Abstract
Leptospirosis is an acute anthropo-zoonotic infection of worldwide significance caused by spirochaete Leptospira interrogans which has 23 serogroups and >200 serovars. Various factors influencing the animal activity, suitability of the environment for the survival of the organism and behavorial and occupational habits of human beings can be the determinants of incidence and prevalence of the disease. The disease was considered inconsequential till recently, but it is emerging as an important public health problem during the last decade or so due to sudden upsurge in the number of reported cases and outbreaks. Since isolation rate of the microorganism from clinical specimens is low due to prior indiscriminate use of antibiotics, serological techniques remain the cornerstone of diagnosis.
India; leptospirosis; world
MINI-REVIEW
Leptospirosis in India and the rest of the world
Rao R. Sambasiva; Gupta Naveen; Bhalla P.; Agarwal S.K.
Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry; Microbiology Department, Pt. BD Sharma Post Graduate Institute of Medical Sciences, Rohtak, Haryana; Microbiology, Maulana Azad Medical College, New Delhi; Department of Medicine, Malulana Azad Medical College, New Delhi, India
Correspondence Correspondence to Dr. Naveen Gupta M.D. D-87, Ashok Vihar, Phase-I Delhi-110052, India Phone: 91-011-27420625 E-mail: micronaveen@hotmail.com
ABSTRACT
Leptospirosis is an acute anthropo-zoonotic infection of worldwide significance caused by spirochaete Leptospira interrogans which has 23 serogroups and >200 serovars. Various factors influencing the animal activity, suitability of the environment for the survival of the organism and behavorial and occupational habits of human beings can be the determinants of incidence and prevalence of the disease. The disease was considered inconsequential till recently, but it is emerging as an important public health problem during the last decade or so due to sudden upsurge in the number of reported cases and outbreaks. Since isolation rate of the microorganism from clinical specimens is low due to prior indiscriminate use of antibiotics, serological techniques remain the cornerstone of diagnosis.
Key Words: India, leptospirosis, world.
A Brief History of Leptospirosis
It is little more than 100 years since Weil, Professor of Medicine at Heidelberg (1886) whose name has been given to the disease in humans first described this disease, which is caused by Leptospira interrogans, serovar icterohaemorrhagiae or copenhageni [1]. Leptospires had been seen at that time, but were not cultured and were named Spirocheta interrogans by Stimson as early as 1907, in silver stained preparations of liver from a patient believed to have died of yellow fever, the viral origins of which were then unrecognized. The patient really had Weil's disease [2]. Its contagious nature and microbial origin were proved independently, first in Japan by Inada et al. (Spirochaeta icterohaemorrhagiae) in 1915 [3], and soon after in Germany (Spirochaeta icterogenes) by Uhlenhuth and Fromme [4]. Both groups isolated, cultivated and described pathogenic Leptospires. Later, a saprophytic leptospira found in fresh water was described in 1914; it was named Spirochaeta biflexa. Noguchi proposed the name 'Leptospira' (thin spirals) in 1918, following detailed microscopical and cultural observations [5]. In the 15 years or so, from discovery until the 1930s, many of the important serovars prevalent throughout the world, and their host sources were discovered [6]. During the 1920s to 1950s, the milder forms of leptospirosis, the numerous related but distinct serotypes and occupational relationships were elucidated in Japan, Indonesia and Germany. Electron microscopy revealed much of the detail of the structure during the 1960s and 1970s [5]. Yanagawa and Faine (1966) showed that Leptospires were analogous to other bacteria in structure and that characteristic antigens are associated with structural elements [7].
Consequently, Leptospirosis researchers became concerned with serological classification, based on absorption and cross agglutination of antisera [8]. ELISA methods were developed to analyse non-agglutinating as well as agglutinating antigens [9] and monoclonal antibodies were used to identify epitopes involved in immunity, or for classification [10]. Historically important developments in the last 15 years include lipopolysaccharide derivation of the antigens involved in immunity and molecular techniques for identification and genetic speciation; currently, PCR methods are being developed for identification and diagnosis [9].
Epidemiology: World Situation
Leptospirosis is a worldwide zoonosis. According to the occupational groups involved and the nature of the disease presentations, different names have been used, e.g. seven-day fever found commonly in Japan, Cane cutter's disease in Australia, Rice field Leptospirosis in Indonesia and Fort Bragg fever, which appeared as an outbreak in the US. Weil's disease, which is one of the severe forms of this disease, occurs in many countries, including India and other South-East Asian Countries, China, continental Europe and England. Leptospirosis exists in all the five inhabited continents and in a large number of countries. It occurs in tropical, subtropical and temperate zones [11].
In November 1961, an outbreak of Leptospirosis occurred among 186 US Army Troops in the canal zone who had engaged in a jungle exercise 10 to 13 days earlier [12].
Epidemiological investigations (1975 to 1977) carried out in Barbados revealed the seroprevalence of Leptospirosis in the various occupational groups to be 29.8% (highest in sanitation workers 42.7% followed by sugarcane workers 39.4%). Fever cases with suspected leptospirosis gave seropositivity of 28.7% in a hospital survey and 15% seropositivity was noted in healthy individuals [13].
In a survey made in northern Trinidad between mid 1977 and mid 1978 leptospiral infection was found to be widespread in the general population, and among occupational groups the highest prevalence of antibodies was found in sugarcane workers (45% infected). From 1977 to 1982, sera were collected from fever cases in Trinidad; 9% were confirmed as current cases and 23% showed evidence of previous infection [14].
A high prevalence of leptospiral antibodies in humans was reported from Somalia in 1982 [15]. Another survey in 1987 in Italy showed a prevalence in rural areas of 11.34%, while it was 3.08% in urban areas of central Italy [16].
In 1987 a seroprevalence as high as 25% (14/56) was reported in patients hospitalised in Karachi, Pakistan [17]. In 1989, serological evidence of leptospiral infection was found in 12.5% of Barbados school children and 9.5% of Trinidad school children aged 7 to 14 years [18].
The Hawaii State Department of Health reported a leptospirosis incidence rate of 2.97 per 100,000 population, compared to a national rate of leptospirosis in the United States of 0.02 per 100,000 in 1992. The proportion of leptospirosis cases in Hawaii related to occupational exposure dropped from 56% during 1971 to 1975 to 29% during 1986 to 1990, whereas cases related to recreational, habitational or vocational exposure increased from 43% during 1971 to 1975 to 71% during 1986 to 1990 [19].
Three papers have been published in Uruguay on ARF in Leptospirosis. The first series of five cases was published in 1972. These five cases represented 8% of the total cases. The second series of 20 cases, published in 1993, showed an incidence of ARF of 15%. The expected frequency of ARF in Uruguay is 0.71.3 cases per 100,000 per year [20].
Symptomatic leptospirosis is particularly frequent and severe in the Seychelles; 80 cases were reported over a two-year period during 1989 to 1990, 65 cases during 1993 to 1994, and 75 cases during 1995 to 1996 [21].
In October, 1995, epidemic hemorrhagic fever, without jaundice or renal manifestations, was reported to be caused by leptospira in rural Nicaragua, following heavy flooding [22].
In 1995, 90 out of 295, i.e. 30.5%, of apparently healthy individuals tested positive for anti-leptospira antibodies by MAT in the Cordillera province of Bolivia [23].
In Turkey, screening of 1,440 people for leptospira antibodies using MAT revealed 5.48% positivity. Among rice field workers seropositivity was found to be 9.6% to 13% [24].
In Vientiane, Laos serological evidence of recent leptospiral infection in 1995 and 1996 was recognised in 21% of the serum samples from 70 acute jaundice cases that were negative for markers of acute hepatitis A and B [25].
An outbreak of leptospirosis among white-water rafters in Costa Rica was reported in September, 1996 [26].
An outbreak of acute febrile illness in 1998 among athletes participating in triathlons in Wisconsin and Illinois was reported to be due to leptospirosis [27].
Epidemiology: The Indian Situation
The serological study of leptospirosis in man has been limited in India. In 1931, an extensive survey of the disease outbreak in the Andaman Islands was made and researcher's isolated L. andamans and L. grippotyphosa [28]. Several others have confirmed the prevalence of leptospirosis in India by isolating leptospires from human material [29-31].
In 1960, serological evidence of L. icterohaemorrhagiae and canicola antigen was found in five cases of jaundice [32].
In 1966, out of 93 sera from PUO cases, three were positive by the agglutination lysis test, one against L. icterohaemorrhagiae and two for L. canicola, and out of 43 cases of jaundice, two were positive for L. icterohaemorrhagiae and one for L. icterohaemorrhagiae and L. pomona [33].
In 1967, in Bombay, one of 150 sera from infective hepatitis cases showed evidence of leptospira infection due to L. pyrogenes. Leptospira agglutinins at significant titres were demonstrated in 5 out of 17 sera from suspected cases of leptospirosis and in 6 cases out of 11 sera from workers of animal farms and piggeries [34].
In 1983, in Madras, the seroprevalence of leptospirosis in jaundiced patients was 18% and it was 24% in PUO cases [35]. In 1983, a serological study was made of a population that consisted mainly of children in a village near Madras, city in Tamil Nadu State, India, following an outbreak of disease in cattle; 35 of 75 (47%) human sera gave positive antibody titres [36].
During 1984 to 1985, acute renal failure due to leptospirosis in 19 human patients was reported in Madras [37]. In 1988, during the peak of the monsoon season, serum and urine samples from 40 patients, with a history of fever, vomiting, jaundice, abdominal pain and renal failure, from various hospitals in Madras city and MAT revealed that 33 (82.5%) had specific leptospiral antibodies, with titres ranging from 1:160 to 1:6400 against different serovars [38].
Leptospirosis MAT titres >1:1600 and > 1:800 occurred in 39 of 54 and 51 of 54 cases, respectively, in patients admitted to the Government General Hospital, Madras, during November and December 1990 to 1991 with symptomatology suggestive of disease [39].
In 1993, a serosurvey of conservancy workers in Madras (using MAT) revealed a prevalence rate of 32.9% [40].
An outbreak of acute febrile illness with hemorrhagic manifestations and pulmonary involvement occurred in Diglipur of North Andamans during October to November 1993; 66.7% of the victims had significant titres of antibodies against leptospira [41].
In 1994, an increase in the number of individuals with uveitis was noted at Aravind Eye hospital, Madurai, India after an epidemic of leptospirosis in South India; the epidemic followed severe flooding of the Tamil Nadu District in the autumn of 1993; 37/46 patients (80%) had leptospira DNA and 33/46 patients (72%) had positive serology [42].
In 1995, a seroprevalence rate of 12% leptospirosis was found among febrile and jaundice patients in Pondicherry [43].
Thirty-eight acute renal failure cases with clinical suspicion of leptospirosis were screened from July to November, 1996 and 27 (71%) seropositive cases were diagnosed by MAT [44].
Morphology
The etiologic agent of leptospirosis is Leptospira interrogans. It is a thin spiral organism 0.1µm x 6 -20µm, with tightly set coils, and it is characterized by very active motility, by rotating ("spinning") and bending. Usually one or both ends of this single-cell organism are bent or hooked, but straight forms also occur that rotate and travel more slowly than hooked forms. Because of their narrow diameter, the leptospires are best visualized by dark-field illumination or phase contrast microscopy and they do not stain readily with aniline dyes. The free living (L. biflexa) and parasitic leptospires (L. interrogans) are morphologically indistinguishable [45].
Antigens and Immune Response
Leptospiroses have a complex antigenic structure. The somatic antigen is genus specific. The surface antigen is a polysaccharide and is serovar specific. The outer membrane is a potent immunogen lipopolysaccharide in nature. It is the major antigen and the target of antibody and complement-mediated bactericidal activity. Antibodies directed against it are protective in nature. Flagellar antigen is composed of genus and serotype specific antigens. Some serovars, e.g. L. icterohaemorrhagie, have an additional Vi antigen associated with virulence.
The immunological response to leptospires is both humoral and cell mediated; after the entry of the organism into the host, both the B and T-cell dependent areas are stimulated. The initial elimination is done by phagocytosis. Most of the leptospires are digested in the vacuoles of macrophages. The phagocytic activity of the polymorphonuclear cells is enhanced by opsonizing antibodies. Cell-mediated immunity plays a role in preventing renal localization.
The antibody response is classical, with peak IgM levels appearing first, quickly followed by IgG antibodies, which persist longer than IgM. High IgM levels can be observed during the first two months of the disease. IgG response in leptospirosis is often erratic and occasionally is not detected. IgA antibodies appear on the fifth day and definitely persist up to nine months, and so may serve as better seroepidemiological markers than IgG. Heterologous, i.e. genus-specific, antibodies appear first but decline faster; homologous, i.e. serovar-specific, antibodies appear later and persist longer.
Recovery from infection is possible after the appearance of lytic and opsonic antibodies and phagocytic clearance of leptospires from blood and tissues [45].
Cultural Characteristics
Leptospires are obligate aerobes. When cultivated in a suitable aerated medium at 30oC and an optimal pH of 7.2 to 7.6 their generation time varies from 7 to 12 hours and yields are 6 to 8 x 109 cells/ml. Vitamins B1 and B12, and long chain fatty acids, are the only organic compounds are required for their growth. Fatty acids are their main source of energy and carbon and are also required as a source of cellular lipids, since leptospira cannot synthesize fatty acids de novo. Owing to the inherent toxicity of free fatty acids, these must be supplied to the leptospires either bound to albumin or in a non-toxic esterified form. Carbohydrates are not a suitable source of energy or carbon. Ammonium salts are an effective source of cellular nitrogen. Leptospires incorporate purine bases, but not pyrimidine bases, into their nucleic acids. Because of this they are resistant to the antibacterial activity of the pyrimidine analogue, 5-fluorouracil. This compound is used in selective media for the isolation of leptospires from contaminated sources.
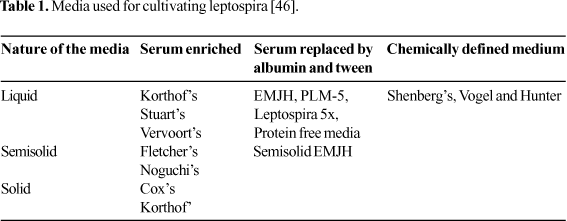
The types of media used for the isolation and cultivation of leptospires are media enriched with rabbit serum or bovine serum albumin (BSA) and protein-free media. Liquid media are necessary for growing the cultures for serological diagnosis of infection and for typing the isolates. Liquid media are converted to a semisolid form by the incorporation of 0.2% agar and to the solid form by the addition of 1% agar. Growth is readily initiated in these media and usually is easily visualized as one or more rings of dense growth several mm to cm below the surface of the medium, although a lack of rings of growth does not necessarily mean an absence of leptospires. Solid media are useful for cloning the strains and for isolating leptospires from contaminated sources. Colonies in 1% agar are subsurface and become visible within 7 to 14 days (Table 1) [45].
Isolates are differentiated from non-pathogenic leptospires by their more fastidious requirements, inability to grow at 13oC and susceptibility to 8-azaguanine.
Pathogenesis
The most frequent sources of infection are urine, kidneys, surface water, mud and soil. Leptospires are presumed to enter via small abrasions or other breaches of the surface integument. They may also enter directly into the bloodstream or lymphatic system via the conjunctiva, the genital tract in some animals, the nasopharyngeal mucosa, possibly through a cribriform plate, the lungs following inhalation of aerosols, or through an invasion of the placenta from the mother to the foetus at any stage of pregnancy in mammals. It is unlikely that penetration of intact skin or other mucosal surfaces occurs. Drinking or inhalation of contaminated water following immersion can also cause leptospirosis [47].
Pathogenic leptospira rapidly invade the bloodstream after penetrating skin or mucous membranes. The primary lesion in leptospirosis is disruption of the integrity of the cell membrane of the endothelial cells lining small blood vessels in all parts of the body. Capillary leakage and hemorrhages result. These effects can be attributed to the action of a glycoprotein (GLP) toxin of leptospires. Widespread petechial hemorrhages are apparent in all organs and tissues, particularly the lungs, omentum and pericardium. Ischaemia from damage to blood vessels in the renal cortex leads to renal tubular necrosis, particularly of the proximal convoluted tubules. The resulting anatomical damage causes renal failure that can be fatal. Liver cell necrosis caused by ischaemia and destruction of hepatic architecture leads to the characteristic jaundice of the severe type of leptospirosis. Blood clotting mechanisms are affected by liver failure, aggravating the hemorrhagic tendencies. There may also be thrombocytopenia. Leptospires enter the cerebrospinal fluid (CSF) in the early septicemia phase of the illness, but there is little evidence of inflammatory response in the CSF [48]. The anterior chamber of the eye is invaded by leptospires during acute infection, but they are trapped there and cannot move out after the local vasodilation and inflammation subside. Antibodies from circulation can enter and cause an acute hypersensitivity uveitis.
Leptospires are able to persist in some anatomically localized and immunologically privileged sites, after antibodies and phagocytes have cleaved leptospires from all other sites. The most significant site of persistence is the renal tubule. Leptospires appear in the kidney 2 to 4 weeks after an acute infection, attached to an interdigitated area in the brush border of proximal renal tubular epithelium. The type of reaction in the tissues ranges from none at all to heavy scarring; animals may excrete leptospires intermittently or regularly for periods of months or years, or for their lifetimes. However, humans do not remain carriers for long, and the urine is free of leptospires at the time of clinical recovery [49].
Clinical Features
Leptospirosis occurs as two clinically recognizable syndromes. The most common syndrome is anicteric leptospirosis, a self-limited illness that occurs in 85% to 90% of the cases. There are two clearly-defined stages in anicteric leptospirosis; the septicemic stage and the immune stage. Icteric leptospirosis, or Weil's syndrome, is a more serious, potentially fatal, syndrome and occurs in 5% to 10% of the cases. The demarcation between the septicemia stage and the immune stage is not as distinct in this syndrome. Although subclinical infection is uncommon, the results of serological testing show that it occurs in some workers who have been occupationally exposed to leptospires [48].
Anicteric Leptospirosis
The incubation period for leptospirosis is usually 7 to 12 days, but it can range from 2 to 20 days. The onset of anicteric leptospirosis is abrupt and is characterized by fever, headache, severe myalgia, chills with rigors, prostration and sometimes, circulatory collapse. The septicemic (or first) phase lasts 3 to 7 days. Fever is high and remitting. Headache is intense, unremitting and possibly throbbing. Anorexia, nausea, vomiting and abdominal pain occur in most patients. The most common physical finding is conjunctival suffusion in the absence of purulent discharge. Other signs include masculopapular skin rash, pharyngeal injection, lymphadenopathy, splenomegaly, hepatomegaly, and muscle tenderness. The symptoms are prominent for 4 to 7 days during the septicemic stage, at which time defervescence due to lysis occurs. Leptospires can be isolated from the blood and the CSF during this phase.
The immune (or second) stage of anicteric leptospirosis is preceded by a one to three-day asymptomatic period. The onset of the immune stage coincides with the appearance of IgM antibodies. Fever, headache and vomiting are less severe at the onset of the immune stage than during the septicemic stage. The duration of the immune stage ranges from 4 to 30 days, and the leptospires are cleared from the blood and the CSF after the first days of this stage. Leptospiruria develops and persists for 1 to 3 weeks.
Aseptic meningitis is the hallmark of the immune stage. Mild pleocytosis is present, with or without meningeal signs and symptoms. The CSF cell count is <500/mm3 in most cases. Polymorphonuclear cells may predominate early in the illness, but mononuclear cells predominate later. The CSF protein levels ranges from <40mg/dl (normal) to 300 mg/dl and the CSF glucose concentration is generally normal. Uveitis, iritis, iridocyclitis and chorioretinitis may also appear during the immune stage [48].
Icteric Leptospirosis
Icteric leptospirosis or Weil's syndrome is a form of disease characterized by symptoms of hepatic, renal and vascular dysfunction. The clinical manifestations vary in terms of severity and symptomatology. Some patients with jaundice may have no renal manifestation. Supportive therapy has reduced mortality to between 5% and 10%. Any serotype of L. interrogans may cause icteric leptospirosis.
During the leptospiraemic phase of icteric leptospirosis, the symptoms do not suggest leptospirosis until the third to seventh day of illness, when jaundice and azotaemia develops. The biphasic course of the disease is obscured by severe and persistent fever, jaundice and azotaemia. Jaundice appears, but there is no evidence of hepatocellular destruction. Hepatic dysfunction occurs, but it resolves and it is rarely the cause of death. The serum bilirubin level is usually <20 mg/dl, but can be as high as 60mg/dL to 80mg/dL. Hypoprothrombinemia occurs in a minority of patients and responds to administration of Vitamin K. Serum transaminase levels are mildly elevated, rarely exceeding 100U/L to 200U/L. Serum bilirubin levels peak within seven days and the increase persists for a few days to several weeks.
Renal involvement is common in both anicteric and icteric leptospirosis, but symptoms are present only in patients with icteric disease. Azotemia, oliguria and anuria commonly occur during the second week of illness, but may appear as early as 3 to 4 days after onset. Blood urea nitrogen levels are below 100mg/dL in most cases, but may occasionally exceed 300mg/dl. Serum creatinine levels are usually 2mg/dL to 8mg/dL, although they may reach 18mg/dL. Results of urinalysis are abnormal in 70% to 80% of cases; proteinuria, hyaline or granular casts, hematuria and pyuria are typical findings. The onset of anuria is a poor prognostic sign and diuresis usually signals resolution.
Hypotension due to vascular collapse occurs only in patients with icteric leptospirosis, hemorrhage occurs only in severe cases, congestive heart failure occurs rarely but non-specific ECG changes are observed in most patients. Changes in sensorium may occur. Other laboratory abnormalities include anemia, thrombocytopenia, leucocytosis with neutrophilia and an increase in the level of creatinine phosphokinase (i.e. the MM fraction) [48].
Laboratory Diagnosis of Leptospirosis
The diverse clinical presentations of this disease make it essential for the laboratory to play a role in diagnosis. Microbiological diagnosis of leptospirosis aims at demonstrating the leptospires, by culturing them or by demonstrating an appreciable antibody response to them [46].
Dark-Field Microscopy
The typical motility of the leptospiroses in the clinical sample (blood, CSF, urine or peritoneal fluid) observed with dark-field microscopes, when correlated with clinical parameters, may aid in early diagnosis. It is a simple method, but it may not be positive if there are few bacteria in the sample. Double centrifugation of the sample at low speed to separate the cellular elements, and then at high speed, help concentrate the leptospires. Artefacts like lysed RBCs, fibrils, etc. may however, be mistaken for leptospires. So it is not recommended as the only diagnostic procedure to be used [46].
Phase contrast microscopy is useful for visualizing leptospires in the laboratory, but, because of its technical limitations in thick suspensions and its optical characteristics, it has no practical purpose whenever dark-field microscopy is available [45].
Direct Evidence
Dark-field microscopy
Phase contrast microscopy
Silver staining
Immunofluorescence
Immunoperoxidase
DNA hybridisation
Polymerase chain reaction
Serovar specific ELISA
Blood
Urine
CSF
Body fluids and tissues
Macroscopic agglutination test (MSAT)
Indirect fluorescent antibody test (IFAT)
Indirect haemagglutination test (IHA)
Counter immuno electrophoresis (CIEP)
Complement fixation test (CFT)
Newer techniques
ELISA
Microcapsule agglutination test (MCAT)
Lepto-Dipstick
Serogroup/serovar specific tests
Microscopic agglutination test (MAT)
Serovar specific ELISA
Staining Methods
Silver deposition techniques. Leptospires in smears of tissues or fluids on slides can be stained using silver deposition methods. The variously described procedures are modifications of Warthin Starry's method for staining. The stain is based on chemically reducing surface properties of leptospires and other spirochaetes. Well-stained preparations show black spirochaetes in pale yellow or brown tissue elements. This method has the same limitation as dark-field procedures, as it is difficult to detect small numbers of organisms in tissue sections, and artefacts may be mistaken for leptospires [45].
Immunofluorescence. Immunofluorescence staining of leptospires is often preferable to silver staining of laboratory, environmental or clinical specimens because it is easier to see leptospires, especially in small numbers, and the serovars or serogroups can be determined presumptively. When a combination of antisera labelled with different fluorochromes is used, more than one serological type of leptospires can be identified in the same preparation. One disadvantage is the need for special fluorescent microscopy equipment; another is that specially prepared labelled antisera are required. A double layer or sandwich method is used with primary specific anti leptospiral antisera and a secondary universal fluorochrome labelled anti rabbit globulin serum [47].
Culture of leptospira. The infecting strains can often be isolated in culture, provided that suitable material is obtained before antibiotics have been administered. Early in the course of illness during the leptospiraemic phase the inoculum of preference is blood or cerebrospinal fluid; later during the phase of leptospiruria it is urine. Blood culture of febrile patients has been extensively used, especially in Australia and New Zealand, and is recommended for routine diagnosis [45]. It is particularly valuable in man, as the serological response can be slow and may be absent altogether if antibiotics are given early. Because serology is usually serogroup specific, isolation is essential to identify the infecting serotype. Such information is essential for epidemiological purposes, for the selection of relevant leptospires for use in diagnostic tests and vaccines and for the assessment of antibiotic sensitivity.
Animal inoculation. Laboratory animals are useful for isolating the organisms from contaminated materials and for maintaining recent isolates. They are also sometimes essential for decontaminating cultures, and with the help of passive protection, they may be used to recover a single serotype from a mixed culture. Young animals, preferably weanlings, should be used because older animals may resist the infection. Stocks must be free from endemic leptospiral infection; guinea pigs, hamsters, gerbils, young rabbits, swiss white mice, albino American deer mice and 1 to 3 day-old chicks may be used. The material should be inoculated intraperitoneally through one of the lower quadrants of the abdominal wall. The animals should be examined twice daily, and a drop of peritoneal fluid can be examined with dark-field microscopy for active leptospires from the 3rd to the 7th day [50].
Serological Methods
Reliable serological diagnosis is now within the capacity of most general-duty laboratories. Serological tests can be a guide to the infecting serum and this information is useful for prognosis and epidemiology. Serological tests do not react until a few days after infection, but reactions persists for months or years. Persistent antibodies allow retrospective diagnosis [51]. but seroconversion or a 4-fold or greater rise in titre in paired serum samples in the presence of a compatible clinical illness is an important criterium for the defective diagnosis of leptospirosis. The wide range of tests that are available are broadly divided into genus-specific and serogroup/serotype-specific tests.
Genus Specific Tests
They become positive earlier in the illness and are ideal for a clinical diagnosis. The antigen for these tests is prepared from the non-pathogenic L. biflexa Patoc 1 strain.
1. Macroscopic agglutination test. A rapid macroscopic slide agglutination test can be used to screen human and animal serum samples. These tests are carried out with a dense suspension of leptospires, which agglutinate into clumps visible to the naked eye. The best method is Galton's macroscopic slide agglutination test, in which 12 antigens were originally proposed, and later supplementary antigens were suggested [52]. In 1966, undiluted serum and pools of 3 antigens were used for screening. If the serum reacts with one or more pools, it is then retested, undiluted with individual antigens present in the reacting pools. Finally, the serum is titrated with the individual suspensions [53]. Strain Patoc 1 may be used in a macroscopic test; this simple test provides a rapid and reliable means of screening human sera for leptospiral-genus-specific antibodies. It allows a provisional diagnosis of acute leptospirosis to be made within a few minutes; it is not suitable for retrospective or survey work [54]. Positive reactions, however, should be confirmed by complement fixation and microscopic agglutination tests.
In 1997, a 100% correlation of a macroscopic slide agglutination test (MSAT), using three serogroups, with MAT was reported. Of the 592 samples received, 317 were positive by IgM ELISA; among these MSAT was positive in 310 (sensitivity 97.8%); 303 samples had MAT titres of >80. In all these patients MSAT was positive. Fourteen samples that had MAT titres of 40 were positive by IgM ELISA. Among these, MSAT was positive in seven. Leptospira autmnalis was the most common serogroup (59.9%), followed by icterhaemorrhagiae (15.5%). Two hundred and seventy five samples that were negative by IgM ELISA were also negative by MSAT. Thus MSAT had good correlation with both IgM ELISA and MAT, and therefore can be used as a valuable and simple screening test. The sensitivity of this test can be enhanced by adding the locally-prevalent serovars [55].
In 1998, a commercially available slide agglutination test (SAT) was evaluated. Leptospirosis was diagnosed in 108 patients on the basis of a >4-fold rise in titre by MAT. Both SAT and IgM ELISA only failed to detect one case of infection (sensitivity 99%). Only 3 of 145 blood donors, and none of the patients with other illnesses, were SAT positive (specificity 99%). SAT and ELISA were significantly more sensitive as initial screening tests. For 22% of the patients, the diagnosis of leptospirosis was made earlier by SAT than by MAT [56].
In a three year study (1995 to 1997) made at Chennai Medical College 1764 of 5614 blood samples (31.4%) were positive by MSAT, using a preparation of patoc, autmnalis and icterohaemorrhagae. In this study, MSAT was found to be a simple, rapid, and sensitive diagnostic test for active leptospirosis; the sensitivity of the test can be improved by the addition of locally prevalent serovars [57].
2. Sensitized erythrocyte tests. Leptospiral extracts (lipopolysaccharides) and erythrocyte sensitising substance (ESS) are used to sensitize sheep and human red blood cells [40]. Two sorts of reaction occur when ESS-sensitised cells are mixed with sera containing the homologous antibodies: haemagglutination (HA) or sensitised erythrocyte agglutination (SEA), and hemolysis (HL) or sensitised erythrocyte lysis (SEL). Chang and Mc Comb (1954) were the first to report Leptospiral ESS, which is broadly reactive with leptospiral antibodies and specific for them [58]. In 1955 it was reported that sheep erythrocytes, sensitised with leptospiral ESS, were hemolyzed when mixed with serum containing antibodies in the presence of complement. This study revealed that ESS was broadly reactive in both HA and HL tests, but the HL test gave much higher titres. In 1957, researchers found that in comparison with the microscopic agglutination test, using various groups of sera, the SEA test was less reactive. A similar conclusion was drawn in a comparison of the SEA, SEL and MAT. In 1957, detailed preparation, standardization and stabilization of ESS extracted from the CDC strain (biflexa complex) was reported; also findings of the HL test agreed very well with MAL test findings. Other researchers in 1958 showed that the SEL test detected antibodies in sera stored at 20oC, whereas Mc Comb et al. (1957) had noted that stored sera became negative in the SEA test. Again in 1959, the HL test was evaluated and it was reported that an increase in sensitivity was achieved by two modifications: Human plasma fraction V was added at a final concentration of 1% to the veronal buffer that was used as a diluent for the complement; and 128 units of ESS (instead of 16 units) were used. Apparently the antibodies detected by ESS are distinct from the agglutinin and from antibodies detected by the CFT and precipitin reactions. The same antigen antibody system is involved in SEA and SEL reactions. Compared to the CFT, the SEL has the disadvantage that the test sera need to be absorbed free from heterophile antibody; but it has the advantage that the complement used in the tests needs to be in excess and therefore it need not be titrated accurately before each test [53].
Indirect Immunofluorescence Test
In 1966, Patoc strain 1 was used in an indirect immunofluorescence test. Immunofluorescence has not been used widely for primary diagnostic tests. It is used to detect leptospirosis in tissues [47]. This is a fast and reliable test, where facilities are available. It has a sensitivity of 91.4% [46].
In 1995, the IFA assay was compared with MAT, and it was found that the IFA test is moderately sensitive and specific for the initial diagnosis of leptospirosis; it could replace more complicated and less sensitive MA assays [59].
1. ELISA test. This is now widely used as a genus-specific screening test in man. Both peroxidase and urease labelled conjugates have been used satisfactorily. Stable reagents are available and form the basis of bedside tests, which are read visually. The use of computer assisted automated readers and the appropriate controls improves the reproducibility and predictive value of this test [51].
In 1985, it was found that all patients reacted to the antigen preparation, but maximal IgG titres were attained only with homologous serum [60]. Other researchers found in 1985 that a simplified dot ELISA test with antigen prepared from the Patoc 1 strains of L. biflexa is as sensitive as multi-antigen MAT [61].
In 1997, dot ELISA was evaluated, using antigen obtained from leptospira interrogans cultures of the serovars brasilinensis, canicola, cynopteri, hebdomadis and icterohaemorrhagiae, for the detection of human IgM, IgG and IgA single serum samples from 63 patients with the icterohaemorrhagic form of leptospirosis in the acute phase. IgM antibodies were detected in 62 (98%) patients and IgG and IgA were observed in 70% and 76% of them, respectively. The negative predictive value of dot ELISA was 98% for IgM, 72% for IgG and 76% for IgA detection. They found that dot ELISA can be used as a laboratory screening test, especially when detecting IgM antibodies and has advantages in terms of yield, time, ease of execution and low cost [62].
In 1988, comparison of dot ELISA and MAT showed that dot ELISA was more sensitive than MAT in the 1st week of illness and MAT was more sensitive than dot ELISA in the 3rd week of illness. Thus both tests could effectively diagnose acute leptospirosis. Dot ELISA required no electrical equipment and only one dilution, whereas a dark-field microscope and several dilutions were needed for the MA test [63].
2. Microcapsule agglutination test (MCAT). This test was developed in 1982 for serodiagnosis of Leptospirosis, based on the passive agglutination of synthetic polymer carriers, sensitized with mixed antigens of sonicated leptospires, by leptospiral antibody. The one point MCAT kit was evaluated for use in humans by six WHO collaborating centres for reference and research on leptospirosis. The MCAT gave positive results earlier in the course of the disease than did MAT or IgM ELISA, but on the other hand it could not detect antibodies against some serovars, e.g. sejroe or the australis serogroup in Slovakia, and it may not detect antibodies in sera collected more than 1 to 2 months after the onset of disease. Other advantages of the one-point MCAT kit are that it is simple and can be performed by relatively unskilled personnel with minimum laboratory facilities; it is also very stable and can be kept for long periods without critical storage requirements [64].
In 1997, evaluation of MCAT indicated that the overall sensitivity and specificity of the test in comparison with MAT were 84.7 and 87.0%, respectively. This test also appeared to have a higher sensitivity than MAT during early stages of the disease (75% vs. 58.3%), though the specificity was less than that of MAT (83.3% vs 100%). The sensitivity of MCAT declined to 61%, 3 to 4 weeks after the onset of illness. Thus MCAT appeared to be a useful screening test for early diagnosis of leptospirosis [65].
In 1998 MCAT was found to be 90.2% sensitive, and 96.3% specific, with a positive predictive value of 94.9%, a negative predictive value of 92.9% and an accuracy of 93.7% [66].
3. Latex agglutination test. This test depends on the sensitisation of commercially available latex particles with a leptospiral antigen. Antiserum will react with the antigen to cause agglutination of the particles. Antigen prepared from L. biflexa serovar Patoc, strain Patoc 1 will cross-react with human convalescent sera to provide a useful screening procedure [45].
4. Lepto dipstick. This dipstick assay for the detection of leptospira-specific IgM antibodies in human sera was evaluated in 1997. Heat stable antigen prepared from leptospira biflexa and coated onto the lower band and internal control was set up in the upper band; detection agent was also incorporated. The sensitivity and specificity of the dipstick assay and the IgM ELISA appeared to agree well. The sensitivity of the dipstick assay for sera collected between days 10 and 30 of the disease was 86.8% and that of IgM ELISA was 88.5%. The specificity of the dipstick assay was calculated to be 92.7% and that of IgM ELISA, 94.2%. The dipstick assay revealed cross reactivity with sera from patients with HIV, Hanta virus, Toxoplasma infection, Lyme borreliosis, malaria, meningococcal meningitis and hepatitis A infection. In contrast, no cross reactivity was observed with these sera in IgM ELISA. The highly stable reagents and simple implementation makes this method suitable for use in clinical and field laboratories in tropical countries. The performance of the dipstick assay is useful for single serum specimens, but it is recommended for use with paired serum samples, because besides strong staining, seroconversion or an increase in staining intensity are consistent with active leptospirosis. An internal control validates the performance of the assay. Thus the numerous practical advantages of the dipstick assay can contribute to an improved diagnosis [67].
5. Microscopic agglutination test (MAT). The MAT is slow, tedious, potentially biohazardous, painstaking and subjective; but it is a very sensitive and reliable assay when used by skilled people. MAT is carried out with suspensions of living cultures or of cultures killed by the addition of neutralized formaldehyde. The clumps of agglutinated living leptospires differ in appearance from clumps of killed cultures. Living leptospires are agglutinated into highly refractile spheroids of various sizes, some of which may be joined to produce elongated masses of confluent spheroids. By contrast, the agglutinated killed leptospires form looser masses with an irregular often angular, outline; these appear flattened, resembling small piles of threads, or snowflakes, or pieces of cotton wool. The degree of agglutination ranges from 100%, when no free leptospires can be seen between the clumps, through lesser degrees, as the serum is more diluted, to nil, as seen in the negative control suspension of leptospires in diluent. The degree of agglutination can only be assessed in terms of the proportion of free leptospires. The accepted endpoint of an agglutination reaction is the final dilution of serum at which 50% or more of the leptospires are aggluatinated [53].
Preparations for MAT require meticulous culture of a collection of the strains used alive as antigen suspensions in the tests, their regular subculture and quality control for authenticity, purity agglutination and skilled educated personnel. A recent advance is the use of standardized preparations of dried leptospires available to accredited diagnostic laboratories from a central reference laboratory [51].
The use of a battery of strains giving comprehensive coverage of all serogroups the multi-antigen MAT provides an alternative to the so-called 'genus-specific' tests as a means of diagnosing leptospirosis; but the necessity to maintain large number of living strains of L. interrogans limits its use to reference laboratories. Wherever possible, local isolates of known identity should be included in the battery of strains because this has repeatedly been shown to increase both the sensitivity and the specificity of the test [51].
Interpretation of diagnostic MAT. In a non-endemic area any level of antibodies, however low, may signify leptospirosis in the 1st week of a clinically compatible illness. The titer will rise in a second specimen taken after 3 to 7 days. If the titer remains below 100, even on repeated testing, it may be assumed that it was due to previous leptospirosis, and not to current illness. A titre of 400 to 800 or more, or a 4-fold rise in titer between 2 tests, is diagnostic when combined with a clinical illness compatible with leptospirosis. In endemic areas, the diagnosis will be confirmed if the titre rises on retesting, but will be negated if it is unchanged, assuming that the infecting serovar was included among the antigens for the MAT [51].
Serotype specific ELISA. Several attempts have been made to develop sero-type specific ELISA tests with a variety of extracted antigens. Tests based on boiled whole cell antigens tend to be genus specific but those based on ultrasound-disintegrated or phenol-extracted preparations show considerable serotype specificity. It is clear that ELISA is more sensitive than live antigen MAT [1].
Molecular Diagnosis of Leptospirosis
DNA restriction enzyme analysis (REA). DNA REA involves the extraction of DNA from a homogenous population of organisms, digestion of the DNA with a restriction endonuclease and electrophoresis of the digested DNA in an agarose gel. The DNA fingerprints thus generated are highly specific for each type of leptospire. The application of REA for the identification of leptospires was first proposed by Marshall and co-workers in 1981. This technique has proven to be sensitive enough to differentiate between leptospiral serovars on the basis of genetic differences [68].
Nucleic acid probes and hybridization. The nucleic acids that contain specific sequences are isolated, cleaved and labelled with a reporter molecule, such as radioactive (32P or 35S) or non-radioactive (biotin, digoxigen) molecules. The labelled DNA in the single-stranded form is then hybridized to ssDNA in tissues (in situ hybridization), in paper (Southern blot hybridization) or in solution (solution hybridization). If the nucleotide sequences in the nucleic acid probe are complementary to those in the sample, hybridization occurs and results in the form of nucleic acid hybridization are monitored by autoradiography in the case of probes labelled with radioactive material, or calorimetrically with non-radioactive material. Terpestra et al., 1968 developed a sensitive and specific diagnostic method for early detection of leptospires using DNA hybridization with 32P and biotin-labelled probes prepared from leptospiral DNA. Slot blot hybridization to study the DNA relatedness among strains of L. biflexa using whole genomic DNA probe was employed by others; 66 pathogenic leptospiral serovars by DNA slot blot hybridization were characterised [68].
Polymerase chain reaction (PCR). PCR involves in vitro enzymatic amplification of a target DNA sequence through a series of polymerisations carried out by a thermostable DNA polymerase, primed with a pair of the short DNA fragments, which bind specifically to the sequence of interest. Amplified DNA fragments produced by this technique can be easily visualized on ethidium-bromide-stained agarose gels with a UV transilluminator. PCR was first developed for the detection of leptospires in urine samples of infected cattle. Urine samples containing as few as 10 leptospires/ml gave positive results. PCR tests detected leptospires using primers that amplify a 631 bp fragment in the 5' region of 165 rDNA samples. As little as 10-1 pg of purified DNA and as few as 10-1 leptospires per ml can be identified by this method. Other's in 1993 reported two sets of primers (G1 and G2, and B641 and B6511) derived from genomic DNA libraries of leptospiral serovar ictero haemorrhagiae (RGA) and Bim (Strain 501) enabled the PCR amplification of target DNA fragments from a leptospiral reference strain. Researchers in 1994 tested urine samples from patients at different stages of leptospirosis and concluded that the detection of leptospires in urine with PCR was a promising approach for the early diagnosis of leptospirosis, and was useful in studying long-term shedding. Others evaluated PCR in 1995 for the early detection of leptospires in clinical samples from patients with acute leptospirosis. They compared PCR, culture and serology, and concluded that PCR was a rapid, sensitive and specific method of diagnosing leptospiral infection, especially during the first few days of the disease. A ligase-mediated PCR (LM-PCR) was developed in 1996, with potential for differentiation of serovars within L. interrogans. The number of fragments that were generated was significantly lower than the number generated by RFLP; the LM-PCR method had the potential to use less template DNA and was quicker than standard RFLP [68].
Pulsed field gel electrophoresis (PFGE). PFGE is a variation of agarose gel electrophoresis that permits analysis of bacterial DNA fragments over an order of magnitude longer than that with conventional REA. This process is technically more demanding, requires more expensive, specialised equipment, but provides highly reproducible restriction profiles with well-resolved fragments. In 1990, fingerprints for 72 reference pathogenic leptospiral serovars by PFGE were made following Notl digestion of chromosomal DNA. PFGE was found to be more rapid than serology and was useful for identification and epidemiological studies [68].
Ribotyping. Recently, ribosomal ribonucleic acid gene restriction patterns have been used for the identification of species or for epidemiological typing. The conserved nature of the rRNA gene allows the use of a single probe for typing bacteria for any phylogenetic comparison. Genetic variation in the L. interrogans serogroup icterohamorrhagiae was found by ribosomal RNA gene restriction fragment patterns. Other researchers examined the ribosomal DNA fingerprints from 103 pathogenic leptospiral strains in 1993, using Eco R1 RFLP of rRNA genes, and described 69 new leptospiral ribotypes in addition to 49 that had been previously observed [68].
Treatment
Leptospirosis in all its forms is amenable to treatment with antibiotics. Leptospires are susceptible in laboratory tests to all clinically useful antibiotics, except chloramphenicol and rifampicin. Leptospires are not usually cultured and tested for susceptibility in individual cases. Resistance in clinical use has not been reported, although failures of treatment have rarely been suggested. The antibiotics most usually recommended are penicillin, at high doses, unless the patient is hypersensitive to penicillin, in which case erythromycin is used. Tetracyclines are used but they have disadvantages and are contraindicated for people with renal insufficiency, for children and for pregnant women. Doxycycline is recommended for treatment and short-term chemoprophylaxis. Penicillin should be administered as early as possible, during the leptospiraemic phase, parenterally in very ill patients. No antibiotic can reverse the destructive effects of leptospirosis in tissues and organs, but penicillin was found to have beneficial effects, reducing mortality and duration of illness in severe leptospirosis, when given intravenously, even at a late stage. Antibiotic treatment may be accompanied by a Jarish-Herxheimer reaction (a transient increase in fever and severity of symptoms immediately following treatment), which does not contraindicate administration of antibiotics [51].
Renal failure is the commonest cause of death; if there are signs that this is developing, peritoneal dialysis or the use of an artificial kidney should not be delayed [1].
Prevention and Control
Prevention of leptospirosis in all situations is not possible, because it is widespread in so many animals and places all over the world. The best that can be done is to limit the effects of leptospirosis on humans and the animals they depend on. This involves identification of sources, containing them and eliminating them or their effects.
Indications that leptospirosis occurs comes from correct diagnosis, which depends on laboratory expertise and quality assurance. Hospital, clinical or practitioner records can point to known or suspected leptospirosis. Clustering of cases can suggest a common source. The prevalent serovar or serovars can likewise suggest animal reservoirs. A history of contact with animals or with surface waters during occupational or recreational travel or pursuits is also important.
The best way to avoid leptospirosis is to keep away from animals and areas that may be contaminated by their urine. People whose occupation, travel or hobbies involve risks should know of the disease and how to avoid it. The main groups at risk are dairy farmers and milkers (hardjo infection from cows); slaughter-house workers, meat inspectors, veterinarians and meat carriers in food industries; people who work habitually in wet occupations (rice farmers, sugar cane harvesters, drainers, sewer workers, miners); adventure travellers (cave exploration, white water rafting, water sports) and military or civil emergency personnel. People should be aware of the dangers and be dissuaded from swimming in rivers or pools suspected to be contaminated. Education through industry or community self help groups can raise awareness and prevent infection in humans and the animals that they keep.
Even in urban centres, civil emergencies that break drains and sewers from which rats and effluents emerge break the spatial barrier between them and urban dwellers. Rat control in and around food storage and preparation areas, crop storage areas, stables, milking sheds, intensive animal production installations and dwellings is difficult but will remove a major source of leptospirosis for humans and domesticated animals. All the people involved in high-risk activities should wear protective clothing and need to adopt a reasonable standard of hygiene. Impervious knee-high boots, aprons, gloves, face masks or eye protection should be used wherever indicated [51].
Active Protection Immunization and Chemoprophylaxis Immunization of animals
The purpose of immunization of domesticated food and breeding animals is to protect them from leptospirosis so that productivity is maximized and to protect humans in contact with these animals. Dogs are immunized to protect them and human companions. Effective vaccines containing suspensions of killed L. borgpetersenii serovar hardjo and L. interrogans serovar pomona are widely available commercially. The use of locally prevalent strains is recommended. The vaccines are given subcutaneously or intramuscularly in two initial doses, one month apart, followed by animal boosters [51].
Immunization of humans. Vaccines composed of killed cultures of leptospires protect people against leptospirosis. Washing or ultrafiltration is used to remove unwanted proteins from the culture media. These vaccines may cause side effects, ranging from local soreness to fever and incapacity for a few days. Two doses are given subcutaneously, 3 to 4 weeks apart, followed by animal boosters. Multivalent combinations effective against several serovars are compounded, as required by local needs. They are made available to selected high-risk groups, wherever the side effects are preferable to severe leptospirosis. Work is progressing towards a non-toxic vaccine, based on requisite lipopolysaccharide epitopes [51].
Chemoprophylaxis. Doxycycline can prevent leptospirosis, if given before and during exposure. Prolonged administration is not recommended [51].
4. Uhlenhuth P., Formme W. Quoted in Topley and Wilson's Principles of Bacteriology, Virology and Immunit. 1916;8(2):617.
Received on 12 September 2002
Revised 20 December 2002
- 1. Edward A., Hodder, Staughton. Leptospirosis. Quoted in Topley and Wilson's Principles of Bacteriology, Virology and Immunity. 8th edn. Vol. 3, 619, 1990
- 2. Stimson A.M. Note on an organism found in yellow fever tissue. Publ Hlth Rep 1907;22:541.
- 3. Inada R., Ido Y., Hoki R., Kaneko R., Ito H. The etiology mode of infection and specific therapy of Weil's disease. J Exp Med 1916;23:377-402.
- 5. Noguchi H. The spirochaetes, in the newer knowledge of bacteriology and immunology, Jordan EO and Falk IS. University of Chicago Press, Chicago, 1928;452-97.
- 6. Kmety E., Dikken H. Classification of the species Leptospira interrogans and the history of its serovars. University Press Groningen, Groningen, 1993
- 7. Yanagawa and Faine. Morphological and serological analysis of Leptospiral structure. Nature (London) 1996;211:823-6.
- 8. Abdussalam M., Alexander A.D., et al. Research need in Leptospirosis. Bull WHO 1972;47:113-22.
- 9. Adler B., Murphy A.M., et al. Detection of specific anti leptospiral immunoglobulins M and G in human serum by solid phase ELISA. JClin Microbiol 1980;11:452-7.
- 10. Adler B., Faine S. A pomona serogroup - specific, agglutinating antigen in leptospira, identified by monoclonal antibodies. Pathology 1983;15:247-50.
- 11. Sehgal S.C. Emergence of leptospirosis as a public health problem. In round table conference series no. 3; 1998:7-16.
- 12. Gale N.B., Alexander A.D., Evans L.B., et al. An outbreak of leptospirosis among US army troops in the canal zone. Am J Trop Med and Hyg. 1966;(15): 64-70.
- 13. Damude D.F., Jones C.J. The problem of human leptospirosis in Barbados. Trans Soc Trop Med & Hyg 1973;(73):169-77.
- 14. Everard C.O., Fraser-Changpong G.M., Everard J.D. The incidence of severe leptospirosis in Trinidad. Trop Geogr Med 1987;39(2):126-32.
- 15. Cacciapuoti B., Nuti M., Pinta A., Sabrie A.M. Human Leptospirosis in Somalia:A serological survey: Trains Roy Soy of Trop Med & Hyg 1982
- 16. Cacciapuoti B., Vellucci A., Ciceroni L., et al. Prevalence of Leptospirosis in man: A pilot survey. Eur J Epidemiol 1987;3(2):137-42.
- 17. Ahmed I.P. Serological studies on Leptospirosis in Pakistan. J Pak Med Assoc 1987;37:233-6.
- 18. Everard C.O.R., Haynes R.J., Edwards C.N. Leptospiral infection in school children from Trinidad and Barbados. Epidem Inf 1989;103:143-56.
- 19. Katz Alan R., Sasaki Davi M., Mumm Col Alam H., et al. Leptospirosis in Oahu: An outbreak among military personnel associated with recreational exposure. Milt Med 1977;162(2):101-4.
- 20. Lombardi Raul. Acute renal failure in leptospirosis in Uruguay. Renal Failure 1977;19(2):315-8.
- 21. Yersin Claude, Bovet Pascal, Merien Fabrice, et al. Human leptospirosis in the Seychelles (Indian Ocean): a population based study. Am J Trop Med Hyg 1998;59(6):930-40.
- 22. Trivejo Rosalie T., Rigan Perez Jose G., Ashford David A., et al. Epidemic leptospirosis associated with pulmonary hemorrhage - Nicaragua, 1995. J Infec Dis 1998;178:1457-63.
- 23. Ciceroni L., Bartoloni A., Pinto A., et al. Prevalence of Leptospiral infection in human in Cordillera Province, Bolivia. Trans Roy Soc Trop Med & Hyg 1995;89:38-6.
- 24. Leblebicioglu Hakan, Sencan Irfan, Sunbul Mustafa, et al. Weils disease, Report of 12 cases. Scand J Infect Dis 1996;28:637-9.
- 25. Bounlu Kathanthong, Insisiengmay Sithat, Vanthanonvong Khempet, et al. Acute jaundice in Vietiane, Lao People's democratic republic. Clin Infect Dis 1998;27:717-21.
- 26. MMWR. Outbreak of leptospirosis among white water rafters - Costa Rica 1996. JAMA 1997;278(10).
- 27. MMWR. Outbreak of acute febrile illness among athletes participating in Triathlons Wisconsin and Illinois, 1998. JAMA 1998;(17):280.
- 28. Taylor J., Goyle A.N. Leptospirosis in the Andaman. Indian Med Res Memoirs 1931;20.
- 29. Das Gupta B.M., Chopra R.N. The occurrence of Weil's disease in Indian. Indian Med Gaz 1937;72:610-2.
- 30. Das Gupta B.M. Leptospirosis in India. Indian Med Gaz 1938;73:449-53.
- 31. Lahiri M.N. A note on the occurrence of Leptospirosis in Bombay, Indian Med Gaz 1941a;76:669-70.
- 32. Dalal P.M. Leptospirosis in Bombay city (report of 5 cases). Indian J Med Sco 1960;14:295-301.
- 33. Joseph K.M. Kalra SL Leptospirosis in India. Ind J Med Res 1966;54(7):611-14.
- 34. Bhatnagar R.K., Sant M.V., Jhala H.L. Prevalence of Leptospirosis in Bombay, Studies in man and Animals, Indian J Path Bact 1967;10:324-31.
- 35. Ratnam S., Sundaraj T., Thyagarajan S.P., et al. Serological evidence of leptospirosis in jaundice and pyrexia of unknown origin. Indian J Med Res 1983;77(4):430.
- 36. Ratnam S., Sundaraj T., Subramanian S. Serological evidence of leptospirosis in a human population following an outbreak of the disease in cattle. Trans Roy Soc. Trop Med & Hyg 1983;77(1):94-8.
- 37. Muthusethupathi M.A., Shivakumar S. Acute renal failure due to Leptospirosis. JAPI 1987;35:631-3.
- 38. Venkataraman K.S., Ramakrishna J., Raghavan N. Human Leptospirosis:a recent study in Madras, India. Trans Roy Soc Trop Med & Hyg 1991;85:304.
- 39. Muthusethupathi M.A., Shivakumar S., Suguna R., et al. Leptospirosis in Madras - A clinical and serological study. JAPI 1995;43(7):456-8.
- 40. Ratnam S., Everard C.O.R., Alex J.C., et al. Prevalence of Leptospiral agglutinins among conservancy workers in Madras City, India J Trop end & Hyg 1993;96:41-5.
- 41. Sehgal S.C., Murhekar M.V., Sugunan A.P. Outbreak of Leptospirosis with pulmonary involvement in North Andaman. Indian J Med Res 1995;102:9-12.
- 42. Chu Kathryn M., Rathinam R., Namperumalsamy P. Identification of leptospira species in the pathogenesis of uveitis and determination of clinical ocular characteristics in South India. J of Infect Dis 1998;177:1314-21.
- 43. Prabhakar P.K., Harish B.N., Rao R. Sambasiva. Seroprevalence of leptospirosis among febrile and jaundice patients. Indian J of Med Micro 1995;13(4):189-91.
- 44. Saravanan R., Rajendran P., Thygarajan S.P. Isolation of leptospira Javanica from urine sample of an acute renal failure case in Chennai: India. Ind J of Med Microbiol 1998;16(2):61-3.
- 45. Faine S. In: Guidelines for the control of leptospirosis. Geneva WHO, 67, 1982
- 46. Bharadwaj R. Microbiology of Leptospirosis. In: Round Table conference series no. 3, 1998;33:40.
- 47. Faine S. Leptospira and Leptospirosis. CRC Press, 1994, Boca Raton, Florida USA.
- 48. Wesley Farr R. Leptospirosis. Clin Infect Dis 1995;21:1-8.
- 49. Faine S. Leptospirosis. Topley & Wilson's Microbiology and Microbial infection. 1998, 9th Edition vol. 2. 1287-1303.
- 50. Sehgal S.C., Vijyachari P., Sharma R.K. Laboratory manual: Hands on training workshop on laboratory diagnosis of leptospirosis. 1999;(May)10.
- 51. Faine S. Leptospirosis. Topley and Wilson's Microbiology and Microbial infections. 1998, 9th Edition Vol. 3, 849-869.
- 52. Galton Menges R.W., Shotts E.B., Nahmias A.J., Health C.W. Leptospirosis: Epidemiology, Clinical manifestation in man and animals and methods in laboratory diagnosis. 1962, USPHS Publication No. 951, Washington DS US Government Printing Office.
- 53. Turner L.H. Leptospirosis II. Trans Soc Med & Hyg 1968;62(6).
- 54. Coghlan Joyee D. Leptospira, Borrelia, Sprillum. Mackie and Mc Cartney. Practical Medical Microbiology 1996, 14th Edition (Vol. 2), 559-562.
- 55. Sumathi G.., Chinari Pradeep K.S., Shivakumar S. Msat-A Screening test for leptospirosis. Ind J Med Microbiol 1997;15(2):84.
- 56. Brandao Angela P., Camargo Eide D., Silva Emilson D., et al. Macroscopic agglutination test for rapid diagnosis of human leptospirosis. J Clin Microbiol 1998;36(11):3138-42.
- 57. Chinari Pradeep K.S., Sumathi G., Rao Vimala Ranga, Kumar S. Shiva. Leptospirosis Laboratory, Chennai Medical College - A three year experience in Serodiagnosis (1995-97). Ind J Med. Micro 1999;17(1):50-1.
- 58. Chang R.S., Mc Comb Dorothy E. Erythrocyte sensitising substances form five strains of leptospirae,. Am J Trop Med Hyg 3:481-9.
- 59. Appassakij Hatsadee, Silpapojakul Khachornsakdi, et al. Evaluation of the immunofluorescent antibody test for the diagnosis of human leptospirosis. Am J Trop Med. & Hyg 1995;52(4):340-43.
- 60. Terpestra W.J., Ligthart G.S., Schoone G.J. ELISA for the detection of specific IgM and IgG in human leptospirosis. J Gen Micro 1985;131:377-85.
- 61. Pappas Michael G., Ballon W., Ripley Gray, et al. Rapid serodiagnosis of leptospirosis using the IgM - specific Dot ELISA: Comparison with the microscopic agglutination test. Am J Trop Med Hyg 1985;34(2):346-54.
- 62. Vinicius D.A., Silva Marcos, Nakamura Paulo Mutuko, et al. Immunodiagnosis of human leptospirosis by DOT-ELISA for the detection of IgM, IgG and IgA antibodies. Am J Trop Med Hyg 1997;56(6):650-5.
- 63 Georage Watt, Alquiza Lily M., Padne Launena P., et al. The rapid diagnosis of leptospirosis: A prospective comparison of the DOT-enzyme linked immunosorbent assay and the genus specific microscopic agglutination test at different stages of illness. J Infect Dis 1998;157(4).
- 64. Arimitsu Y., Kmety E., Ananyina Y., et al. Evaluation of the one point microcapsule agglutination test for diagnosis of leptospirosis. Bulletin WHO 1994;72(3):395-9.
- 65. Sehgal S.C., Vijyachari P., Subramaniam V. Evaluation of leptospira microcapsule agglutination test (MCAT) for serodiagnosis of leptospirosis. Ind J Med Res 1997;106;504-7.
- 66. Suputtamongkol Y., Sarawish S., Silpasakorn S. Microcapsule agglutination test for the diagnosis of leptospirosis in Thailand. Ann Trop Med & Parasit 1998;92(7):797-801.
- 67. Gussenhoven George C., Hoorn Menno A.W.G., Goris Marga G.A., et al. Leptodipstick, a dipstick assay for detection of leptospira specific immunoglobulin M antibodies in human sera. J Clin Micro 1997;35(1):92-7.
- 68. Ramadas P. Biotechnology in leptospirosis in Round Table Conference Series, 1998;(3):41-7.
Publication Dates
-
Publication in this collection
08 Dec 2003 -
Date of issue
June 2003
History
-
Reviewed
20 Dec 2002 -
Received
12 Sept 2002