm which corresponding to the absorption of phenoxazine and pyrene moieties, respectively. Moreover, the spectra showed a linear correlation between concentration and absorbance, and the molar extinction coefficient was determined through the Lambert – Beer law. In addition, the PHP exhibited the high intensity light absorption at wavelength λmax1 = 441 nm, λmax2 = 417 nm with coefficient is ε1 = 40870 (M-1.cm-1), ε2 = 36980 (M-1.cm-1). This results confirmed that 10-(Perylene-3-yl-10H-Phenoxazine (PHP) catalyst can be activated in visible light (violet/blue light).
To see more clearly, we consider the same concentration of perylene precursor and PHP at 50 µM (Figure 3)
The spectrum showed that PHP has higher absorbance than perylene in the same range of wavelength. For example, at a wavelength of λ = 440 nm, the absorbance of perylene was 0.536 while PHP was 2.093. This result suggested that the high absorption intensity of PHP is higher than those of perylene that lead to the activation efficiency for organic photocatalyst polymerization.
According to the pioneer work of Hawker[1616 Treat, N. J., Sprafke, H., Kramer, J. W., Clark, P. G., Barton, B. E., Read de Alaniz, J., Fors, B. P., & Hawker, C. J. (2014). Metal-free atom transfer radical Polymerization. Journal of the American Chemical Society, 136(45), 16096-16101. http://dx.doi.org/10.1021/ja510389m. PMid:25360628.
http://dx.doi.org/10.1021/ja510389m...
], Matyjaszewski[55 Matyjaszewski, K. (2012). Atom Transfer Radical Polymerization (ATRP): Current status and future perspectives. Macromolecules, 45(10), 4015-4039. http://dx.doi.org/10.1021/ma3001719.
http://dx.doi.org/10.1021/ma3001719...
,1313 Matyjaszewski, K., & Tsarevsky, N. V. (2014). Macromolecular engineering by atom transfer radical polymerization. Journal of the American Chemical Society, 136(18), 6513-6533. http://dx.doi.org/10.1021/ja408069v. PMid:24758377.
http://dx.doi.org/10.1021/ja408069v...
], and Miyake[88 Theriot, J. C., Lim, C. H., Yang, H., Ryan, M. D., Musgrave, C. B., & Miyake, G. M. (2016). Organocatalyzed atom transfer radical polymerization driven by visible light. Science, 352(6289), 1082-1086. http://dx.doi.org/10.1126/science.aaf3935. PMid:27033549.
http://dx.doi.org/10.1126/science.aaf393...
,1717 Pearson, R. M., Lim, C. H., McCarthy, B. G., Musgrave, C. B., & Miyake, G. M. (2016). Organocatalyzed atom transfer radical polymerization using N-Aryl Phenoxazines as Photoredox Catalysts. Journal of the American Chemical Society, 138(35), 11399-11407. http://dx.doi.org/10.1021/jacs.6b08068. PMid:27554292.
http://dx.doi.org/10.1021/jacs.6b08068...
], we carried out the following process for metal-free ATRP where PHP is used as the organic photocatalyst. Under UV irradiation, PHP is excited to form a reductant PHP*, which activates phenyl 2-bromo-2-methylpropanoate (PBMP) initiator and generates radicals. The generated radical can be added to methyl methacrylate monomers (MMA) to form alkyl radicals which are deactivated by the oxidized radical cation PHP* to regenerate the ground state of PHP. In this typical O-ATRP, we also investigated the solvents, the molar ratio of PHP catalyst with initiator, monomers which impact to the efficiency of polymerization.
The polymerization process is performed in the presence of the PHP catalyst following the O-ATRP procedure. The content of catalyst at 0.5; 0.1; 0.05; 0.02 equivalent has been investigated for polymerization of methyl methacrylate. The reactions were carried out in THF solvent under UV irradiation of 365 nm, 24 hours. The results of O-ATRP for MMA polymerization using PHP catalyst are presented in the following Table 1.
The result showed that the amount of PHP about 5% molar ratio comparing with initiator give the high monomer conversion of 77.61% for 24 h. On the other hand, the conversion of monomer in the polymerization was decreased if the amount of PHP catalyst decreased. It should be noted that the resulted PMMA exhibited the Mn of 30.450 g/mol which is with the polydispersity index (Đ) of obtained PMMA exhibited the value of 1.28 which is reasonable for controlled polymerization (normally Đ is required below 1.5) (Figure 4).
4. Conclusion
10-(perylen-yl)-10H-phenoxazine (PHP) has been synthesized based on phenoxazine and pyrene has been proved to be an efficient metal-free catalyst for O-ATRP which produced polymethacrylates with controlled molecular weight of 30.457 g/mol as well as narrow polydispersity of 1.28 by UV irradiation. This research enables the synthesis procedure for potential bio/electronic polymeric materials which will eliminate the trace metal element in final polymeric compound.
5. Acknowledgement
This research is funded by Vietnam National University Ho Chi Minh City (VNU-HCM) under grant number NV2019-20-03.
-
How to cite: Vo, T. H., Le, H. T., Nguyen, T. A., Ho, N. Q., Le, T. V., Tran, D. H., Truong, T. T., & Nguyen, H. T. (2020). Synthesis of novel organocatalyzed phenoxazine for free metal atom transfer radical polymerization. Polímeros: Ciência e Tecnologia, 30(2), e2020018. https://doi.org/10.1590/0104-1428.10119.
6. References
-
1Zetterlund, P. B., Kagawa, Y., & Okubo, M. (2008). Controlled/Living radical polymerization in dispersed systems. Chemical Reviews, 108(9), 3747-3794. http://dx.doi.org/10.1021/cr800242x PMid:18729519.
» http://dx.doi.org/10.1021/cr800242x -
2Moad, G., Rizzardo, E., & Thang, S. H. (2008). Radical addition-fragmentation chemistry in polymer synthesis. Polymer, 49(5), 1079-1131. http://dx.doi.org/10.1016/j.polymer.2007.11.020
» http://dx.doi.org/10.1016/j.polymer.2007.11.020 -
3Keddie, D. J., Carlos, G. S., Moad, G., Rizzardo, E., & Thang, S. H. (2011). Switchable Reversible Addition–Fragmentation Chain Transfer (RAFT) polymerization in aqueous solution, N, N-Dimethylacrylamide. Macromolecules, 44(17), 6738-6745. http://dx.doi.org/10.1021/ma200760q
» http://dx.doi.org/10.1021/ma200760q -
4Matyjaszewski, K., & Xia, J. (2001). Atom transfer radical Polymerization. Chemical Reviews, 101(9), 2921-2990. http://dx.doi.org/10.1021/cr940534g PMid:11749397.
» http://dx.doi.org/10.1021/cr940534g -
5Matyjaszewski, K. (2012). Atom Transfer Radical Polymerization (ATRP): Current status and future perspectives. Macromolecules, 45(10), 4015-4039. http://dx.doi.org/10.1021/ma3001719
» http://dx.doi.org/10.1021/ma3001719 -
6Shanmugam, S., Xu, J., & Boyer, C. (2017). Photocontrolled living polymerization systems with reversible deactivations through electron and energy transfer. Macromolecular Rapid Communications, 38(13), 1700143. http://dx.doi.org/10.1002/marc.201700143 PMid:28556363.
» http://dx.doi.org/10.1002/marc.201700143 -
7Miyake, G. M., & Theriot, J. C. (2014). Perylene as an organic photocatalyst for the radical polymerization of functionalized vinyl monomers through oxidative quenching with alkyl bromides and visible light. Macromolecules, 47(23), 8255-8261. http://dx.doi.org/10.1021/ma502044f
» http://dx.doi.org/10.1021/ma502044f -
8Theriot, J. C., Lim, C. H., Yang, H., Ryan, M. D., Musgrave, C. B., & Miyake, G. M. (2016). Organocatalyzed atom transfer radical polymerization driven by visible light. Science, 352(6289), 1082-1086. http://dx.doi.org/10.1126/science.aaf3935 PMid:27033549.
» http://dx.doi.org/10.1126/science.aaf3935 -
9Corrigan, N., Yeow, J., Judzewitsch, P., Xu, J., & Boyer, C. (2019). Seeing the light: advancing materials chemistry through Photopolymerization. Angewandte Chemie International Edition, 58(16), 5170-5189. http://dx.doi.org/10.1002/anie.201805473 PMid:30066456.
» http://dx.doi.org/10.1002/anie.201805473 -
10Pintauer, T., Zhou, P., & Matyjaszewski, K. (2002). General method for determination of the activation, deactivation, and initiation rate constants in transition metal-catalyzed atom transfer radical processes. Journal of the American Chemical Society, 124(28), 8196-8197. http://dx.doi.org/10.1021/ja0265097 PMid:12105893.
» http://dx.doi.org/10.1021/ja0265097 -
11Dadashi‐Silab, S., Pan, X., & Matyjaszewski, K. (2017). Phenyl Benzo [b] Phenothiazine as a visible light Photoredox Catalyst for metal‐free atom transfer radical Polymerization. Chemistry (Weinheim an der Bergstrasse, Germany), 23(25), 5972-5977. http://dx.doi.org/10.1002/chem.201605574 PMid:28009492.
» http://dx.doi.org/10.1002/chem.201605574 -
12Corrigan, N., Shanmugam, S., Xu, J., & Boyer, C. (2016). Photocatalysis in organic and polymer synthesis. Chemical Society Reviews, 45(22), 6165-6212. http://dx.doi.org/10.1039/C6CS00185H PMid:27819094.
» http://dx.doi.org/10.1039/C6CS00185H -
13Matyjaszewski, K., & Tsarevsky, N. V. (2014). Macromolecular engineering by atom transfer radical polymerization. Journal of the American Chemical Society, 136(18), 6513-6533. http://dx.doi.org/10.1021/ja408069v PMid:24758377.
» http://dx.doi.org/10.1021/ja408069v -
14McCarthy, B., & Miyake, G. M. (2018). Organocatalyzed atom transfer radical polymerization catalyzed by core modified N-Aryl Phenoxazines Performed under Air. ACS Macro Letters, 7(8), 1016-1021. http://dx.doi.org/10.1021/acsmacrolett.8b00497 PMid:31827976.
» http://dx.doi.org/10.1021/acsmacrolett.8b00497 -
15McCarthy, B. G., Pearson, R. M., Lim, C. H., Sartor, S. M., Damrauer, N. H., & Miyake, G. M. (2017). Structure-Property relationships for Tailoring Phenoxazines as reducing Photoredox Catalysts. Journal of the American Chemical Society, 140(15), 5088-5101. http://dx.doi.org/10.1021/jacs.7b12074 PMid:29513533.
» http://dx.doi.org/10.1021/jacs.7b12074 -
16Treat, N. J., Sprafke, H., Kramer, J. W., Clark, P. G., Barton, B. E., Read de Alaniz, J., Fors, B. P., & Hawker, C. J. (2014). Metal-free atom transfer radical Polymerization. Journal of the American Chemical Society, 136(45), 16096-16101. http://dx.doi.org/10.1021/ja510389m PMid:25360628.
» http://dx.doi.org/10.1021/ja510389m -
17Pearson, R. M., Lim, C. H., McCarthy, B. G., Musgrave, C. B., & Miyake, G. M. (2016). Organocatalyzed atom transfer radical polymerization using N-Aryl Phenoxazines as Photoredox Catalysts. Journal of the American Chemical Society, 138(35), 11399-11407. http://dx.doi.org/10.1021/jacs.6b08068 PMid:27554292.
» http://dx.doi.org/10.1021/jacs.6b08068
Publication Dates
-
Publication in this collection
04 Sept 2020 -
Date of issue
2020
History
-
Received
09 Feb 2020 -
Reviewed
21 June 2020 -
Accepted
22 June 2020