ABSTRACT
The development of tissue cultured-based protocols applied to braúna, an endangeres species, would be though highly desirable. However, contamination-free explants are determinant for the success of this technique, which might be vulnerable to the toxicity of disinfectants. Here, we aimed at establishing an efficient protocol for in vitro production of axenic seedlings of braúna and to evaluate the toxicity of disinfectant agents in the Lactuca sativa model species. Experiments I and II: Seeds were treated with sodium hypochlorite (NaOCl) and fungicide captan, with and without residue, by different immersion times, respectively. The following were analyzed: contamination; germination and normal and abnormal seedlings. Experiment III: Lettuce seeds placed in Petri dishes were exposed to 2.5 mL captan at 0.5; 1; 2; 4 and 8%; 0.01% glyphosate and distilled water. The germination, length of seedlings, cell cycle, nuclear and chromosomal alterations of the cells of the root meristem were assesed. The isolated use of NaOCl was not efficient in the disinfestation of braúna seeds. However, the immersion of the seeds in captan, for 10 minutes with its residue, led to higher germination and vigor indexes; however, resulted in the formation of abnormal seedlings. This compound exhibited toxicity in the lettuce model seeds because affected the germination and the whole development of the seedlings, showing clastogenic and aneugenic action in the meristematic cell cycle.
Keywords:
Abnormal seedlings; Disinfestation; Melanoxylon braun; Plant tissue culture; Seeds
INTRODUCTION
The species Melanoxylon brauna Schott (Fabaceae - Caesalpinioideae), commonly known as braúna, is a native tree of the Atlantic Forest, which has great ecological and economic value (Lorenzi, 2014LORENZI, H. Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas nativas do Brasil, 6. ed., Nova Odessa: Instituto Planta rum, 2014. 384 p.). Its wood is widely used in the navigation and furniture manufacturing industry, light poles and fence posts (Carvalho, 2007CARVALHO, F.A.; NASCIMENTO, M.T.; BRAGA, J.M.A. Estrutura e composição florística do estrato arbóreo de um remanescente de Mata Atlântica submontana no município de Rio Bonito, RJ, Brasil (Mata Rio Vermelho). Revista Árvore, v. 31, n. 4, p. 717-730, 2007. ). However, due to its intense exploitation and the lack of replanting, this species has rarely been registered in floristic surveys, being classified as vulnerable in the Official List of Flora Threatened with Extinction (IBAMA, 2018INSTITUTO BRASILEIRO DO MEIO AMBIENTE (IBAMA). Lista oficial de flora ameaçada de extinção. Available at: <Available at: http://www.ibama.gov.br/flora
>. Acessed on: Apr. 04 2018.
http://www.ibama.gov.br/flora...
).
The propagation of the M. brauna is currently carried out ex vitro through seeds (Santos et al., 2017SANTOS, M.M.; BORGES, E.E.L.; ATÍDE, G.M.; SOUZA, G.A. Germination of seeds of Melanoxylon brauna Schott. under heat stress: production of reactive oxygen species and antioxidant activity. Forests, v. 8, n. 11, p. 1-13, 2017. ). Aiming to speed up the availability of propagative material, plant tissue culture is an alternative technique in the production of high genetic-sanitary quality plantlets and maintenance in germplasm collections (Villalobos and Engelmann, 1995VILLALOBOS, V.M.; ENGELMANN, F. Ex situ conservation of plant germplasm using biotechnology. World Journal of Microbiology and Biotechnology , v. 11, n. 4, p. 375-382, 1995. ). However, to make it routine, some particular limitations on the use plant tissue culture appear. One of them relates to the presence of microorganisms in the explants, which need to be eliminated with disinfecting substances (Chaves et al., 2005CHAVES, A.C.; SCHUCH, M.W.; ERIG, A.C. Estabelecimento e multiplicação in vitro de Physalis peruviana L. Ciência e Agrotecnologia , v. 29, n. 6, p. 1281-1287, 2005. ). However, variations induced by tissue culture and then inherited by progeny, for example, somaclonal variation may be a function of the oxidative stress results of the explant preparation (Bednarek and Orlowska, 2019BEDNAREK, P.T.; ORLOWSKA, R. Plant tissue culture environment as a switch-key (epi)genetic changes. Plant Cell, Tissue and Organ Culture , v. 1, p. 1-13, 2019.).
Ethanol, sodium and calcium hypochlorite, carboxin, thiram and carbendazim are the most commonly used substances for surface-sterilization of the explants in vitro propagation procedures, and their concentrations and exposure time vary according to species and type of explant, and, therefore, the adequacy of protocols is needed for an efficient disinfestation (Andrade et al., 2000ANDRADE, M.W.; LUZ, J.M.Q.; LACERDA, A.S.; MELO, P.R. Micropropagação da aroeira (Myracrodruon urundeuva Fr. All). Ciência e Agrotecnologia, v. 24, n. 1, p. 174-180, 2000. ; Chaves et al., 2005CHAVES, A.C.; SCHUCH, M.W.; ERIG, A.C. Estabelecimento e multiplicação in vitro de Physalis peruviana L. Ciência e Agrotecnologia , v. 29, n. 6, p. 1281-1287, 2005. ; Souza et al., 2011SOUZA, L.S.; FIOR, C.S.; SOUZA, P.V.D.; SCHWARZ, S.F. Desinfestação de sementes e multiplicação in vitro de guabijuzeiro a partir de segmentos apicais juvenis (Myrcianthes pungens O. Berg) D. Legrand. Revista Brasileira de Fruticultura, v. 33, n. 7, p. 691-697, 2011.). The main substances used for explant disinfestation are ethanol, sodium and calcium hypochlorite, hydrogen peroxide, and silver nitrate, and sodium hypochlorite is widely known to be an efficient bactericidal agent (Oyebanji et al., 2009OYEBANJI, O.B.; NWEKE, O.; ODEBUNMI, O.; GALADIMA, N.B.; IDRIS, M.S.; NNODI, U.N.; AFOLABI, A.S.; OGBADU, G.H. Simple, effective and economical explant-surface sterilization protocol for cowpea, rice and sorghum seeds. African Journal of Biotechnology, v. 8, n. 20, p. 5395-5399, 2009.). Sodium hypochlorite and sodium dichloroisocyanurate are also used in chemical sterilization of culture media (Pais et al., 2016PAIS, A.K.; SILVA, A.P. da; SOUZA, J.C. de; TEIXEIRA, S.L.; RIBEIRO, J.M.; PEIXOTO, A.R.; PAZ, C.D. Sodium hypochlorite sterilization of culture medium in micropropagation of Gerbera hybrida cv. Essandre. African Journal of Biotechnology , v. 15, n. 36, p. 1995-1998, 2016.; Urtiga et al., 2019URTIGA, C. da C.; SILVA-CARDOSO, I.M. de A.; FIGUEIREDO, S.A. Low sodium isocyanurate concentrations as a substitute to medium autoclaving in plant tissue culture. Plant Cell, Tissue and Organ Culture , v. 139, n. 3, p. 601-604, 2019.), which according to Pais et al. (2016) may be a substitute for thermal sterilization (autoclaving). By adding active chlorine (0.003%) to the culture medium, these authors verified total control of contaminants, with no significant differences in relation to gerbera growth in autoclaved media.
Other disinfectant agents are used in tissue culture, such as fungicides with fungitoxicity inherent to their chemical composition (mechanism of action), concentration and exposure time (Edgington et al., 1971EDGINGTON, L.V.; KHEW, K.L.; BARRON, G.L. Fungitoxic spectrum of benzimidazole compounds. Phytopathology, v. 61, p. 42- 44, 1971. ). The action of fungicides on the metabolism of microorganisms is particularly noteworthy in protein synthesis, in which the chemical substances inhibit the conversion of free amino acids into proteins and the synthesis and polymerization of nucleic acids (Sisler, 1969SISLER, H.D. Effect of fungicides on protein and nucleic acid synthesis. Annual Review ofPhytopathology , v. 7, n. 1, p. 311-330, 1969.).
In spite of its effectiveness, the agents used in disinfestation can be toxic to both microorganisms and explants. Captan, the active principle of some fungicides, for instance captan, is a fungicide used in seed treatment, and may cause damage to the DNA and affect the normality of seedlings (Bolognesi, 2003BOLOGNESI, C. Genotoxicity of pesticides: a review of human biomonitoring studies. Mutation Research/Reviews in Mutation Research, v. 543, n. 3, p. 251-272, 2003.). However, macro and microscopic analyzes are excellent alternatives to study the phytotoxic, cytotoxic and genotoxic effects of substances on bioindicator species (Kumari et al., 2011KUMARI, M.; KHAN, S.S.; PAKRASHI, S.; MUKHERJEE, A.; CHANDRASEKARAN, N. Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. Journal of Hazardous Materials, v. 190, n. 1-3, p. 613-621, 2011. ).
It was aimed with the present work establish an efficient protocol for the in vitro establishment of axenic seedlings of Melanoxylon brauna using sodium hypochlorite and captan as disinfecting agents and to evaluate the toxicity this agent in the Lactuca sativa model.
MATERIAL AND METHODS
The experiments were performed at the Plant Tissue Culture Laboratories of the Department of Forestry and Wood Sciences and at the Laboratory of Cytogenetics and Plant Tissue Culture of the Center for Agrarian Sciences and Engineering, at the Federal University of Espírito Santo (UFES). Braúna (Melanoxylon brauna Schott) seeds were obtained from matrices of the municipality of Leopoldina-MG, at 21° 59’ 75” S, 42° 49’ 42” W.
Experiment I: In vitro germination of braúna seeds affected by immersion times in sodium hypochlorite (NaOCl)
Braúna seeds were initially washed in running water and neutral detergent. Following, in a laminar flow chamber, seeds were submerged in 70% alcohol, for one minute, and sodium hypochlorite (NaOCl) at 2.5% of active chlorine (Candura®) in different immersion times (0; 5; 10; 15; 20 and 25 minutes). The NaOCl residue from the seeds was removed by triple rinse in distilled water, which was previously autoclaved for 30 minutes at 121 °C, 1 atm.
Subsequently, seeds were individually inoculated into test tubes (25 x 150 mm) containing 10 mL of Woody Plant Medium - WPM (2.41 g L-1, Sigma®) (Lloyd and McCown 1980LLOYD, G.; McCOWN, B. Commercially feasible micropropagation of Mountain Laurel, Kalmia latifolia, by use of shoot tip culture. International Plant Propagators’ Society, v. 30, p. 421-427, 1980. ), sucrose (30 g L-1, Dinamic®), myo-inositol (0.1 mg L-1, Sigma®), and agar (7 g L-1, Kasvi®). The pH of the medium was adjusted to 5.7±0.1 and then autoclaved for 20 minutes at 121 ºC, 1 atm. The in vitro cultures were maintained in a growth room for 30 days at 27±2 °C, photoperiod of 16 hours and irradiance of 50 µmol m-2 s-1.
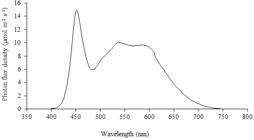
The irradiance in the growth room was provided by 20W tubular LED bulbs, which emit wavelengths within the visible spectrum (450 to 700 nm) (Figure 1). The irradiance and spectrum were measured with the equipment Spectrapen LM500-VIS, Photon Systems Instruments®.
The analyzes were: contamination total (%); bacterial contamination (%); fungal contamination (%); germination (%); germination speed index (ISG); normal seedlings (%); abnormal seedlings (%) and dormancy (%).
Germination (%) was conducted with four replications of 25 seeds for each treatment. Germinated seeds were counted daily for 30 days. Germination was characterized by the emission of the primary root with a length greater than or equal to two millimeters (Brasil, 2009). Results were expressed as percentage of germination.
The germination speed index (ISG) was determined concomitantly with the germination test, being daily computed the number of seeds that showed protrusion of the primary root equal to or greater than 2 mm (Maguire, 1962), being counted until 30th day. The ISG was calculated by summing the number of seeds germinated each day, divided by the number of days elapsed between sowing and germination: ISG = (G1/N1) + (G2/N2) + (G3/N3) + .. . + (Gn/Nn), where: ISG= germination rate index; G1, G2, G3, ..., Gn = number of germinated seeds computed at the first, second, third and last count; N1, N2, N3, ..., Nn = number of days from sowing to first, second, third and last count.
The percentage of normal seedlings was calculated considering the seedlings with all the formed structures (root, hypocotyl and cotyledons), in relation to the germination percentage (seedlings with primary root protrusion), according to Brasil (2009), after 30 days of seeding. Abnormal seedlings were considered to be those that did not follow the normality pattern mentioned above, such as the rosette shape of the aerial part.
The experiment was performed in a completely randomized design (CRD), with six treatments and four replicates with 25 seeds each. Data were submitted to analysis of variance and regression (p≤0.01). For the effect of regression analysis and for the adjustment of equations (Ŷ), the significance of the betas (p≤0.05) was used as criteria. For all analyzes we used the statistical program R (2017).
Experiment II: In vitro germination of braúna seeds after different times of immersion and maintenance of captan residue
The same disinfestation protocol of experiment I was used, with the best seed immersion time in 2.5% NaOCl, which was 15 minutes. Subsequently, the residue was removed by triple rinse in sterile distilled water. Then the seeds were submerged in 2% p.a. Captan® at different exposure times (0; 5; 10; 15; 20; 25 and 30 minutes).
The experiment was performed in a completely randomized design (CRD), in a 7 x 2 factorial scheme with seven exposure times to captan and absence or presence of the fungicide residue, with four replicates of 25 seeds each, which were inoculated individually into test tubes (25 x 150 mm) containing 10 mL-aliquots of WPM. In the plots without the captan residue, the seeds were washed three times in distilled and autoclaved water for subsequent inoculation, and in the plots with the residue maintenance, the seeds were inoculated into test tubes immediately after immersion in the fungicide.
The experiments were kept in a growth room at room temperature 27±2 °C, 16 hour photoperiod with irradiance of 50 µmol m-2 s-1 and wavelengths in the visible spectrum (Figure 1).
For all experiments the following parameters were assessed: contamination (%); germination (%); germination speed index (ISG); normal seedlings (%); abnormal seedlings (%) and dormancy (%). Data were submitted to analysis of variance and Skott-Knott mean grouping test (p≤0.01). The analyzes were made using the statistical program R (2017).
Experiment III: Evaluation of captan toxicity in bioassay with Lactuca sativa L. seeds, plant model.
Among the various plant bioassays that are commonly used for toxicity testing, lettuce presents itself as a great option. It correlates with data obtained from tests with other plants, animals (Bianchi et al., 2015BIANCHI, J.; CABRAL-DE-MELLO, D.C.; MARIN-MORALES, M.A. Toxicogenetic effects of low concentrations of the pesticides imidacloprid and sulfentrazone individually and in combination in in vitro tests with HepG2 cells and Salmonella typhimurium. Ecotoxicology and Environmental Safety, v.120, p.174-183, 2015.), even human cells (Palmieri et al., 2016PALMIERI, M.J.; ANDRADE-VIEIRA, L.F.; TRENTO, M.V.C.; ELEUTÉRIO, M.W.F.; LUBER, J.; DAVIDE, L. C.; MARCUSSI, S. Cytogenotoxic effects of Spent Pot Liner (SPL) and its main components on human leukocytes and meristematic cells of Allium cepa. Water, Air, & Soil Pollution , v. 227, n. 5, p. 1-10, 2016.). It has fast germination rates, is easy to cultivate, is found on seed stores and groceries alike, and has been shown to be as reliable as the vastly known and commonly used Allium cepa (Silveira et al., 2017SILVEIRA, G. L.; LIMA, M. G. F.; REIS, G. B.; PALMIERI, M. J.; ANDRADE-VIERIA, L. F. Toxic effects of environmental pollutants: Comparative investigation using Allium cepa L. and Lactuca sativa L. Chemosphere, v. 178, p. 359-367, 2017.). Also, the availability is high and up to 90% can germinate within 24 hours. In addition, this species is exhibited for its high number of seeds, large chromosomes and high sensitivity to mutagenic and genotoxic compounds (Ribeiro et al., 2013RIBEIRO, L.R.; SANTOS, M.F.; SILVA, Q.M.; PALMIERI, M.J.; ANDRADE-VIEIRA, L.F.; DAVIDE, L.C. Cytogenotoxic effects of ethanolic extracts of Annona crassiflora (Annonaceae). Biologia, v. 68, n. 3, p. 433-438, 2013.).
The experiment was carried out in a completely randomized design (CRD) with five concentrations of Captan® (0.5; 1; 2; 4 and 8% p.a.), 0.01% glyphosate solution (positive control) and distilled water (negative control), with five replicates of 25 seeds each. Seeds of lettuce (Lactuca sativa L. cultivar Manteiga (Feltrin Sementes®) were placed in Petri dishes lined with Germitest® paper, moisturized with 2.5 mL of solution of each treatment, subsequently kept in a BOD (Biochemical Oxigen Demand) chamber, under photoperiod of 12 hours and temperature of 24±2 °C. Germination analyses were performed every eight hours for 48 hours.
After 48 hours, the root length was measured with a digital caliper (0.01 mm) and ten seedlings of each treatment were randomly selected for fixation in methanol and acetic acid solution (3:1), and stored in a freezer. After 120 hours, the shoot length of the seedlings that remained in the Petri dishes was measured with a digital caliper.
The fixed roots were submitted to the crushing technique (adapted from Guerra and Souza, 2002GUERRA, M.; SOUZA, M.J. Como observar cromossomos: um guia de técnica em citogenética vegetal, animal e humana. Funpec. São Paulo, 2002. 131 p. ), in which, at room temperature, triple rinsing was carried out with distilled water (10 minutes each rinse) followed by immersion in 5N HCl solution for 18 minutes and again in distilled water for 10 minutes.
After washing, the apical meristem of each individual root was removed and transferred to a microscopy slide, then a drop of the 2% acetic orcein dye was added. After two minutes, the stained meristem was crushed between coverslip and slide. The slides were observed under a microscope with objective lens (Nikon® BE Plan 40x/0.65). Each treatment had 1000 cells counted per replicatet. Mitotic index (MI) was obtained by dividing the number of dividing cells (prophase, metaphase, anaphase and telophase) by the total cells evaluated in each treatment.
The frequencies of chromosomal and nuclear alterations were obtained by dividing the number of chromosomal and nuclear alterations, respectively, by the total number of cells evaluated. The frequency of changes, which represents the occurrence of each individual change, was evaluated by dividing the number of individual changes (c-metaphase, bridge, sticky, polyploidization, broken and lost) and the number of cells per division. The data were submitted to analysis of variance and the means were compared by the Dunnett’s test (p≤0.05).
RESULTS AND DISCUSSION
Experiment I: In vitro germination of braúna seeds after different immersion times in sodium hypochlorite (NaOCl)
The most widely used explants in tissue culture are derived from in vitro plants, which in turn were established from surface disinfected seeds with removal of the integument or aseptically excised zygotic embryos (Silva et al., 2019SILVA, J. A. T. da; ZENG, S.; GODOY-HERNÁNDEZ, G.; RIVERA-MADRID, R.; DOBRÁNSZKI, J. Bixa orellana L. (achiote) tissue culture: a review. In Vitro Cellular & Developmental Biology - Plant, v. 55, n. 3, p. 231-241, 2019.). In the control treatment (absence of NaOCl), there was 87.36% of seed contamination. The lowest contamination in the braúna (Melanoxylon brauna Schott) seeds was 39.11%, at the time of maximum technical effectiveness, which corresponds to the minimum point obtained in the quadratic model of the regression: 20.73 minutes of immersion in NaOCl (Figure 2a).
Contamination (%, a), contamination bacterial (%, b), contamination fungal (%, c), germination (%, d), germination speed index (ISG, e), abnormal seedlings (%, f), normal seedlings (%, g), in vitro dormancy (%, h) of braúna seeds (Melanoxylon brauna Schott) after immersion times in sodium hypochlorite (NaOCl). *(p≤0.01); TMTE. Time of Maximum Technical Effectiveness.
For braúna seeds, the non-use of disinfestation agents makes it impossible to proceed with in vitro propagation processes. NaOCl reduced contamination and consequently favored a higher percentage of normal seedlings up to a maximum exposure time of 25 minutes (29.27%) (Figure 2e). Despite the bacterial control (Figure 2b), the reduced normality of the seedlings (Figure 2c) is due to fungi activity (Figure 2a); thus requiring another disinfectant agent with effectiveness in the control of this type of microorganism.
Chlorine, the active principle of sodium hypochlorite, when diluted in water, promotes the formation of hypochlorous acid (HClO) and chlorine gas (Cl2). Hypochlorous acid is able to readily penetrate the bacterial cell through the cytoplasmic membrane and acts on proteins and nucleic acids of these microorganisms by oxidizing the sulfhydryl groups and the amino, indole and hydroxyphenol groups of tyrosine (Ingram et al., 2003INGRAM, P.R.; HOMER, N.Z.M.; SMITH, R.A.; PITT, A.R.; WILSON, C.G.; OLEJNIK, O.; SPICKETT, C.M. The interaction of sodium chlorite with phospholipids and glutathione: a comparison of effects in vitro, in mammalian and in microbial cells. Archives of Biochemistry and Biophysics, v. 410, n. 1, p. 121-133, 2003. )
The percentage and the germination velocity of the seeds presented increasing linear regression (Figure 2b and c, respectively) with the increase of immersion time in NaOCl until reaching maximum germination (51.88 %), with 25 minutes (Figure 2b). This suggests that the reduction in contamination allowed the seeds of brauna to partly express their germination potential, however, as the seed immersion time in NaOCl increased, the percentage of abnormal seedlings increased, reaching 29.24% in the TMTE of 25 minutes (Figure 2d).
Activation of seed metabolism is necessary for a series of physiological and biochemical processes to take place, whereby through the absorption of water by the seeds it promotes disruption of the integument by the embryo causing germination to occur (Flores et al., 2014FLORES, A.V.; BORGES, E.E.L.; GUIMARÃES, V.M.; GONÇALVES, J.F.C.; ATAÍDE, G.M.; BARROS, D.P. Atividade enzimática durante a germinação de sementes deMelanoxylon braunaSchott sob diferentes temperaturas. Cerne, v. 20, n. 3, p. 401-408, 2014.).
Germination and vigor are influenced by external and internal factors, and in M. brauna seeds size and color are important characteristics, with large seeds, independent of color, and small seeds showing the highest percentages and velocities. germination. These results corroborate the observations of Flores et al. (2014FLORES, A.V.; BORGES, E.E.L.; GONÇALVES, J.F.C.; GUIMARAES, V.M.; ATAIDE, G.M.; BARROS, D.P.; PEREIRA, M.D. Efeito do substrato, cor e tamanho de sementes na germinação e vigor de Melanoxylon brauna. Pesquisa Florestal Brasileira, v. 34, n. 78, p. 141-147, 2014.), that coloration coloration may be a morphological indicator of maturity stage for seeds of this species. Santos et al. (2017SANTOS, M.M.; BORGES, E.E.L.; ATÍDE, G.M.; SOUZA, G.A. Germination of seeds of Melanoxylon brauna Schott. under heat stress: production of reactive oxygen species and antioxidant activity. Forests, v. 8, n. 11, p. 1-13, 2017. ) studying thermal stress on the germination of M. brauna seeds, observed that when soaking them in water at 25 ºC there was 80% germination. In the present study, a maximum germination of 51.88% was observed (Table 2). However, the use of NaOCl as a disinfectant may have negatively influenced seed germination due to long exposure of cell tissues at different times. NaOCl acts by increasing the integument permeability and leaching of germination inhibitors. However, it can act negatively at high concentrations and cause damage to embryonic tissue (Carnelossi et al., 1995CARNELOSSI, M.A.G.; LAMOUNIER, L.; RANAL, M.A. Efeito da luz, hipoclorito de sódio, escarificação e estratificação na germinação de sementes de alface (Lactuca sativa L.), c.v. Maioba e Moreninha-de-Uberlândia. Pesquisa Agropecuária Brasileira, v. 30, n. 6, p. 779-787, 1995.). In Solanum lycopersicum L. seeds, sterilization with NaOCl interfered with the absorption and incorporation of leucine into proteins (Abdul-Baki, 1974ABDUL-BAKI, A.A. Hypochlorite and tissue sterilization. Planta, v. 115, n. 4, p. 373-376, 1974.). According to Khanna and Sharma (2013KHANNA, N.; SHARMA, S. Allium cepa root chromosomal aberration assay: a review. Indian Journal of Pharmaceutical Biology, v. 1, n. 3, p. 105-119, 2013. ), substances with toxic effects on plants, besides delayed root growth also cause genetic changes in cells.
The reduced efficiency of NaOCl in the control of contamination can be attributed to its greater effectiveness against bacteria, especially in the present study, where the persistent contamination after disinfestation was largely fungal (Figure 3a, b) and the presence of bacteria after this process was reduced or absent. Contamination in seeds of native forest species is associated with the presence of the contaminating agent on the surface of the integument and, when under favorable conditions, the contaminant agent thrives and colonizes the whole surface of the seed, preventing water absorption, which is primordial for the germination process (Botelho et al., 2008BOTELHO, L.S.; MORAES, M.H.D.; MENTEN, J.O. Fungos associados às sementes de ipê-amarelo (Tabebuia serratifolia) e ipê-roxo (Tabebuia impetiginosa): incidência, efeito na germinação e transmissão para as plântulas. Summa Phytopathologica, v. 34, n. 4, p. 343-348, 2008. ). Bacterial contamination was less than 30% in banana explants disinfested with NaOCl (Musa AAB cv. Maçã); however, the treatment was not efficient in the control of fungal contamination (Carneiro et al., 2000CARNEIRO, M.F.; SILVA, G.D.; XIMENES, P.A.; CARNEIRO, I.F.; BORGES, J.D. Avaliação de produtos na descontaminação de explantes de banana (Musa AAB cv. Maçã). Pesquisa Agropecuária Tropical, v. 30, p. 29-35, 2000.), corroborating with Aziz et al. (2018AZIZ, N.A.; TAN, B.C.; OTHMAN, R.Y.; KHALID, N. Efficient micropropagation protocol and genome size estimation of an important cover crop, Mucuna bracteata DC. ex Kurz. Plant Cell, Tissue and Organ Culture, v. 132, n. 2, p. 267-278, 2018. ), who observed that mucuna seeds treated with 50% Clorox® (commercial product with 5.25% w/v NaOCl) presented high percentage of contaminated seeds.
a, b. Contaminated Melanoxylon brauna seeds in vitro; c. Axenic seedling six days after in vitro germination; d. Abnormal seedling 30 days after in vitro germination. e. Normal seedling 30 days after in vitro germination. Bars: 1 cm.
Bacteria are prokaryotic organisms, characterized by being unicellular and small in size, as well as lacking organelles such as mitochondria, Golgi complex and mitotic spindle, and presenting genetic material that is dispersed in the cytoplasm (Murat et al., 2010MURAT, D.; BYRNE, M.; KOMEILI, A. Cell biology of prokaryotic organelles. Cold Spring Harbor Perspectives in Biology, v. 2, n. 10, p. 1-18, 2010.). It is believed that the absence of the nuclear envelope makes proteins and nucleic acids more vulnerable to NaOCl, since this disinfecting agent acts on these molecules. Bevilacqua et al. (2011BEVILACQUA, C.B.; REINIGER, L.R.S.; GOLLE, D.P.; ROSA, F.C. Desinfestação superficial, germinação e regeneração in vitro a partir de sementes de calêndula. Ciência Rural, v. 41, n. 5, p. 761-766, 2011.) ratified the efficiency of the antibacterial action of NaOCl in seeds of Calendula officinalis, which after being treated with 2.5% NaOCl for 30 minutes had 93.9% of the seeds free of this microorganism.
Experiment II: In vitro germination of braúna seeds after different times of immersion and maintenance of captan residue
Microbes throughout the life cycle of plants can be initiated by a pollinating agent and floral microbiomes, some of which will finally be recruited into developing seeds, where they multiply in the spermosphere during seed germination (Nelson, 2018NELSON, E.B. The seed microbiome: Origins, interactions, and impacts. Plant and Soil, v. 422, n. 1-2, p. 7-34, 2018.).
When these micro-organisms are not efficiently controlled by disinfectant agents, they develop mainly when in culture media (Table 1). The lowest contamination, the highest germination and the highest ISG occurred with the maintenance of the captan residue in the seeds, regardless of the time of immersion in the fungicide. Maintenance of the captan residue in the seeds after 10; 20; 25 and 30 minutes of immersion resulted in the highest percentages of normal seedlings (Figure 3e) (68; 65; 61 and 59%, respectively). The drastic reduction of contamination with captan residues allows the expression of seeds as to germination and vigor. According to Marcos Filho (2015)MARCOS FILHO, J. Seed vigor testing: an overview of the past, present and future perspective. Scientia Agricola, v. 72, n. 4, p. 363-374, 2015. , the factors that influence the physiological potential of seeds include germination and vigor, which govern the ability of seeds to express their vital functions under favorable and unfavorable environmental conditions. However, it was verified that the maintenance of the captan residue, in the majority of the immersion times, promoted a greater abnormality of the seedlings (Figure 3d) (Table 1).
The difference in contamination in the control treatment (alcohol 70%, one minute + NaOCl 2.5% active chlorine, 15 minutes) compared to the addition of captan is significant, suggesting that only NaOCl is not sufficient for the disinfestation of braúna seeds.
The addition of captan to the disinfestation process of the braúna seeds promoted a satisfactory increase of uncontaminated seeds (Figure 3c) (40%), and when its residue was maintained in the seeds, this value increased to 53%. The fungitoxicity of captan is due to its multisite action, which irreversibly blocks molecules containing the thiol group, resulting in reduced activity of several enzymes essential for fungal survival (Arce et al., 2010ARCE, G.T.; GORDON, E.B.; COHEN, S.M.; SINGH, P. Genetic toxicology of folpet and Captan. Critical Reviews in Toxicology, v. 40, n. 6, p. 546-574, 2010.). Unlike sodium hypochlorite, captan is a water-insoluble chemical compound, and because of this apolar characteristic, it is able to penetrate more easily into the nucleus of fungal cells and initiate the toxicological process. In a study with Saccharomyces cerevisiae treated with captan, it was found that 81.7% of these fungi presented cell death, caused by loss of cell membrane integrity (Scariot et al., 2017SCARIOT, F.J.; JAHN, L.; DELAMARE, A.P.L.; ECHEVERRIGARAY, S. Necrotic and apoptotic cell death induced by Captan on Saccharomyces cerevisiae. World Journal of Microbiology and Biotechnology, v. 33, n. 8, p. 159, 2017.).
The maintenance of the captan residue reduced the contamination, due to the longer contact with the seeds, since this fungicide has a protective action, inhibiting the growth and development of possible microorganisms that persist in the seeds, even after the disinfestation processes. The short exposure time combined with the low permeability characteristics of the integument due to the cerosity found in braúna seeds reduced the efficiency of the captan treatment without residue.
The effectiveness of protective fungicides is closely related to their contact performance and, for successful disinfestation, it is important that captan covers the entire surface of the seed (Wagner et al., 2003WAGNER, P.; FURSTNER, R.; BARTHLOTT, W.; NEINHUIS, C. Quantitative assessment to the structural basis of water repellency in natural and technical surfaces. Journal of Experimental Botany, v. 54, n. 385, p. 1295-1303, 2003.; Koller et al., 2004KOLLER, W.; PARKER, D.M.; TURECHEK, W.W.; AVILA-ADAME, C.; CRONSHAW, K. A two-phase resistance response to Venturia inaequalis populations to the QoI fungicides kresoxim-methyl and trifloxystrobin. Plant Disease, v. 88, n. 5, p. 537-544, 2004. ). Removal of the residue after treatment with captan reduced its fungitoxic effect because the contact fungicides cannot persist on plant surfaces after being washed out with water 24 hours after treatment, leaving them unprotected (Abbott and Beckerman, 2018ABBOTT, C.P.; BECKERMAN, J.L. Incorporating adjuvants with Captan to manage common apple diseases. Plant Diseases, v. 102, n. 1, p. 231-236, 2018.).
In spite of the efficiency of captan in the disinfestation with the maintenance of its residue, it also caused abnormalities in braúna seedlings, with an average of 17% abnormal seedlings. This observation suggests that captan promoted some cytotoxic effect on braúna seedlings.
Experiment III: Evaluation of captan toxicity in Lactuca sativa L. seeds
The effects of captan at all concentrations tested and all characteristics analyzed, except for shoot length, were more drastic than those caused by glyphosate (positive control). Mitotic index at concentrations 1; 2; 4 and 8 % of captan was statistically similar to the mitotic index of the positive control (glyphosate), and the MI of the root meristematic cells treated with 0.5% captan was reduced to 39.43%, compared to the negative control (water) (Figure 4).
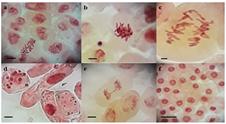
Genotoxic damage in meristematic cells of Lacuta sativa L. exposed to different concentrations of the fungicide captan. a. C-metaphase; b. Sticky chromosomes; c. Multipolar anaphase; d. Micronuclei; e. Anaphasic bridge; f. Interphase. Bars: 5 μm.
Captan reduced germination and root length, decreased mitotic index and increased the frequency of chromosomal alterations of Lactuca sativa cells, suggesting, respectively, the phytotoxic, cytotoxic and genotoxic power of this fungicide.
The macroscopic analyzes (germination, ISG, root length and shoot length) are closely related to the microscopic ones (cell divisions and chromosomal alterations), since germination and the growth of root and shoot depend on the increase of cell number and elongation (Harashima and Schnittger, 2010HARASHIMA, H.; SCHNITTGER, A. The integration of cell division, growth and differentiation. Current Opinion in Plant Biology, v. 13, n. 1, p. 66-74, 2010. ), which, in turn, rely upon successive mitotic cycles. In this sense, if there is no blockage in the transition of mitosis phases, cell division normally occurs, as well as root and shoot growth.
The results obtained in this work corroborate with those obtained with the fungicides difeconazol and tebuconazole used in Allium cepa seeds, which determined a decrease in root growth, reduction of mitotic index and increase of chromosomal and nuclear alterations (Bernardes et al., 2015BERNARDES, P.M.; ANDRADE-VIEIRA, L.F.; ARAGÃO, F.B.; FERREIRA, A.; FERREIRA, M.F.S. Toxicity of difenoconazole and tebuconazole in Allium cepa. Water, Air, & Soil Pollution, v. 226, n. 7, p. 1-11, 2015.). Mitotic index can provide information on the cytotoxicity of a particular substance, in which its reduction is related to cell cycle paralysis evidencing cytotoxic activity, whereas its increase may indicate tumor processes (Souza et al., 2010SOUZA, L.F.B.; LAUGHINGHOUSE, H.D.; PASTORI, T.; TEDESCO, M.; KUHN, A.W.; CANTO-DOROW, T.S.; TEDESCO, S.B. Genotoxic potential of aqueous extracts of Artemisia verlotorumon the cell cycle of Allium cepa. International Journal of Environmental Studies, v. 67, n. 6, p. 871-877, 2010. ), both of which are detrimental to the normal development of the organism.
The number of cells in interphase for concentrations 1; 2 and 4 % was similar to the positive control. On the other hand, the number of prophase cells submitted to the 0.5 % concentration was similar to the negative control, and the other concentrations were similar to the positive control. The number of cells in metaphase and anaphase at concentrations 1; 2; 4 and 8% resembled the positive control. At telophase, cells at all concentrations of captan were similar to the positive control (Table 2).
The similarity of mitotic index at concentrations 1; 2; 4 and 8% of captan with the positive control (glyphosate, inhibitor of the action of the enzyme 5-enolpyruvate-shikimate-3 phosphate synthase (EPSPs), interfering in the synthesis of chorismate - immediate precursor of the amino acids tryptophan, tyrosine and phenylalanine) (Funke et al., 2006FUNKE, T.; HAN, H.; HEALY-FRIED, M.L.; SCHONBRUNN, E. Molecular basis for the herbicide resistance of Roundup ready crops. Proceedings of the National Academy of Sciences, v. 103, n. 35, p. 13010-13015, 2006. ), suggests that in these concentrations the fungicide and glyphosate paralyzed the cell division of the lettuce apical root meristem. It can be seen in figure 4 that all cells exposed to captan at the concentrations of 2 and 4 % are in the interphase (Figure 4f), and the cytotoxic effect of captan at 8% was so severe as to not allow germination at all, so the effect on the cell cycle on the meristem for this treatment could not be evaluated.
There were alterations in the cell cycle (C-metaphase, sticky chromosomes, multipolar anaphase, anaphase bridge and micronuclei) at the concentrations of 0.5 and 1% of captan and germination of seeds, with only 41% germinating at the lowest concentration; whereas no germination was observed in the highest concentration (Table 2).
However, because at the concentration of 0.5% the cytotoxic effect of captan was lower, some cells were able to advance in the division, and it is possible to identify greater chromosomal changes in the phases of the cell cycle. At this concentration, the frequency of chromosomal changes was three times higher than the negative control, suggesting the genotoxic potential of captan.
Figure 4 shows the different chromosomal changes caused by the fungicide, and it is possible to infer its mechanism of action on lettuce. Changes in chromosome structure such as bridges (Figure 4e) and breakage indicate clastogenic action, and when changes are numerical and encompass the set of chromosomes, such as lost chromosome, adherent chromosome or sticky (Figure 4b), delays, multipolarity (Figure 4c) and C-metaphase (Figure 4a), there is an indication of aneugenic action (Vidakovic-Cifrek et al., 2002VIDAKOVIC-CIFREK, Z.; PAVLICA, M.; REGULA, I.; PAPES, D. Cytogenetic damage in shallot (Allium cepa) root meristems induced by oil industry “high-density brines”. Archives of Environmental Contamination and Toxicology, v. 43, n. 3, p. 284-291, 2002. ; Leme et al., 2009LEME, D.M.; MARIN-MORALES, M.A. Allium cepa test in environmental monitoring: A review on its application. Mutation Research , v. 682, n. 1, p. 71-81, 2009. ).
In this sense, since it was possible to observe both chromosomal alterations that cover the structure and those that interfered in the chromosomal set, it can be affirmed that the mechanism of action of captan on lettuce is clastogenic and aneugenic, with predominance of the latter.
The occurrence of sticky chromosomes (Figure 4b) in cells was due to changes in chromosome structure, and it promoted the loss of normal condensation characteristics forming clusters, whereas c-metaphase is due to mitotic spindle malformation or abnormal cell division (Fenech, 2000FENECH, M. The in vitro micronucleus technique. Mutation Research, v. 455, n. 1-2, p. 81-95, 2000.). The chromosome bridges are due to adhesion and a subsequent failure to separate the chromosomes in the anaphase, and once the chromatids are adhered, they remain united in the anaphase, and when separated, they can lead to the formation of micronuclei (Marcano et al., 2004MARCANO, L.; CARRUYO, I.; DEL CAMPO, A.; MONTIEL, X. Cytotoxicity and mode of action of maleic hydrazide in root tips of Allium cepa L. Environmental Research, v. 94, n. 2, p. 221-226, 2004.). These and other chromosomal and nuclear alterations are detrimental to cells and can cause them to enter the cell death process (Palmieri et al., 2014PALMIERI, M.J.; LUBER, J.; ANDRADE-VIEIRA, L.F.; DAVIDE, L.C. Cytotoxic and phytotoxic effects of the main chemical components of spent pot-liner: A comparative approach. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, v. 763, p. 30-35, 2014.).
CONCLUSIONS
The isolated use of NaOCl is not efficient in the disinfestation of braúna seeds. The fungicide captan at 2% p.a. applied for 10 minutes, with the maintenance of its residue, improved germination and vigor, resulting in greater and lesser formation of normal and abnormal seedlings, respectively. Captan has high phytotoxic, cytotoxic and genotoxic effect and its mechanism of action in the cell cycle of Lactuca sativa is clastogenic and aneugenic, suggesting the formation of abnormal seedlings in M. brauna.
AKNOWLEDGEMENTS
The authors thank FAPES/SEAG Notice No. 06/2015, for the research funding and CNPq for granting the research scholarship.
REFERENCES
- ABBOTT, C.P.; BECKERMAN, J.L. Incorporating adjuvants with Captan to manage common apple diseases. Plant Diseases, v. 102, n. 1, p. 231-236, 2018.
- ABDUL-BAKI, A.A. Hypochlorite and tissue sterilization. Planta, v. 115, n. 4, p. 373-376, 1974.
- ANDRADE, M.W.; LUZ, J.M.Q.; LACERDA, A.S.; MELO, P.R. Micropropagação da aroeira (Myracrodruon urundeuva Fr. All). Ciência e Agrotecnologia, v. 24, n. 1, p. 174-180, 2000.
- ARCE, G.T.; GORDON, E.B.; COHEN, S.M.; SINGH, P. Genetic toxicology of folpet and Captan. Critical Reviews in Toxicology, v. 40, n. 6, p. 546-574, 2010.
- AZIZ, N.A.; TAN, B.C.; OTHMAN, R.Y.; KHALID, N. Efficient micropropagation protocol and genome size estimation of an important cover crop, Mucuna bracteata DC. ex Kurz. Plant Cell, Tissue and Organ Culture, v. 132, n. 2, p. 267-278, 2018.
- BEDNAREK, P.T.; ORLOWSKA, R. Plant tissue culture environment as a switch-key (epi)genetic changes. Plant Cell, Tissue and Organ Culture , v. 1, p. 1-13, 2019.
- BERNARDES, P.M.; ANDRADE-VIEIRA, L.F.; ARAGÃO, F.B.; FERREIRA, A.; FERREIRA, M.F.S. Toxicity of difenoconazole and tebuconazole in Allium cepa Water, Air, & Soil Pollution, v. 226, n. 7, p. 1-11, 2015.
- BEVILACQUA, C.B.; REINIGER, L.R.S.; GOLLE, D.P.; ROSA, F.C. Desinfestação superficial, germinação e regeneração in vitro a partir de sementes de calêndula. Ciência Rural, v. 41, n. 5, p. 761-766, 2011.
- BIANCHI, J.; CABRAL-DE-MELLO, D.C.; MARIN-MORALES, M.A. Toxicogenetic effects of low concentrations of the pesticides imidacloprid and sulfentrazone individually and in combination in in vitro tests with HepG2 cells and Salmonella typhimurium Ecotoxicology and Environmental Safety, v.120, p.174-183, 2015.
- BOTELHO, L.S.; MORAES, M.H.D.; MENTEN, J.O. Fungos associados às sementes de ipê-amarelo (Tabebuia serratifolia) e ipê-roxo (Tabebuia impetiginosa): incidência, efeito na germinação e transmissão para as plântulas. Summa Phytopathologica, v. 34, n. 4, p. 343-348, 2008.
- BOLOGNESI, C. Genotoxicity of pesticides: a review of human biomonitoring studies. Mutation Research/Reviews in Mutation Research, v. 543, n. 3, p. 251-272, 2003.
- CARNEIRO, M.F.; SILVA, G.D.; XIMENES, P.A.; CARNEIRO, I.F.; BORGES, J.D. Avaliação de produtos na descontaminação de explantes de banana (Musa AAB cv. Maçã). Pesquisa Agropecuária Tropical, v. 30, p. 29-35, 2000.
- CARNELOSSI, M.A.G.; LAMOUNIER, L.; RANAL, M.A. Efeito da luz, hipoclorito de sódio, escarificação e estratificação na germinação de sementes de alface (Lactuca sativa L.), c.v. Maioba e Moreninha-de-Uberlândia. Pesquisa Agropecuária Brasileira, v. 30, n. 6, p. 779-787, 1995.
- CARVALHO, F.A.; NASCIMENTO, M.T.; BRAGA, J.M.A. Estrutura e composição florística do estrato arbóreo de um remanescente de Mata Atlântica submontana no município de Rio Bonito, RJ, Brasil (Mata Rio Vermelho). Revista Árvore, v. 31, n. 4, p. 717-730, 2007.
- CHAVES, A.C.; SCHUCH, M.W.; ERIG, A.C. Estabelecimento e multiplicação in vitro de Physalis peruviana L. Ciência e Agrotecnologia , v. 29, n. 6, p. 1281-1287, 2005.
- EDGINGTON, L.V.; KHEW, K.L.; BARRON, G.L. Fungitoxic spectrum of benzimidazole compounds. Phytopathology, v. 61, p. 42- 44, 1971.
- FENECH, M. The in vitro micronucleus technique. Mutation Research, v. 455, n. 1-2, p. 81-95, 2000.
- FLORES, A.V.; BORGES, E.E.L.; GONÇALVES, J.F.C.; GUIMARAES, V.M.; ATAIDE, G.M.; BARROS, D.P.; PEREIRA, M.D. Efeito do substrato, cor e tamanho de sementes na germinação e vigor de Melanoxylon brauna Pesquisa Florestal Brasileira, v. 34, n. 78, p. 141-147, 2014.
- FLORES, A.V.; BORGES, E.E.L.; GUIMARÃES, V.M.; GONÇALVES, J.F.C.; ATAÍDE, G.M.; BARROS, D.P. Atividade enzimática durante a germinação de sementes deMelanoxylon braunaSchott sob diferentes temperaturas. Cerne, v. 20, n. 3, p. 401-408, 2014.
- FUNKE, T.; HAN, H.; HEALY-FRIED, M.L.; SCHONBRUNN, E. Molecular basis for the herbicide resistance of Roundup ready crops. Proceedings of the National Academy of Sciences, v. 103, n. 35, p. 13010-13015, 2006.
- GUERRA, M.; SOUZA, M.J. Como observar cromossomos: um guia de técnica em citogenética vegetal, animal e humana. Funpec. São Paulo, 2002. 131 p.
- HARASHIMA, H.; SCHNITTGER, A. The integration of cell division, growth and differentiation. Current Opinion in Plant Biology, v. 13, n. 1, p. 66-74, 2010.
- INGRAM, P.R.; HOMER, N.Z.M.; SMITH, R.A.; PITT, A.R.; WILSON, C.G.; OLEJNIK, O.; SPICKETT, C.M. The interaction of sodium chlorite with phospholipids and glutathione: a comparison of effects in vitro, in mammalian and in microbial cells. Archives of Biochemistry and Biophysics, v. 410, n. 1, p. 121-133, 2003.
- INSTITUTO BRASILEIRO DO MEIO AMBIENTE (IBAMA). Lista oficial de flora ameaçada de extinção. Available at: <Available at: http://www.ibama.gov.br/flora >. Acessed on: Apr. 04 2018.
» http://www.ibama.gov.br/flora - KHANNA, N.; SHARMA, S. Allium cepa root chromosomal aberration assay: a review. Indian Journal of Pharmaceutical Biology, v. 1, n. 3, p. 105-119, 2013.
- KOLLER, W.; PARKER, D.M.; TURECHEK, W.W.; AVILA-ADAME, C.; CRONSHAW, K. A two-phase resistance response to Venturia inaequalis populations to the QoI fungicides kresoxim-methyl and trifloxystrobin. Plant Disease, v. 88, n. 5, p. 537-544, 2004.
- KUMARI, M.; KHAN, S.S.; PAKRASHI, S.; MUKHERJEE, A.; CHANDRASEKARAN, N. Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa Journal of Hazardous Materials, v. 190, n. 1-3, p. 613-621, 2011.
- LEME, D.M.; MARIN-MORALES, M.A. Allium cepa test in environmental monitoring: A review on its application. Mutation Research , v. 682, n. 1, p. 71-81, 2009.
- LLOYD, G.; McCOWN, B. Commercially feasible micropropagation of Mountain Laurel, Kalmia latifolia, by use of shoot tip culture. International Plant Propagators’ Society, v. 30, p. 421-427, 1980.
- LORENZI, H. Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas nativas do Brasil, 6. ed., Nova Odessa: Instituto Planta rum, 2014. 384 p.
- MARCANO, L.; CARRUYO, I.; DEL CAMPO, A.; MONTIEL, X. Cytotoxicity and mode of action of maleic hydrazide in root tips of Allium cepa L. Environmental Research, v. 94, n. 2, p. 221-226, 2004.
- MARCOS FILHO, J. Seed vigor testing: an overview of the past, present and future perspective. Scientia Agricola, v. 72, n. 4, p. 363-374, 2015.
- MURASHIGE, T. Plant propagation through tissue cultures. Annual Review of Plant Physiology, v. 25, n. 1, p. 135-166, 1974.
- MURAT, D.; BYRNE, M.; KOMEILI, A. Cell biology of prokaryotic organelles. Cold Spring Harbor Perspectives in Biology, v. 2, n. 10, p. 1-18, 2010.
- NELSON, E.B. The seed microbiome: Origins, interactions, and impacts. Plant and Soil, v. 422, n. 1-2, p. 7-34, 2018.
- OYEBANJI, O.B.; NWEKE, O.; ODEBUNMI, O.; GALADIMA, N.B.; IDRIS, M.S.; NNODI, U.N.; AFOLABI, A.S.; OGBADU, G.H. Simple, effective and economical explant-surface sterilization protocol for cowpea, rice and sorghum seeds. African Journal of Biotechnology, v. 8, n. 20, p. 5395-5399, 2009.
- PAIS, A.K.; SILVA, A.P. da; SOUZA, J.C. de; TEIXEIRA, S.L.; RIBEIRO, J.M.; PEIXOTO, A.R.; PAZ, C.D. Sodium hypochlorite sterilization of culture medium in micropropagation of Gerbera hybrida cv. Essandre. African Journal of Biotechnology , v. 15, n. 36, p. 1995-1998, 2016.
- PALMIERI, M.J.; ANDRADE-VIEIRA, L.F.; TRENTO, M.V.C.; ELEUTÉRIO, M.W.F.; LUBER, J.; DAVIDE, L. C.; MARCUSSI, S. Cytogenotoxic effects of Spent Pot Liner (SPL) and its main components on human leukocytes and meristematic cells of Allium cepa Water, Air, & Soil Pollution , v. 227, n. 5, p. 1-10, 2016.
- PALMIERI, M.J.; LUBER, J.; ANDRADE-VIEIRA, L.F.; DAVIDE, L.C. Cytotoxic and phytotoxic effects of the main chemical components of spent pot-liner: A comparative approach. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, v. 763, p. 30-35, 2014.
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, 2017.
- RIBEIRO, L.R.; SANTOS, M.F.; SILVA, Q.M.; PALMIERI, M.J.; ANDRADE-VIEIRA, L.F.; DAVIDE, L.C. Cytogenotoxic effects of ethanolic extracts of Annona crassiflora (Annonaceae). Biologia, v. 68, n. 3, p. 433-438, 2013.
- SANTOS, M.M.; BORGES, E.E.L.; ATÍDE, G.M.; SOUZA, G.A. Germination of seeds of Melanoxylon brauna Schott. under heat stress: production of reactive oxygen species and antioxidant activity. Forests, v. 8, n. 11, p. 1-13, 2017.
- SCARIOT, F.J.; JAHN, L.; DELAMARE, A.P.L.; ECHEVERRIGARAY, S. Necrotic and apoptotic cell death induced by Captan on Saccharomyces cerevisiae World Journal of Microbiology and Biotechnology, v. 33, n. 8, p. 159, 2017.
- SILVA, J. A. T. da; ZENG, S.; GODOY-HERNÁNDEZ, G.; RIVERA-MADRID, R.; DOBRÁNSZKI, J. Bixa orellana L. (achiote) tissue culture: a review. In Vitro Cellular & Developmental Biology - Plant, v. 55, n. 3, p. 231-241, 2019.
- SILVEIRA, G. L.; LIMA, M. G. F.; REIS, G. B.; PALMIERI, M. J.; ANDRADE-VIERIA, L. F. Toxic effects of environmental pollutants: Comparative investigation using Allium cepa L. and Lactuca sativa L. Chemosphere, v. 178, p. 359-367, 2017.
- SISLER, H.D. Effect of fungicides on protein and nucleic acid synthesis. Annual Review ofPhytopathology , v. 7, n. 1, p. 311-330, 1969.
- SOUZA, L.F.B.; LAUGHINGHOUSE, H.D.; PASTORI, T.; TEDESCO, M.; KUHN, A.W.; CANTO-DOROW, T.S.; TEDESCO, S.B. Genotoxic potential of aqueous extracts of Artemisia verlotorumon the cell cycle of Allium cepa International Journal of Environmental Studies, v. 67, n. 6, p. 871-877, 2010.
- SOUZA, L.S.; FIOR, C.S.; SOUZA, P.V.D.; SCHWARZ, S.F. Desinfestação de sementes e multiplicação in vitro de guabijuzeiro a partir de segmentos apicais juvenis (Myrcianthes pungens O. Berg) D. Legrand. Revista Brasileira de Fruticultura, v. 33, n. 7, p. 691-697, 2011.
- URTIGA, C. da C.; SILVA-CARDOSO, I.M. de A.; FIGUEIREDO, S.A. Low sodium isocyanurate concentrations as a substitute to medium autoclaving in plant tissue culture. Plant Cell, Tissue and Organ Culture , v. 139, n. 3, p. 601-604, 2019.
- VIDAKOVIC-CIFREK, Z.; PAVLICA, M.; REGULA, I.; PAPES, D. Cytogenetic damage in shallot (Allium cepa) root meristems induced by oil industry “high-density brines”. Archives of Environmental Contamination and Toxicology, v. 43, n. 3, p. 284-291, 2002.
- VILLALOBOS, V.M.; ENGELMANN, F. Ex situ conservation of plant germplasm using biotechnology. World Journal of Microbiology and Biotechnology , v. 11, n. 4, p. 375-382, 1995.
- WAGNER, P.; FURSTNER, R.; BARTHLOTT, W.; NEINHUIS, C. Quantitative assessment to the structural basis of water repellency in natural and technical surfaces. Journal of Experimental Botany, v. 54, n. 385, p. 1295-1303, 2003.
HIGHLIGHTS
-
1
Sodium hypochlorite (NaOCl) alone is not efficient for disinfesting brauna seeds.
-
2
Brauna seeds also require the use of captan fungicide (2%) 10 min-1 in disinfestation and maintenance of its residue.
-
3
This compound exhibited toxicity in the lettuce seed model.
Publication Dates
-
Publication in this collection
30 Mar 2020 -
Date of issue
Oct-Dec 2019
History
-
Received
12 Nov 2019 -
Accepted
10 Dec 2019