Abstract
It is well-recognized that different sulphur curing systems have greatly influenced to the final properties of the rubber vulcanizates. In this study, the properties of vulcanizates with conventional vulcanization (CV) and efficiency vulcanization (EV) systems were correlated in the aspect of stress relaxation and thermal stability. The stress relaxation behaviour and thermal stability were studied with the temperature scanning stress relaxation (TSSR) and with thermogravimatric analysis (TGA) techniques, respectively. Thermo-oxidative degradation of rubber chains in the CV system was greater than the EV system, leading to easier stress relaxation and poorer aging resistance of the CV system. Also, thermal stability of the rubber crosslinked with CV system was poorer than that with the EV system as corroborated by the activation energy of degradation. TSSR result correlated well with TGA result, and both revealed type of crosslinkages governed the thermo-oxidative degradation and thermal stability of vulcanizates.
Keywords:
natural rubber; stress relaxation; thermal stability; crosslink system
1. Introduction
It is well known that unvulcanised NR has low strength, is unstable over a wide range of temperatures, and cannot recover its original shape after a large deformation[11 Coran, A. Y. (2013). Vulcanization. In B. Erman, J. E. Mark, & C. M. Roland (Eds.), The science and technology of rubber (pp. 337-381). Amsterdam: Elsevier. http://dx.doi.org/10.1016/B978-0-12-394584-6.00007-8.
http://dx.doi.org/10.1016/B978-0-12-3945...
,22 Khan, I., & Bhat, A. H. (2014). Micro and nano calcium carbonate filled natural rubber composites and nanocomposites. In S. Thomas, C. H. Chan, L. Pothen, J. Joy, & H. Maria (Eds.), Natural rubber materials, vol 2: Composites and nanocomposites (pp. 467-487). Cambridge: Royal Society of Chemistry.]. Therefore, all typical NR products require vulcanizing. This is a chemical process, converting viscous rubber materials into three-dimensional elastic crosslinked networks by using chemicals and heat[33 Coran, A. Y. (1995). Vulcanization: Conventional and dynamic. Rubber Chemistry and Technology, 68(3), 351-375. http://dx.doi.org/10.5254/1.3538748.
http://dx.doi.org/10.5254/1.3538748...
]. As a result, the vulcanizates are less sensitive to heat or cold and have elasticity, strength and stability in the ranges needed in applications[44 Hoover, F. I., & To, B. H. (2004). Vulcanization. In B. Rodgers (Ed.), Rubber compounding: Chemistry and applications (pp. 505-568). New York: Marcel Dekker Inc.]. By far, sulfur is the most extensively used crosslinking agent in the rubber industries as it is cost-effective, broadly compatible with compounding ingredients, and allows to predict the eventual vulcanizate properties[55 Ciullo, P. A., & Hewitt, N. (1999). The rubber formulary. New York: William Andrew.]. In general, sulfur vulcanization systems are either conventional (CV), semi-efficient (Semi-EV), or efficient (EV) types, and the linkages generated by vulcanization reactions can be either mono- (C-S-C), di- (C-S2-C), or polysulfidic (C-Sx-C; x>2) crosslinks, depending on accelerator/sulfur ratio. The CV systems have accelerator/sulfur ratio below 0.7; EV has this ratio above 2.5; and semi-EV is used to label the remaining cases[66 Datta, R. N. (2002). Rubber curing systems. Shawbury: Smithers Rapra Technology.,77 Linhares, F. N., Kersch, M., Niebergall, U., Moreira Leite, M. C. A., Atlstadt, V., & Furtado, C. R. G. (2017). Effect of different sulphur-based crosslink networks on the nitrile rubber resistance to biodiesel. Fuel, 191, 130-139. http://dx.doi.org/10.1016/j.fuel.2016.11.060.
http://dx.doi.org/10.1016/j.fuel.2016.11...
]. It has been proven that the CV systems give a large proportion of polysulfidic linkages with bond strengths less than 262 kJ/mol, while EV systems tend to create more monosulfidic linkages with bond strengths of about 280 kJ/mol[88 Pimolsiriphol, V., Saeoui, P., & Sirisinha, C. (2007). Relationship among thermal ageing degradation, dynamic properties, cure systems, and antioxidants in natural rubber. Polymer-Plastics Technology and Engineering, 46(2), 113-121. http://dx.doi.org/10.1080/03602550601152861.
http://dx.doi.org/10.1080/03602550601152...
,99 Bhowmick, A. K., & Mangaraj, D. (1994). Vulcanization and curing techniques. In A. K. Bhowmick, M. M. Hall, & H. A. Benarey (Eds.), Rubber products manufacturing technology (pp. 315-396). New York: Marcel Dekker Inc.]. Due to the lower bond strength, poorer thermal ageing is typical with the CV system. Several prior studies have assessed the properties of NR with different types of crosslinks. Pimolsiriphol et al.[88 Pimolsiriphol, V., Saeoui, P., & Sirisinha, C. (2007). Relationship among thermal ageing degradation, dynamic properties, cure systems, and antioxidants in natural rubber. Polymer-Plastics Technology and Engineering, 46(2), 113-121. http://dx.doi.org/10.1080/03602550601152861.
http://dx.doi.org/10.1080/03602550601152...
] investigated thermal aging degradation of CV and EV crosslinked NR, and found that the thermal ageing properties of NR vulcanizates depend strongly on crosslink density. On the other hand, Larpkasemsuk et al.[1010 Larpkasemsuk, A., Raksaksri, L., Chuayjuljit, S., Chaiwuthinan, P., & Boonmahidthisud, A. (2019). Effects of sulfur vulcanization system on cure characteristics, physical properties and thermal aging of epoxidized natural rubber. Journal of Metals Materials and Minerals, 29(1), 49-57.] reported that for epoxidized natural rubber the highest tensile strength and oil resistance were obtained with a CV system, but the highest thermal stability was achieved with a semi-EV system due to high crosslink density with thermally comparatively stable mono- and disulfidic linkages. Boonkerd et al.[1111 Boonkerd, K., Deeprasertkul, C., & Boonsomwong, K. (2016). Effect of sulfur to accelerator ratio on crosslink structure reversion, and strength in natural rubber. Rubber Chemistry and Technology, 89(3), 450-464. http://dx.doi.org/10.5254/rct.16.85963.
http://dx.doi.org/10.5254/rct.16.85963...
] found that adding polysulfidic linkages gave the vulcanizate a higher tensile strength but a lower reversion resistance. Rattanasom et al.[1212 Rattanasom, N., Poonsuk, A., & Makmoon, T. (2005). Effect of curing system on the mechanical properties and heat aging resistance of natural rubber/tire tread reclaimed rubber blends. Polymer Testing, 24(6), 728-732. http://dx.doi.org/10.1016/j.polymertesting.2005.04.008.
http://dx.doi.org/10.1016/j.polymertesti...
] also found that NR/tire tread reclaimed rubber blend vulcanizates had better heat aging resistance with an EV system than with CV, due to the better thermal stability of mono- and di-sulfidic crosslinks compared to polysulfidic linkages. Although various studies have reported on mechanical and thermal properties achieved with CV and EV systems but the discussions remained in contradiction.
Temperature scanning stress relaxation (TSSR) measurement is a well-known technique used for determining stress relaxation behaviors of thermoplastic elastomers[1313 Vennemann, N., Bokamp, K., & Broker, D. (2006). Crosslink density of peroxide cured TPV. Macromolecular Symposia, 245-246(1), 641-650. http://dx.doi.org/10.1002/masy.200651391.
http://dx.doi.org/10.1002/masy.200651391...
]. However, it was also useful for analysing relaxation behaviour or thermo-mechanical behaviour of unfilled rubbers vulcanizates[1414 Vennemann, N., Schwarze, C., & Kummerlowe, C. (2014). Determination of crosslink density and network structure of NR Vulcanizates by means of TSSR. Advanced Materials Research, 844, 482-485. http://dx.doi.org/10.4028/www.scientific.net/AMR.844.482.
http://dx.doi.org/10.4028/www.scientific...
15 Oncel, S., Kurtoglu, B., & Karaagac, B. (2019). An alternative antioxidant for sulfur-vulcanized natural rubber: henna. Journal of Elastomers and Plastics, 51(5), 440-456. http://dx.doi.org/10.1177/0095244318796594.
http://dx.doi.org/10.1177/00952443187965...
-1616 Karaagac, B., Cengiz, S. C., Bayram, T., & Sen, M. (2018). Identification of temperature scanning stress relaxation behaviors of new grade ethylene propylene diene elastomers. Advances in Polymer Technology, 37(8), 3027-3037. http://dx.doi.org/10.1002/adv.21973.
http://dx.doi.org/10.1002/adv.21973...
]. Vennemann et al.[1414 Vennemann, N., Schwarze, C., & Kummerlowe, C. (2014). Determination of crosslink density and network structure of NR Vulcanizates by means of TSSR. Advanced Materials Research, 844, 482-485. http://dx.doi.org/10.4028/www.scientific.net/AMR.844.482.
http://dx.doi.org/10.4028/www.scientific...
] determined the relaxation behavior of NR crosslinked with different vulcanization systems by using TSSR. They found that the ratio of sulfur to accelerator has a large influence on the relaxation behavior of vulcanizates. Furthermore, they also found that the change of stress relaxation behavior was caused by the cleavage of crosslinks or scission of main chains. Similar observation was also noticed by Oncel et al.[1515 Oncel, S., Kurtoglu, B., & Karaagac, B. (2019). An alternative antioxidant for sulfur-vulcanized natural rubber: henna. Journal of Elastomers and Plastics, 51(5), 440-456. http://dx.doi.org/10.1177/0095244318796594.
http://dx.doi.org/10.1177/00952443187965...
] when they studied the oxidative thermal aging behaviour of the NR containing new type of antioxidants. Karaagac et al.[1616 Karaagac, B., Cengiz, S. C., Bayram, T., & Sen, M. (2018). Identification of temperature scanning stress relaxation behaviors of new grade ethylene propylene diene elastomers. Advances in Polymer Technology, 37(8), 3027-3037. http://dx.doi.org/10.1002/adv.21973.
http://dx.doi.org/10.1002/adv.21973...
] used TSSR to investigate the stress relaxation of NR and ethylene propylene diene rubber and revealed the effect of molecular weight on the pattern of stress relaxation. For the same polymer, the higher molecular weight shifted the pattern of stress relaxation to higher temperature. Though there have been reports on the relaxation behaviour of the NR but an attempt to correlate low temperature relaxation behaviour (lower than 300 °C) with thermal properties tested are still largely unexplored.
To address this aspect, alternative sulfur vulcanizing systems were used to experimentally assess the effect of vulcanization system on stress relaxation and thermal properties of NR, along with other properties. The aim is to reveal the consistency between relaxation behavior and thermal properties of two different crosslink systems. Unfilled NR is crosslinked with two alternative sulfur vulcanization systems, namely of CV and EV types, with fixed total contents of the crosslinking agent and accelerator, the relation of stress relaxation behavior with thermal degradation resistance is discussed.
2. Materials and Methods
2.1 Materials
The NR (STR 5L) was purchased from Chalong Concentrated Natural Rubber Latex Industry Co., Ltd., Thailand. Stearic acid and zinc oxide (ZnO) used as activators for the sulfur curing system were manufactured by Imperial Chemical Co. Ltd., Pathumthani, Thailand and Global Chemical Co. Ltd., Samutprakarn, Thailand, respectively. N-cyclohexyl-benzothiazyl-sulphenamide (CBS) purchased from Flexsys America L.P., West Virginia, USA, was used as accelerator, and the sulfur was manufactured by Siam Chemical Co., Ltd., Samut Prakan, Thailand.
2.2 Sample preparation
NR was compounded with other ingredients, namely stearic acid, ZnO, CBS and sulfur, using an internal mixer (Brabender® GmbH & Co. KG, Duisburg, Germany) at a fixed fill factor of 0.8, initial temperature of 40 °C and a rotor speed of 60 rpm. The chemical formulations, mixing steps and mixing times are displayed in Table 1. Total ingredient contents were kept constant at 107 part(s) per hundred parts of rubber (phr) while mixing time of all samples was fixed at 5 min. The mixing torque was recorded and the compounds were finally compression molded at 160 °C using a laboratory hot press according to their respective curing times. In this study, the compound formulation was designed to incorporate only necessary additives in order to minimize undesired properties. Thus, the effect of crosslink types on thermal property can be truly estimated.
2.3 Characterization
2.3.1 Curing characteristics
The curing characteristics of different NR compounds were measured at 160 °C for 20 minutes, using a Moving die rheometer, MDR 3000 Basic (Montech, Germany). The rheometric parameters scorch time (Ts1), cure time (Tc90), maximum torque (MH), torque difference (MH-ML) and cure rate index (CRI) were determined. Tc90 is the time at which 90% of cure has taken place and can be estimated as:
The cure rate indexes (CRIs) for the compounds were calculated from:
where, Tc90 is the time at 90% vulcanization (min) and Ts1 is the scorch time (min).
2.3.2 Aging resistance
The aging resistance of NR vulcanizates at high temperatures was quantified in terms of the percentage of reversion in rubber vulcanizates after 300s from maximum torque value (R300). The percentage of reversion was comparatively calculated according to the equations given by Khang and Ariff and Kok[1717 Khang, T. H., & Ariff, Z. M. (2012). Vulcanization kinetics study of natural rubber compounds having different formulation variables. Journal of Thermal Analysis and Calorimetry, 109(3), 1545-1553. http://dx.doi.org/10.1007/s10973-011-1937-3.
http://dx.doi.org/10.1007/s10973-011-193...
,1818 Kok, C. M. (1987). The effects of compounding variables on the reversion process in the sulphur vulcanization of natural rubber. European Polymer Journal, 23(8), 611-615. http://dx.doi.org/10.1016/0014-3057(87)90006-1.
http://dx.doi.org/10.1016/0014-3057(87)9...
] which was expressed as follows:
where MH is the maximum torque in rheometric test and M300s is the torque 300 seconds after the maximum torque peak[1717 Khang, T. H., & Ariff, Z. M. (2012). Vulcanization kinetics study of natural rubber compounds having different formulation variables. Journal of Thermal Analysis and Calorimetry, 109(3), 1545-1553. http://dx.doi.org/10.1007/s10973-011-1937-3.
http://dx.doi.org/10.1007/s10973-011-193...
].
Here, Tmax is maximum torque, Tt is torque at t seconds after maximum and Tmin is minimum torque[1818 Kok, C. M. (1987). The effects of compounding variables on the reversion process in the sulphur vulcanization of natural rubber. European Polymer Journal, 23(8), 611-615. http://dx.doi.org/10.1016/0014-3057(87)90006-1.
http://dx.doi.org/10.1016/0014-3057(87)9...
].
2.3.3 Temperature Scanning Stress Relaxation (TSSR) Measurement
The TSSR measurement (Brabender, Duisburg, Germany) was performed in order to investigate thermo-mechanical behavior of the NR vulcanizates[1919 Srinivasan, N., Bokamp, K., & Vennemann, N. (2005). New test method for the characterisation of filled elastomers. KGK. Kautschuk, Gummi, Kunststoffe, 2005(58), 650.]. The NR specimens were placed in the electrical heating chamber at temperature of about 23 °C and at the strain of 50% for 2 h. Non-isothermal test was then performed with heating rate of 2 K/min until the specimens were ruptured.
The crosslink density (υ) was estimated from the initial part of the normalized force curve according to the following correlations[1313 Vennemann, N., Bokamp, K., & Broker, D. (2006). Crosslink density of peroxide cured TPV. Macromolecular Symposia, 245-246(1), 641-650. http://dx.doi.org/10.1002/masy.200651391.
http://dx.doi.org/10.1002/masy.200651391...
]:
where, R is the universal gas constant, λ is the strain ratio, σ is mechanical stress and T is absolute temperature.
2.3.4 Thermal properties
Thermal properties of the crosslinked NR were investigated from thermogravimetric analysis with TGA4000 (PerkinElmer, USA). The samples were tested from 30 to 500 °C with a heating rate of 10 °C/min and N2 flushing at 30 ml/min. The degradation kinetics of crosslinked NR was also determined from thermogravimetric analysis with TGA4000 (PerkinElmer, USA) done at the heating rates of 5 °C/ min, 10 °C/ min and 20 °C/ min. The formula of Flynn-Wall Ozawa (FWO) was applied to estimate the activation energy (Ea) of degradation as follows[2020 Alwaan, I. M., & Hassan, A. (2014). Pyrolysis, kinetic and kinetic model study of epoxidized natural rubber. Progress in Rubber, Plastics and Recycling Technology, 30(3), 153-168. http://dx.doi.org/10.1177/147776061403000303.
http://dx.doi.org/10.1177/14777606140300...
,2121 Chrissafis, K. (2009). Kinetics of thermal degradation of polymer, complementary use of isoconversional and model-fitting methods. Journal of Thermal Analysis and Calorimetry, 95(1), 273-283. http://dx.doi.org/10.1007/s10973-008-9041-z.
http://dx.doi.org/10.1007/s10973-008-904...
]:
Here, β is the heating rate, Ea is the activation energy, R is the universal gas constant (8.314 j.mol-1.K-1), g(α) is a degree of conversion and T is the absolute temperature. By plotting the Ln (β) against 1/T, the value of E can be evaluated from the slope -0.4567 Ea /R.
3. Results and Discussions
3.1 Torque-time curves of mixing
Mixing torque profiles of the NR compounds were recorded in order to investigate changes in their molecular weights. Figure 1 shows time profiles of torque during mixing. In all cases the first peak comes from introduction of NR into the mixer. The sudden drops of torque after 2 to 3 min was due to ram opening to add activator and curing agents. The torque at the end of mixing was almost constant, and it is widely accepted that for polymer mixes the final torque in an internal mixer relates to the weight-average molecular weight as follows[2222 Jung, C., Jana, S. C., & Gunes, I. S. (2007). Analysis of polymerization in chaotic mixers using time scales of mixing and chemical reactions. Industrial & Engineering Chemistry, 46(8), 2413-2422. http://dx.doi.org/10.1021/ie0613319.
http://dx.doi.org/10.1021/ie0613319...
,2323 Verhoeven, V. W. A., van Vondel, M. P. Y., Ganzeveld, K. J., & Janssen, L. P. B. M. (2004). Rheo‐kinetic measurement of thermoplastic polyurethane polymerization in a measurement kneader. Polymer Engineering and Science, 44(9), 1648-1655. http://dx.doi.org/10.1002/pen.20163.
http://dx.doi.org/10.1002/pen.20163...
].
and
Here C and N are a factor related to the geometry and the rotor speed, respectively. Mw is the weight-averaged molecular weight, η0 is the zero-shear viscosity, B (T) is a temperature dependent factor, and n is determined from:
where K is the consistency index, τ refers to the shear stress and γ is the rate of strain. Since the final mixing torques were almost identical across the cases, it is reasonable to assume that the differences in weight-average molecular weights were negligible.
3.2 Curing characteristics
Rheometer curves for NR crosslinked with CV and EV systems are shown in Figure 2. It can be seen that the curves show differently. As for the EV system, the torque increased steeply with vulcanization time until reaching its maximum, after which it remained constant (plateau). This is not similar to CV system where the torque decreased (reversion) after its maximum. This pattern of behaviour is frequently found when NR is crosslinked with CV and EV systems[2424 Rabiei, S., & Shojaei, A. (2016). Vulcanization kinetics and reversion behavior of natural rubber/styrene-butadiene rubber blend filled with nanodiamond - The role of sulfur curing system. European Polymer Journal, 81, 98-113. http://dx.doi.org/10.1016/j.eurpolymj.2016.05.021.
http://dx.doi.org/10.1016/j.eurpolymj.20...
]. It has been reported that the majority crosslink formation in the CV systems was poly and disulfidics (> 90%), while the monosulfidic was dominant in the EV system (> 80%)[2525 Akiba, M., & Hashim, A. S. (1997). Vulcanization and crosslinking in elastomer. Progress in Polymer Science, 22(3), 475-521. http://dx.doi.org/10.1016/S0079-6700(96)00015-9.
http://dx.doi.org/10.1016/S0079-6700(96)...
]. The plateau behaviour with EV system is thus due to thermally stable crosslinks of monosulfidic types, while decreasing torque after the maximum with the CV system is attributed to breakdown of some thermally unstable polysulfidic crosslinks with lesser bond strength (˂ 262 kJ/mol) than the monosulfidic linkages (~ 280 kJ/mol)[88 Pimolsiriphol, V., Saeoui, P., & Sirisinha, C. (2007). Relationship among thermal ageing degradation, dynamic properties, cure systems, and antioxidants in natural rubber. Polymer-Plastics Technology and Engineering, 46(2), 113-121. http://dx.doi.org/10.1080/03602550601152861.
http://dx.doi.org/10.1080/03602550601152...
,99 Bhowmick, A. K., & Mangaraj, D. (1994). Vulcanization and curing techniques. In A. K. Bhowmick, M. M. Hall, & H. A. Benarey (Eds.), Rubber products manufacturing technology (pp. 315-396). New York: Marcel Dekker Inc.]. The curing parameters in term of maximum torque (MH) and torque difference (MH-ML) obtained with the CV and EV systems are shown in Table 2. The Ts1 and Tc90 were shorter with EV than with CV systems, indicating that the EV system had shorter scorch safety and vulcanization times. As a result of fast curing, the CRI value of EV system is higher than that of the CV system. This was probably resulted from high dosages of accelerator to sulfur. Furthermore, the MH and MH-ML values were much higher with CV than with EV systems. The higher value of the MH indicates the higher stiffness of the CV sample after fully vulcanization, while the greater MH-ML is the higher the crosslink density[2626 Surya, I., & Ismail, H. (2016). Alkanolamide as a novel accelerator and vulcanising agent in carbon black-filled polychloroprene rubber compounds. Plastics, Rubber and Composites, 45(7), 287-293. http://dx.doi.org/10.1080/14658011.2016.1187477.
http://dx.doi.org/10.1080/14658011.2016....
]. The higher stiffness and crosslink density with CV system is due to the higher concentration of sulfur for initiating crosslink reactions than in the EV system. As a result, more extensive vulcanization takes place with the CV system.
Curing properties in terms of Ts1, Tc90, CRI, MH and MH-ML for NR crosslinked with CV and EV systems.
3.3 Reversion resistance
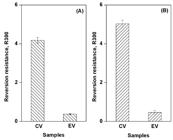
Reversion resistance of NR crosslinked with CV and EV systems was investigated by exposure to shear and elevated temperature for a certain period of time. Figure 3 displays the percentage of reversion according to the equations given by Khang and Ariff [1717 Khang, T. H., & Ariff, Z. M. (2012). Vulcanization kinetics study of natural rubber compounds having different formulation variables. Journal of Thermal Analysis and Calorimetry, 109(3), 1545-1553. http://dx.doi.org/10.1007/s10973-011-1937-3.
http://dx.doi.org/10.1007/s10973-011-193...
] and Kok[1818 Kok, C. M. (1987). The effects of compounding variables on the reversion process in the sulphur vulcanization of natural rubber. European Polymer Journal, 23(8), 611-615. http://dx.doi.org/10.1016/0014-3057(87)90006-1.
http://dx.doi.org/10.1016/0014-3057(87)9...
].
Reversion resistances (R300) of NR crosslinked with CV and EV systems calculated according to (A) Ref 17, and (B) Ref 18.
As the percentage of reversion was estimated after a certain time past the maximum torque, a greater value means higher reversion. A larger value R300 was detected for the CV system, meaning that the NR crosslinked with CV system had poorer reversion resistance as compared with the EV system. As previously mentioned, polysulfidic linkages are dominant with the CV system but monosulfidic linkages are dominant with the EV system. The polysulfidic linkages have poorer bond strength (<265 kJ mol−1)[88 Pimolsiriphol, V., Saeoui, P., & Sirisinha, C. (2007). Relationship among thermal ageing degradation, dynamic properties, cure systems, and antioxidants in natural rubber. Polymer-Plastics Technology and Engineering, 46(2), 113-121. http://dx.doi.org/10.1080/03602550601152861.
http://dx.doi.org/10.1080/03602550601152...
,99 Bhowmick, A. K., & Mangaraj, D. (1994). Vulcanization and curing techniques. In A. K. Bhowmick, M. M. Hall, & H. A. Benarey (Eds.), Rubber products manufacturing technology (pp. 315-396). New York: Marcel Dekker Inc.], and were easier to break by shear and heat.
3.4 Temperature scanning stress relaxation (TSSR)
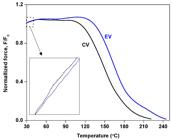
Figure 4 shows normalized force curves of the samples crosslinked with CV and EV systems as a function of temperature. The embedded figure was a magnifying of the initial curves in the temperature range of 30 to 40 °C. It is generally seen from Figure 4 that the initial normalized force of both samples increased slightly at temperatures of 30- 40 °C due to the entropy effect[2727 Barbe, A., Bokamp, K., Kummerlowe, C., Sollmann, H., Vennemann, N., & Vinzelberg, S. (2005). Investigation of modified SEBS-based thermoplastic elastomers by temperature scanning stress relaxation measurements. Polymer Engineering and Science, 45(11), 1498-1507. http://dx.doi.org/10.1002/pen.20427.
http://dx.doi.org/10.1002/pen.20427...
] and the increase of force was sharper in the CV system (see embedded figure), implying the higher crosslink density of the CV sample. The crosslink density of the CV and EV systems was about 111.25 and 87.32 mol/m3. At elevated temperature, the force decrease toward zero due to chain scission caused by thermo-oxidative reaction occurs[1919 Srinivasan, N., Bokamp, K., & Vennemann, N. (2005). New test method for the characterisation of filled elastomers. KGK. Kautschuk, Gummi, Kunststoffe, 2005(58), 650.]. It is also seen that the force at any given temperature of the EV system was shifted to higher temperature, compared to the CV system. In some cases, the shift of these parameters was attributed to the molecular weight differences[1616 Karaagac, B., Cengiz, S. C., Bayram, T., & Sen, M. (2018). Identification of temperature scanning stress relaxation behaviors of new grade ethylene propylene diene elastomers. Advances in Polymer Technology, 37(8), 3027-3037. http://dx.doi.org/10.1002/adv.21973.
http://dx.doi.org/10.1002/adv.21973...
] and crosslink densities[88 Pimolsiriphol, V., Saeoui, P., & Sirisinha, C. (2007). Relationship among thermal ageing degradation, dynamic properties, cure systems, and antioxidants in natural rubber. Polymer-Plastics Technology and Engineering, 46(2), 113-121. http://dx.doi.org/10.1080/03602550601152861.
http://dx.doi.org/10.1080/03602550601152...
,1010 Larpkasemsuk, A., Raksaksri, L., Chuayjuljit, S., Chaiwuthinan, P., & Boonmahidthisud, A. (2019). Effects of sulfur vulcanization system on cure characteristics, physical properties and thermal aging of epoxidized natural rubber. Journal of Metals Materials and Minerals, 29(1), 49-57.], but these two reasons were not applicable for this study due to the negligible change in molecular weight(see Figure 1) and a higher crosslink was gained from the CV system. Thus, influence of crosslink degree and molecular weight can be eliminated. As previously mentioned, the EV system contains majority high thermal stability monosulfidic linkages while the less thermal stability polysulfidic linkages are dominant in the CV system. Thus, it is reasonable to conclude that the lower thermo-oxidative degradation level in the EV system was attributed to the higher thermal stability of monosulfidic linkages. The result clearly confirmed that the type of crosslinks is the main parameter controlling thermal behaviour of the rubber vulcanizates.
The TSSR relaxation spectra of various samples are shown in Figure 5. In the unfilled vulcanizates, the relaxation peak was usually caused by cleavage of sulfur bridges and/or scission of the polymer main chain[1414 Vennemann, N., Schwarze, C., & Kummerlowe, C. (2014). Determination of crosslink density and network structure of NR Vulcanizates by means of TSSR. Advanced Materials Research, 844, 482-485. http://dx.doi.org/10.4028/www.scientific.net/AMR.844.482.
http://dx.doi.org/10.4028/www.scientific...
]. It is seen that the peak of relaxation spectrum of EV system was shifted toward higher temperature, revealing that the breakage of sulfur bridges and/or chain scission of the main chains occur at higher temperature as compared to the CV system. This was attributed to the fact that the higher thermal stability of monosulfidic linkages formed with the EV system[88 Pimolsiriphol, V., Saeoui, P., & Sirisinha, C. (2007). Relationship among thermal ageing degradation, dynamic properties, cure systems, and antioxidants in natural rubber. Polymer-Plastics Technology and Engineering, 46(2), 113-121. http://dx.doi.org/10.1080/03602550601152861.
http://dx.doi.org/10.1080/03602550601152...
,99 Bhowmick, A. K., & Mangaraj, D. (1994). Vulcanization and curing techniques. In A. K. Bhowmick, M. M. Hall, & H. A. Benarey (Eds.), Rubber products manufacturing technology (pp. 315-396). New York: Marcel Dekker Inc.]. As a result of stronger bonding energy of monosulfidic linkage, the stress relaxation behaviour of the EV system was found at higher temperature than the CV system. Therefore, the aging resistance of the CV was poorer than that of the EV system can be confirmed.
It is widely accepted that within temperature range of TSSR measurement, the changes in mechanical properties due to crosslink scission of the rubber structures can be detected. But at higher temperature, crosslink scission and main chain degradation occur simultaneously. To further understand the degradation of CV and EV systems at high temperature and to correlate high temperature properties with low temperature behaviors, TGA was performed.
3.5 Thermal properties
TGA analysis was performed in order to observe thermal degradation behavior and thermal stability for NR crosslinked with CV and EV systems. In this study, the thermal stability of the NR vulcanizates was evaluated from that temperature at which the sample had lost 5% of its initial weight (Td5). The relationship of weight loss (%) with temperature and the derivative weight loss curves at heating rate of 10 °C/min are shown in Figure 6B. It is seen from Figure 6Athat the crosslinked NR samples showed two regions of degradation: a minor weight loss at 180 – 300 °C, and a major loss at 300 – 470 °C. The former weight loss was attributed to volatile substances including stearic acid and moisture, and the latter was due to the degradation of rubber molecules[2828 Hayeemasae, N., Ismail, H., Matchawet, S., & Masa, A. (2019). Kinetic of thermal degradation and thermal stability of natural rubber filled with titanium dioxide nanoparticles. Polymer Composites, 40(8), 3149-3155. http://dx.doi.org/10.1002/pc.25163.
http://dx.doi.org/10.1002/pc.25163...
]. The Td5 with the CV system appeared at 328 °C, which is lower than the corresponding temperature with the EV system (331 °C), meaning that the CV system gave poorer thermal stability than the EV system.
Thermogravimetric curves: (A) weight loss (%), and (B) derivative weight loss versus temperature of NR samples at 10 °C/min heating rate.
Considering the derivative weight loss curves (Figure 6B), the maximum degradation rate with the EV system (-14.19 wt %/min) was slower than with the CV system (-15.47 wt %/min). The degradation of NR chains crosslinked with the EV system was slightly harder than with the CV system. Again, this was attributed to the higher thermal stability of linkages formed with the EV system. As the CV system provided higher crosslinking degree but the thermal properties with CV were poorer than with the EV system. Therefore, the degree of crosslinking does not determine the thermal stability (or resistance to degradation). Only type of crosslinkage is responsible for this change. Higher thermal stability of monosulfidic linkage formed with the EV system may retard both the scission of crosslink and degradation of main chain. The TGA results are in accordance well with the TSSR result as previously shown.
To gain further confirm the degradation process of NR crosslinked with CV and EV systems, activation energies associated with degradation was estimated. The activation energy is the minimum energy required of molecules for them to undergo a phase transition during heating[2929 Tripathy, S. P., Mishra, R., Fink, D., & Dwivedi, K. K. (2004). Irradiation effect on the activation energy of thermal decomposition of polymers. Radiation Effects and Defects in Solids, 159(11-12), 607-612. http://dx.doi.org/10.1080/10420150412331330502.
http://dx.doi.org/10.1080/10420150412331...
]. Plots of Ln (β) versus 1/T for NR crosslinked with the two alternative systems are shown in Figure 7, and the estimates of activation energies are shown in Figure 8. The degradation after curing with the CV system required a lesser activation energy than with the EV system, so the former case was easier to degrade. The higher activation energy with the EV system clearly confirms that the NR crosslinked with EV system had harder degradation and better thermal stability than with the CV system. This is here tentatively attributed to the crosslink system.
Since the high temperature results obtained from TGA measurement agreed well with the low temperature behavior tested by TSSR technique, it is reasonable to assume that the thermal behavior at higher temperature can be partially estimated by using temperature stress relaxation technique which was tested at lower temperature.
4. Conclusions
In this study, influences of two alternative sulfur vulcanization systems, of CV and EV types, on curing reaction, stress relaxation behavior and thermal properties of NR vulcanizates were investigated. The CV system provided higher maximum toque and torque different. From TSSR measurements, the degree of crosslinking was greater with the CV system. However, higher crosslink and chain scissions caused by thermo-oxidative occurs in the CV system due to the polysulfidic crosslinks having comparatively poorer thermal stability. The monosulfidic linkage in the EV system tentatively provided better resistance to the scission of both crosslink and rubber chains as revealed by TGA analysis. The degradation of the rubber main chain was found to hinder by the EV system as was later confirmed by higher activation energy. Both TSSR measurement and TGA analysis showed well agreement that the better aging property, thermo-oxidative resistance and thermal stability were greater with the EV system.
5. Acknowledgements
The authors gratefully acknowledged Prince of Songkla University for financial support (Grant no. RDO6202102S). Research and Development Office (RDO) of Prince of Songkla University and Assoc. Prof. Dr. Seppo Karrila are also acknowledged for assistance in editing the English language in this manuscript.
-
How to cite: Hayeemasae, N., & Masa, A. (2020). Relationship between stress relaxation behavior and thermal stability of natural rubber vulcanizates. Polímeros: Ciência e Tecnologia, 30(2), e2020016. https://doi.org/10.1590/0104-1428.03120.
6. References
-
1Coran, A. Y. (2013). Vulcanization. In B. Erman, J. E. Mark, & C. M. Roland (Eds.), The science and technology of rubber (pp. 337-381). Amsterdam: Elsevier. http://dx.doi.org/10.1016/B978-0-12-394584-6.00007-8
» http://dx.doi.org/10.1016/B978-0-12-394584-6.00007-8 -
2Khan, I., & Bhat, A. H. (2014). Micro and nano calcium carbonate filled natural rubber composites and nanocomposites. In S. Thomas, C. H. Chan, L. Pothen, J. Joy, & H. Maria (Eds.), Natural rubber materials, vol 2: Composites and nanocomposites (pp. 467-487). Cambridge: Royal Society of Chemistry.
-
3Coran, A. Y. (1995). Vulcanization: Conventional and dynamic. Rubber Chemistry and Technology, 68(3), 351-375. http://dx.doi.org/10.5254/1.3538748
» http://dx.doi.org/10.5254/1.3538748 -
4Hoover, F. I., & To, B. H. (2004). Vulcanization. In B. Rodgers (Ed.), Rubber compounding: Chemistry and applications (pp. 505-568). New York: Marcel Dekker Inc.
-
5Ciullo, P. A., & Hewitt, N. (1999). The rubber formulary New York: William Andrew.
-
6Datta, R. N. (2002). Rubber curing systems Shawbury: Smithers Rapra Technology.
-
7Linhares, F. N., Kersch, M., Niebergall, U., Moreira Leite, M. C. A., Atlstadt, V., & Furtado, C. R. G. (2017). Effect of different sulphur-based crosslink networks on the nitrile rubber resistance to biodiesel. Fuel, 191, 130-139. http://dx.doi.org/10.1016/j.fuel.2016.11.060
» http://dx.doi.org/10.1016/j.fuel.2016.11.060 -
8Pimolsiriphol, V., Saeoui, P., & Sirisinha, C. (2007). Relationship among thermal ageing degradation, dynamic properties, cure systems, and antioxidants in natural rubber. Polymer-Plastics Technology and Engineering, 46(2), 113-121. http://dx.doi.org/10.1080/03602550601152861
» http://dx.doi.org/10.1080/03602550601152861 -
9Bhowmick, A. K., & Mangaraj, D. (1994). Vulcanization and curing techniques. In A. K. Bhowmick, M. M. Hall, & H. A. Benarey (Eds.), Rubber products manufacturing technology (pp. 315-396). New York: Marcel Dekker Inc.
-
10Larpkasemsuk, A., Raksaksri, L., Chuayjuljit, S., Chaiwuthinan, P., & Boonmahidthisud, A. (2019). Effects of sulfur vulcanization system on cure characteristics, physical properties and thermal aging of epoxidized natural rubber. Journal of Metals Materials and Minerals, 29(1), 49-57.
-
11Boonkerd, K., Deeprasertkul, C., & Boonsomwong, K. (2016). Effect of sulfur to accelerator ratio on crosslink structure reversion, and strength in natural rubber. Rubber Chemistry and Technology, 89(3), 450-464. http://dx.doi.org/10.5254/rct.16.85963
» http://dx.doi.org/10.5254/rct.16.85963 -
12Rattanasom, N., Poonsuk, A., & Makmoon, T. (2005). Effect of curing system on the mechanical properties and heat aging resistance of natural rubber/tire tread reclaimed rubber blends. Polymer Testing, 24(6), 728-732. http://dx.doi.org/10.1016/j.polymertesting.2005.04.008
» http://dx.doi.org/10.1016/j.polymertesting.2005.04.008 -
13Vennemann, N., Bokamp, K., & Broker, D. (2006). Crosslink density of peroxide cured TPV. Macromolecular Symposia, 245-246(1), 641-650. http://dx.doi.org/10.1002/masy.200651391
» http://dx.doi.org/10.1002/masy.200651391 -
14Vennemann, N., Schwarze, C., & Kummerlowe, C. (2014). Determination of crosslink density and network structure of NR Vulcanizates by means of TSSR. Advanced Materials Research, 844, 482-485. http://dx.doi.org/10.4028/www.scientific.net/AMR.844.482
» http://dx.doi.org/10.4028/www.scientific.net/AMR.844.482 -
15Oncel, S., Kurtoglu, B., & Karaagac, B. (2019). An alternative antioxidant for sulfur-vulcanized natural rubber: henna. Journal of Elastomers and Plastics, 51(5), 440-456. http://dx.doi.org/10.1177/0095244318796594
» http://dx.doi.org/10.1177/0095244318796594 -
16Karaagac, B., Cengiz, S. C., Bayram, T., & Sen, M. (2018). Identification of temperature scanning stress relaxation behaviors of new grade ethylene propylene diene elastomers. Advances in Polymer Technology, 37(8), 3027-3037. http://dx.doi.org/10.1002/adv.21973
» http://dx.doi.org/10.1002/adv.21973 -
17Khang, T. H., & Ariff, Z. M. (2012). Vulcanization kinetics study of natural rubber compounds having different formulation variables. Journal of Thermal Analysis and Calorimetry, 109(3), 1545-1553. http://dx.doi.org/10.1007/s10973-011-1937-3
» http://dx.doi.org/10.1007/s10973-011-1937-3 -
18Kok, C. M. (1987). The effects of compounding variables on the reversion process in the sulphur vulcanization of natural rubber. European Polymer Journal, 23(8), 611-615. http://dx.doi.org/10.1016/0014-3057(87)90006-1
» http://dx.doi.org/10.1016/0014-3057(87)90006-1 -
19Srinivasan, N., Bokamp, K., & Vennemann, N. (2005). New test method for the characterisation of filled elastomers. KGK. Kautschuk, Gummi, Kunststoffe, 2005(58), 650.
-
20Alwaan, I. M., & Hassan, A. (2014). Pyrolysis, kinetic and kinetic model study of epoxidized natural rubber. Progress in Rubber, Plastics and Recycling Technology, 30(3), 153-168. http://dx.doi.org/10.1177/147776061403000303
» http://dx.doi.org/10.1177/147776061403000303 -
21Chrissafis, K. (2009). Kinetics of thermal degradation of polymer, complementary use of isoconversional and model-fitting methods. Journal of Thermal Analysis and Calorimetry, 95(1), 273-283. http://dx.doi.org/10.1007/s10973-008-9041-z
» http://dx.doi.org/10.1007/s10973-008-9041-z -
22Jung, C., Jana, S. C., & Gunes, I. S. (2007). Analysis of polymerization in chaotic mixers using time scales of mixing and chemical reactions. Industrial & Engineering Chemistry, 46(8), 2413-2422. http://dx.doi.org/10.1021/ie0613319
» http://dx.doi.org/10.1021/ie0613319 -
23Verhoeven, V. W. A., van Vondel, M. P. Y., Ganzeveld, K. J., & Janssen, L. P. B. M. (2004). Rheo‐kinetic measurement of thermoplastic polyurethane polymerization in a measurement kneader. Polymer Engineering and Science, 44(9), 1648-1655. http://dx.doi.org/10.1002/pen.20163
» http://dx.doi.org/10.1002/pen.20163 -
24Rabiei, S., & Shojaei, A. (2016). Vulcanization kinetics and reversion behavior of natural rubber/styrene-butadiene rubber blend filled with nanodiamond - The role of sulfur curing system. European Polymer Journal, 81, 98-113. http://dx.doi.org/10.1016/j.eurpolymj.2016.05.021
» http://dx.doi.org/10.1016/j.eurpolymj.2016.05.021 -
25Akiba, M., & Hashim, A. S. (1997). Vulcanization and crosslinking in elastomer. Progress in Polymer Science, 22(3), 475-521. http://dx.doi.org/10.1016/S0079-6700(96)00015-9
» http://dx.doi.org/10.1016/S0079-6700(96)00015-9 -
26Surya, I., & Ismail, H. (2016). Alkanolamide as a novel accelerator and vulcanising agent in carbon black-filled polychloroprene rubber compounds. Plastics, Rubber and Composites, 45(7), 287-293. http://dx.doi.org/10.1080/14658011.2016.1187477
» http://dx.doi.org/10.1080/14658011.2016.1187477 -
27Barbe, A., Bokamp, K., Kummerlowe, C., Sollmann, H., Vennemann, N., & Vinzelberg, S. (2005). Investigation of modified SEBS-based thermoplastic elastomers by temperature scanning stress relaxation measurements. Polymer Engineering and Science, 45(11), 1498-1507. http://dx.doi.org/10.1002/pen.20427
» http://dx.doi.org/10.1002/pen.20427 -
28Hayeemasae, N., Ismail, H., Matchawet, S., & Masa, A. (2019). Kinetic of thermal degradation and thermal stability of natural rubber filled with titanium dioxide nanoparticles. Polymer Composites, 40(8), 3149-3155. http://dx.doi.org/10.1002/pc.25163
» http://dx.doi.org/10.1002/pc.25163 -
29Tripathy, S. P., Mishra, R., Fink, D., & Dwivedi, K. K. (2004). Irradiation effect on the activation energy of thermal decomposition of polymers. Radiation Effects and Defects in Solids, 159(11-12), 607-612. http://dx.doi.org/10.1080/10420150412331330502
» http://dx.doi.org/10.1080/10420150412331330502
Publication Dates
-
Publication in this collection
04 Sept 2020 -
Date of issue
2020
History
-
Received
16 Mar 2020 -
Reviewed
17 June 2020 -
Accepted
18 June 2020