Abstract
The search for snake venom antitumor efficacy has attracted the interest of scientists since the beginning of last century. Snake venom possesses a wide spectrum of biological activities. In this study, we evaluated the effect of Echis coloratus crude venom on the evolution of Ehrlich ascites carcinoma cells (EAC). Normal and EAC-bearing mice were treated with 0.2 mg/kg body weight of crude venom. Crude venom was seen to suppress tumor growth by significantly decreasing EAC cell count and cell viability (p<0.01). There was also a significant increase in survival time of the venom-treated tumor-bearing mice (52.3%, p<0.05) in comparison to the non-treated tumor-bearing counterparts. The study of venom effect and/or tumor inoculation on some important biochemical parameters and enzyme activities showed that the expected venom toxic effect disappeared due to the low (sublethal) dose; treatment of the tumor-bearing mice with this low dose could correct and restore biochemical parameters to normal levels which had been altered due to tumor growth. It can be concluded that Echis coloratus crude venom antitumor efficiency exceeded or camouflaged its toxic effect.
snake venom; Echis coloratus; Ehrlich ascites carcinoma; tumor growth; biochemistry
Original paper
ANTITUMOR AND BIOCHEMICAL EFFECTS OF Echis coloratus CRUDE VENOM ON EHRLICH ASCITES CARCINOMA CELLS IN VIVO
E. A. MADY1 CORRESPONDENCE TO:
E. A Mady - Dept. of Chemistry, Faculty of Education, King Faisal University, P. O. Box 1759, Al-Hassa 31982, K., Saudi Arabia.
eamady1@hotmail.com
CORRESPONDENCE TO:
E. A Mady - Dept. of Chemistry, Faculty of Education, King Faisal University, P. O. Box 1759, Al-Hassa 31982, K., Saudi Arabia.
eamady1@hotmail.com
1 Department of Biochemistry, Faculty of Science, Ain Shams University, Cairo, Egypt.
ABSTRACT: The search for snake venom antitumor efficacy has attracted the interest of scientists since the beginning of last century. Snake venom possesses a wide spectrum of biological activities. In this study, we evaluated the effect of Echis coloratus crude venom on the evolution of Ehrlich ascites carcinoma cells (EAC). Normal and EAC-bearing mice were treated with 0.2 mg/kg body weight of crude venom. Crude venom was seen to suppress tumor growth by significantly decreasing EAC cell count and cell viability (p<0.01). There was also a significant increase in survival time of the venom-treated tumor-bearing mice (52.3%, p<0.05) in comparison to the non-treated tumor-bearing counterparts. The study of venom effect and/or tumor inoculation on some important biochemical parameters and enzyme activities showed that the expected venom toxic effect disappeared due to the low (sublethal) dose; treatment of the tumor-bearing mice with this low dose could correct and restore biochemical parameters to normal levels which had been altered due to tumor growth. It can be concluded that Echis coloratus crude venom antitumor efficiency exceeded or camouflaged its toxic effect.
KEYWORDS: snake venom, Echis coloratus, Ehrlich ascites carcinoma, tumor growth, biochemistry.
INTRODUCTION
The search for biological antitumor agents has attracted the interest of scientists since the beginning of last century. The cytolytic activity of most available antitumor chemicals is due to inhibition of DNA synthesis and replication in tumor cells. It is of great interest to change cancer treatment from chemotherapy to biotherapy, using biological agents with minimal or no adverse effects (26).
Snake venom is a complex mixture of many substances, such as toxins, enzymes, growth factors, activators, and inhibitors with a wide spectrum of biological activities (34). They are also known to cause different metabolic disorders by altering the cellular inclusions and enzymatic activities of different organs (1). The search for snake venoms in relation to malignancy dates back to 1911. However, it was not until 1933 that venoms were recognized as antitumor agents when Calmette et al., (10) reported an antitumor effect of Naja naja venom on adenocarcinoma cells. From that time on, many investigations have been performed using venoms of different snake species (6,7,11,12,25,28,33,37-39,45). It has been reported that venoms from the snake families Elapidae, Viperidae, and Crotalidae caused lysis of Yoshida sarcoma and KB cells (44). There are reports showing the cytotoxic activity of various snake venoms in vivo and in vitro using melanoma and chondrosarcoma cells (11). It was further demonstrated that a purified protein from cobra venom was selectively cytotoxic to cancer cells (7). Lipps (26) isolated two cancer cell inhibitor proteins, atroporin and kaotree from Crotalus atrox and Naja n. kaouthia venoms. The two components showed killing effects on various types of human (breast, colon, liver, ovary) and animal cancer cells at concentrations as low as 0.5 μg/ml. However, controversy still exists regarding the action of snake venoms on tumor growth; the exact mechanism that causes tumor regression after treatment with snake venom is still unknown (38,39).
This study was performed to evaluate the antitumor activity of the venom of one of the most common snakes in Saudi Arabia, Echis coloratus, of the family Viperidae family. The crude venom was tested on one of the most devastating experimental tumor models, the Ehrlich ascites carcinoma cells (EAC) in mice.
MATERIALS AND METHODS
Animals
Adult female Swiss albino mice weighing 22-25g were used throughout this study. They were maintained on commercial standard pellet diet and tap water ad libitum.
Tumor
The initial inoculum of EAC cells was kindly provided by the National Cancer Institute (NCI) of Cairo, Egypt. The EAC cells were thereafter propagated in our laboratory by weekly intraperitoneal (IP) injections of 3x106 cells, freshly drawn from a donor mouse bearing 7-9 day-old ascites tumor suspended in 0.3 ml sterile saline solution.
Venom
Echis coloratus crude venom was kindly supplied by the toxins unit, Zoology Dept., Faculty of Science, King Saud University, Saudi Arabia.
Toxicity Studies
Determination of the acute median lethal dose (LD50) of crude venom was carried out according to Meier and Theakston (30).
Experimental Design
Four groups of 25 mice each were used:
Group 1: Normal control (NC) - Mice received sterile saline solution;
Group 2: Tumor control (TC) - Mice were inoculated IP with 6x106 EAC cells/0.3ml saline solution;
Group 3: Venom treated (V-Tr) - Mice received two IP injections of 0.2 mg/kg body weight of crude venom on days 1 and 5; and
Group 4: Tumor treated (T-Tr) - Mice were inoculated with 6x106 EAC cells/0.3 ml saline solution; later each animal received a single IP injection of 0.2 mg/kg body weight of crude venom on the first and the fifth day after tumor inoculation.
Ten days after tumor inoculation, 15 animals of each group were chosen by lot to be sacrificed after 16-hour fast. Blood samples were collected; serum samples were obtained by centrifugation at 5000 rpm for 10 minutes and stored at -20°C for biochemical analysis. Peritoneal fluid was aspirated for the evaluation of the total number of EAC cells, and the percentage of EAC cell viability. They were tested by the trypan blue exclusion technique according to Boyse et al. (5). The remaining animals (10 in each group) were kept in the laboratory for evaluation of survival percentage (life span prolongation).
Biochemical analysis
Serum glucose, total proteins, total lipids, and the activity of aspartate transferase (AST), alanine transferase (ALT), lactate dehydrogenase (LDH), gamma glutamyle transferase (GGT), and arylsulfatase were assayed according to Trinder (43), Markwell et al. (29), Knight et al. (22), Reitman and Frankel (35), Cabaud and Wroblewski (9), Szasz and Persijin (41), and Gniot and Dzialazynaki (17), respectively.
Statistical analyses
The results were statistically analyzed using SPSS package (Echo Soft Corp., USA, 1993). All results were expressed as means ±standard error (SE). The Student's t test was used for statistical analysis. Correlations of data were evaluated by Spearman's test (rs). Difference was considered statistically significant when p<0.05.
RESULTS
Figure 1 shows the results for the total number of EAC cells and the number and percentage of viable cells. The TC group presented a significantly high total EAC cell count and a significantly higher percentage of viable cells. The T-Tr group showed a significantly lower number of EAC (p<0.05) and a significantly lower percentage of viable EAC cells (p<0.01). It is evident that the dose of the crude venom used resulted in a significant tumor growth inhibition.
Effect of crude venom on total number and cell viability (x 106) of EAC cells. 1) TC, 2) T-Tr.
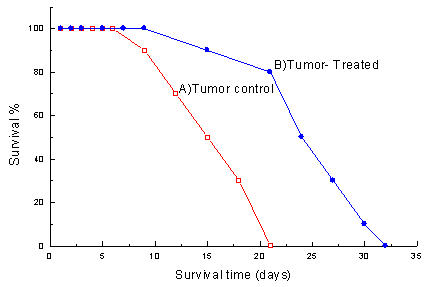
Figure 2 shows the effect of the venom on survival time. The T-Tr group showed a significantly higher survival time than the TC group (52.3%, p<0.05). In addition to increasing life span, treating tumor-bearing animals with crude venom caused a considerable decrease in mortality percentage in comparison to non-treated tumor-bearing animals.
Table 1 shows the serum biochemical profile of the four groups. In relation to the biochemical outcome of tumor implantation against normal controls, there was a significant decrease in serum glucose and total proteins (p<0.001, 0.05); there was a significant increase in total lipids and urea nitrogen (p<0.001). True intracellular enzyme activity showed significant increases in the activity of AST, LDH, GGT, and arylsulfatase (p<0.001); serum ALT, however, showed a significant decrease (p<0.01). There was also significant positive correlation between AST and LDH (rs= 0.65, p<0.05); LDH and GGT (rs=0.85, p<0.04); and GGT and arylsulfatase (rs=0.68, p<0.05). There were no significant changes for the V-Tr animals in serum levels of glucose, total proteins, urea, nitrogen, ALT, GGT, and arylsulfatase; on the other hand, there was a significant increase in total lipids, AST, and LDH (p<0.001, 0.001, and 0.01 respectively).
The effect of tumor and venom together is a tendency to correct and restore the levels of the studied parameters that were altered by either the tumor or the venom.
DISCUSSION
Although the fundamental understanding of cancer is progressing rapidly, there is still no academic breakthrough in therapy. There is a lot of scope for the discovery of naturally occurring biological agents that can be therapeutic and generally important to oncology (26).
Snake venoms have been used scientifically to elucidate physiological mechanisms, and they can be used as the starting point in the design of new therapeutic agents(2). Due to the numerous investigations into snake venoms as anticancer agents, they might hold the next miracle for treating cancer.
In this study, Echis coloratus crude venom was evaluated for its antitumor efficiency on one of the most devastating experimental tumor models, the Ehrlich ascites carcinoma (EAC). Animals bearing EAC cells treated with crude venom showed a significant decrease in the total EAC cell count in comparison to non-treated tumor bearing animals. These data suggest that the venom interfered with the EAC cell growth. This is greatly supported by the significant decrease in the number of viable cells and the corresponding significant increase in dead cell population. Similar results have been previously reported (12,15,19,24,45).
Analysis of survival time for venom-treated and non-venom-treated tumor-bearing animals contributes and confirms the antitumor efficiency of this venom, as it could significantly increase survival of the venom treated tumor-bearing animals by 52.3% (p<0.05). These findings are greatly supported by Hernandez Plata et al. and Silva et al (18,39).
On the other hand, Baldi et al (3) reported results that disagree with this work and those of Hernandez Plata et al. and Silva et al. Baldi et al. (3) did not observe any antitumor effect after treating EAC tumor with Crotalus durissus terrificus venom fractions. This may be explained by the different treatment procedures used. This led us to conclude that, in spite of many investigations, controversy still exists regarding the antitumor efficiency of snake venoms, and the exact mechanism that causes tumor regression in experimental animals after treatment with snake venom is still unknown.
Markland (28) conducting research on thrombin-like enzyme, crotalase from Crotalus adamanteus venom proposed that tumor regression could be explained by malignant cells producing a microenvironment around themselves using host substances, which protected them against defensive responses of the immune system. This microenvironment would be composed of fibrin deposits from the nearby blood vessels. Crotalase apparently destroyed the microenvironment produced by the tumor cells. On the other hand, Silva et al. (39) proposed an indirect mechanism based on the stimulation of the inflammatory response to inhibit tumor growth and to promote its rejection. Lipps (26) isolated two cancer cell inhibitors, atroporin and kaotree from Crotalus atrox and Naja n. kaouthia that revealed a direct and selective cytolytic effect on tumor cells.
In addition to the antitumor activity of Echis coloratus crude venom presented in this work, several biochemical parameters and enzyme activities were assayed in order to evaluate its toxicological effects.
The dose of venom used was based on the LD50, and the experiment was designed to include four groups of animals in order to give an illustrative profile of the biochemical alterations, which may be due to venom and/or tumor. The significant decrease in serum glucose in tumor-bearing animals compared to normal controls could be explained by the fact that tumors have high rate of glycolysis. This is greatly supported by the systemic significant elevation in LDH in tumor-bearing animals. The development of hypoglycemia in tumor-bearing animals has been previously reported (13,16,31,40). This has a way of triggering the body into a constant state of glycogenolysis and gluconeogenesis.
Accelerated gluconeogenesis could be expected from the significant decrease in serum proteins, significant increased serum urea, nitrogen, and AST and LDH. These are greatly supported by previous findings (1,16,32,42).
Treatment of tumor-bearing mice with this low dose of crude venom was found to restore serum glucose levels towards normal levels (Table 1). This may be attributed to accelerated gluconeogenesis, which is greatly supported by the significant increase in LDH activity in the T-Tr group. It has been previously reported that LDH is markedly elevated in EAC-bearing animals, and it is usually used as a tumor marker for follow up and monitoring of tumor patients (4,8,16).
Tumor-bearing animals showed a significant decrease in serum total proteins compared to normal controls. Similar results have been previously reported (21,27,36). Both venom and tumor caused a significant increase in serum total lipids. These findings are greatly supported by previous data (16,20,23).
Regarding the serum GGT and arylsulfatase, which were measured as important parameters of drug toxicity and hepatotoxicity (14), they showed a non-significant change in the V-Tr group. This means that the level of crude venom dose used has no toxic effect. Interestingly, venom treatment of tumor-bearing mice significantly reduced the tumor-enhanced activity of both enzymes.
Taken together, the profile of the studied biochemical parameters in the groups V-Tr and T-Tr, it is evident that treating tumor-bearing animals with low doses of crude venom could restore and correct biochemical parameter levels that have been altered due to tumor growth. Accordingly, it could be concluded that the antitumor activity of Echis coloratus crude venom exceeded or camouflaged its toxicity.
Received November 5, 2001
Accepted December 7, 2001
-
1AIESENBERG AC. The glycolysis and respiration of tumors. Acad. Press, 1981, 21, 314-7.
-
2AWADALLA R., RAHMY TR., EL-SHAMY. Intraperitoneal envenomation of rats with the LD50 of Echis carinatus snake venom: a histological and histochemical study. J. Union Arab. Biol., 1994, 1, 121-47.
-
3BALDI A., MORDOH J., MEDRANO EE., BONAPARTE YP., LUSTIG ES., RUMII L. Estudios tendientes a determinar las posibles propriedades antitumorales del veneno de cobra y del complejo crotoxina A y B. Medicina (Buenos Aires), 1988, 48, 337-44.
-
4BENGTSSON G., ANDERSSON G. The effect of Ehrlich ascites tumor growth on lactate dehydrogenase activities in tissues and physiological fluids of tumor-bearing mice. Int. J. Biochem., 1981, 13, 54-61.
-
5BOYSE E., OLD E., CHOUROUBNKOV I. Cytotoxic test of determination of mouse antibody. In: EISEN M. Ed. Methods in medical research. Chicago: Medical Publisher, 1964: 39-40.
-
6BRAGANCA BM. Biologically active components of cobra venom in relation to cancer research. Indian J. Med. Res., 1976, 64, 1197-207.
-
7BRAGANCA BM., PATEL NT., BRADINATH PG. Isolation and properties of cobra venom factor selectively cytotoxic to Yoshida sarcoma cells. Biochem. Biophys. Acta, 1967, 146, 508-20.
-
8BURGESS EA., SYLVEN B. Lactic dehydrogenase activity in plasma and interstitial fluid during growth of mouse tumors. Cancer Res., 1963, 23, 714-9.
-
9CABAUD G., WROBLEWSKI F. Colorimetric determination of LDH activity of body fluids. Am. J. Clin. Pathol., 1958, 30, 234-7.
-
10CALMETTE A., SAENZ A., COSTIL L. Effects du venin de cobra sur les greffes cancereuses et sur le cancer spontane (adeno-carcinome) de la souris. C. R. Acad. Sci., 1933, 197, 205.
-
11CHAIM-MATYAS A., OVADIA M. Cytotoxic activity of various snake venoms on melanoma B16F10 and chondrosarcoma. Life Sci., 1987, 40, 1601-7.
-
12COTTE CA., ESSENFELD-YAHR E., LAIRET C. Effects of Crotalus and Bothrops venom on normal and malignant cells cultivated in vitro. Toxicon, 1972, 10, 157-61.
-
13CRAWFORD WH. Hypoglycemia. Am. J. Med. Sci., 1931, 181, 496-510.
-
14EL-MERZABANI MM., EL-AASER AA., OSMAN AM., ISMAIL N., ABOUL-ELA F. Potentiation of therapeutic effect of methanesulfonate and protection against its organ cytotoxicity by vitamin C in Ehrlich ascites carcinoma bearing mice. J. Pharm. Belg., 1989, 44, 109-16.
-
15FAHIM FA., ZAHRAN F., MADY EA. Effect of N. nigricollis venom and its fraction on EAC in mice. In: INTERNATIONAL CONFERENCE OF THE EGYPTIAN SOCIETY OF TUMOR MARKERS ONCOLOGY, 1, Cairo, 1988. Abstracts...Cairo: Ain Shams University- Faculty of Medicine, 1988. 375-94.
-
16FAHIM FA., ABD-ALLAH NM., ESMAT AY. Some metabolic aspects in normal and tumor-bearing mice treated with a natural anthraquinone. J. Tumor Marker Oncol., 1993, 8, 35-42.
-
17GNIOT J., DZIALOSZYNSKI LM. Arylsulphatase of human placenta. Clin. Chim. Acta, 1964, 9, 334-41.
-
18HERNANDEZ PLATA G., REY L., HERNANDEZ H., VALERO N., CENDALI G., ANEZ MF., ALVARADO M. Contribution al estudio sobre los efectos antineoplasicos del complejo crotoxina-A-B y cobramine (CFA) en sarcomas de rata. In: CONGRESSO LATINO AMERICANO DE HERPETOLOGIA, 3, São Paulo, 1993. Abstracts... São Paulo: Universidade Estaual de Campinas, 1993. 16.
-
19IWAGUCHI T., TACHECHI M., HAYASHI K. Cytotoxic activity of cytotoxin isolated from Indian cobra venom against experimental tumor cells. Biochem. Int., 1985, 10, 343-9.
-
20KANNAN R., WILSON L., BAKER N. The role of dietary fat and hepatic triglyceride secretion in cancer-induced hypertriglyceridemia. Lipids, 1987, 13, 887-91.
-
21KIND PRN., GORDON M., LAVERICK M., NIAS AH., SLAVIN BM. The effect of C3H mouse mammary tumor on the levels of serum and urine analytes in vivo. Br. J. Cancer, 1985, 52, 607-12.
-
22KNIGHT JA., ANDERSON S., RAWLE JM. Chemical basis of sulfo-phospho-vanillin reaction in estimating total lipids. Clin. Chem., 1972, 18, 199-204.
-
23KREMMER T., HOLOZINGER L. A study of hyperlipidemia induced by ascites tumors. Acta Morphol. Acad. Sci. Hung., 1976, 24, 369-79.
-
24LIPPS BV. Selective cytotoxic activity of snake venom proteins, atroporin and kaotree on various types of cancer cells. In: WORLD CONGRESS ON ANIMAL, PLANT AND MICROBIAL TOXINS, 11, Tel aviv, 1994. Abstracts... Tel Aviv: International Society on Toxinology, 1994. 17.
-
25LIPPS BV. Biological and immunological properties of nerve growth factor from snake venoms. J. Nat. Toxins, 1998, 7, 121-30.
-
26LIPPS BV. Novel snake venom proteins cytolytic to cancer cells in vitro and in vivo systems. J. Venom. Anim. Toxins, 1999, 5, 172-83.
-
27MACINTOSH LC., THOMSON AW., WHITING PH. Serum biochemical changes in C. parvum injected mice bearing the Landschutz ascites carcinoma. Br. J. Exp. Pathol, 1982, 63, 639-43.
-
28MARKLAND JR FS. Antitumor action of crotalase, a defibrinogenating snake venom enzyme. Semin. Thromb. Hemost., 1986, 12, 284-90.
-
29MARKWELL KM., HASS SM., BREBER LL., TOLBERT NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem, 1987, 87, 206-10.
-
30MEIER J., THEAKSTON RDG. Approximate LD50 determination of snake venoms using eight to ten experimental animals. Toxicon, 1986, 24, 395-401.
-
31NADLER WH., WALFER JA. Biochemistry of metabolic process in rats bearing tumors. Arch. Intern. Med., 1929, 44, 700-6.
-
32OSCAR C. Biopharmacological studies on certain new anticancer drugs. Biochem. J., 1988, 110, 701-7.
-
33PATEL TA., BRAGANCA BM., BELLARE RA. Changes produced by cobra venom cytotoxin on morphology of Yoshida sarcoma. Exp. Cell Res., 1969, 57, 287-97.
-
34RAHMY TR., HEMMAID KZ. Histological and histochemical alterations in the liver following intramuscular injection with sublethal dose of the Egyptian cobra venom. J. Nat. Toxins, 2000, 9, 21-32.
-
35REITMAN S., FRANKEL S. A colorimetric method for determination of serum glutamic-oxaloacetic and glutamic-pyruvic transaminases. Am. J. Clin. Pathol., 1957, 28, 56-61.
-
36SHERRY BA., GELIN J., FONG Y., MARANO M., GERAMI HA., LOWRV SF. Anticachectin/tumor necrosis factor - a antibodies attenuate development of cachexia in tumor models. FASEB J., 1989, 3, 1956-62.
-
37SHIAU LIN SY., SU SC., LEE CY. Comparative studies on cytotoxin from the Indian cobra venom and cardiotoxin from the Forosan cobra venom, with special reference to the effect on the growth of the Ehrlich ascites tumor cells in mice. J. Formos. Med. Assoc., 1976, 75, 328-36.
-
38SILVA RJ., FECCHIO D., BARRAVIERA B. Antitumor effect of snake venoms. J. Venom. Anim. Toxins, 1996, 2, 79-90.
-
39SILVA RJ., FECCHIO D., BARRAVIERA B. Effect of Crotalus durissus terrificus (Laurenti, 1768) venom on the evolution of Ehrlich ascites tumor. J. Venom. Anim. Toxins, 1997, 3, 324-41.
-
40SILVERSTEIN H., DERVOT K., OSCAR D. Studies on carbohydrate metabolism and different types of tumors bearing animals. Lancet, 1988, 22, 40-5.
-
41SZASZ G., PERIJN JP. A kinetic colorimetric method for determination of G-glutamyl transpeptidase in serum. Z. Klin. Chem. Biochem., 1974, 12, 228-32.
-
42TAGMIZYAN WA. Metabolism of impaired glucose in patients with neoplasia. Am. Biol., 1990, 2, 53-8.
-
43TRINDER P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem., 1969, 6, 24-8.
-
44TU A., GILTNER JB. Cytotoxic effects of snake venoms on KB and Yoshida cells. Res. Commun. Chem. Pathol. Pharmacol., 1974, 9,783-6.
-
45YOSHIKURA H., OGAWA A., OMORI-SATOH T. Action of Trimeresurus flavoviridus venom and partially purified hemorrhagic principles on animal cells cultivated in vitro. Toxicon, 1966, 4, 183-90.
Publication Dates
-
Publication in this collection
16 Sept 2002 -
Date of issue
2002
History
-
Accepted
07 Dec 2001 -
Received
05 Nov 2001