Abstract
The flowers of the species belonging to the genus Passiflorashow a range of features that are thought to have arisen as adaptations to different pollinators. Some Passiflora species belonging to the subgenus Decaloba sect. Xerogona, show touch-sensitive motile androgynophores. We tested the role of auxin polar transport in the modulation of the androgynophore movement by applying auxin (IAA) or an inhibitor of auxin polar transport (NPA) in the flowers. We recorded the movement of the androgynophore during mechano-stimulation and analyzed the duration, speed, and the angle formed by the androgynophore before and after the movement, and found that both IAA and NPA increase the amplitude of the movement in P. sanguinolenta. We hypothesize that auxin might have a role in modulating the fitness of these Decaloba species to different pollination syndromes and demonstrate that an interspecific hybrid between insect- and hummingbird-pollinated Xerogona species present a heterosis effect on the speed of the androgynophore movement.
Passiflora; androgynophore; IAA; NPA; thigmotropism
Introduction
The genus Passiflora comprises about 500 species which are mostly woody vines that present a huge diversity in flower shape, colors and sizes. Consequently it is a good model for studying plant-pollinator interactions and co-evolution, since it displays all kinds of pollination syndromes. Although this diversity exists, the flowers always exhibit unique features that characterize and cluster the species in the genus. One of them is the corona filaments, which comprises one or more extra whorls that can play different roles in different species, functioning as nectar guide, or forming a floral tube in flowers adapted for bird pollination, and even serving as a landing platform for insects (Ulmer and MacDougal, 2004Ulmer T and MacDougal JM (eds) (2004) Passiflora: Passion-flowers of the World. Timber Press, Inc., Portland, 430 pp.). Another common feature is a nectary system containing an operculum and a membrane structure called limen that encloses a nectary chamber. Finally, the androgynophore, a column in the center of the flower that elevates the androecium and the gynoecium, is present in different sizes and even in shapes. In P. edulis, which has flowers adapted to insect pollination, the androgynophore is a short and straight column. In contrast, in P. mucronata, which is bat pollinated, the androgynophore is a long curved column (Ulmer and MacDougal, 2004Ulmer T and MacDougal JM (eds) (2004) Passiflora: Passion-flowers of the World. Timber Press, Inc., Portland, 430 pp.).
Recently, we showed that in some Passiflora species, the androgynophore can also be a thigmotropic structure, i.e., it has the capability to move in response to touch and the movement is dependent on the direction of the stimulus (Scorza and Dornelas, 2014Scorza LC and Dornelas MC (2014) Rapid touch-stimulated movement in the androgynophore of Passiflora flowers (subgen. Decaloba; Sect. Xerogona): An adaptation to enhance cross-pollination? Plant Signal Behav 9:e27932.; Scorza et al., 2014Scorza LC and Dornelas MC (2014) Rapid touch-stimulated movement in the androgynophore of Passiflora flowers (subgen. Decaloba; Sect. Xerogona): An adaptation to enhance cross-pollination? Plant Signal Behav 9:e27932.). When mechanically stimulated, the androgynophore inclines to the same side where the stimulus came in about 2 seconds. These species, P. sanguinolenta, P. citrina, P. capsularis and P. rubra are in the subgenus Decaloba, and the movement is believed to be related to the pollination system of these species. The motile androgynophore would enhance the chances of pollen deposition on pollinators that would approach the flower and touch the column, which in turn would curves in the pollinator’s direction upon the mechanical stimulus (Scorza and Dornelas, 2014Scorza LC and Dornelas MC (2014) Rapid touch-stimulated movement in the androgynophore of Passiflora flowers (subgen. Decaloba; Sect. Xerogona): An adaptation to enhance cross-pollination? Plant Signal Behav 9:e27932.; Scorza et al., 2014Scorza LC and Dornelas MC (2014) Rapid touch-stimulated movement in the androgynophore of Passiflora flowers (subgen. Decaloba; Sect. Xerogona): An adaptation to enhance cross-pollination? Plant Signal Behav 9:e27932.).
Plant fast movements have been widely studied (for reviews, see Braam, 2005Braam J (2005) In touch: Plant responses to mechanical stimuli. New Phytol 165:373–389.; Scorza and Dornelas, 2011Scorza LCT and Dornelas MC (2011) Plants on the move: Towards common mechanisms governing mechanically-induced plant movements. Plant Signal Behav 6:1979–1986.), in particular pulvinar movements in Fabaceae species, such as Mimosa pudica, where the leaflets of compound leaves shows a fast closing movement in response to touch (thigmonastism) or light regime (photonastism and nyctinastism) (Samejima and Sibaoka, 1980Samejima M and Sibaoka T (1980) Changes in the extracellular ion concentration in the main pulvinus of Mimosa pudica during rapid movement and recovery. Plant Cell Physiol 21:467–479.; Moran, 2007Moran N (2007) Osmoregulation of leaf motor cells. FEBS Lett 581:2337–2347.; Volkov et al., 2010aVolkov AG, Foster JC, Ashby TA, Walker RK, Johnson JONA and Markin VS (2010a) Mimosa pudica: Electrical and mechanical stimulation of plant movements. Plant Cell Environ 33:163–173.). Additionally, carnivore plants such as Drosera, Dionaea (Droseraceae) and Utricularia(Lentibulariaceae), have adapted organs that can produce active movements to capture preys (Sibaoka, 1991Sibaoka T (1991) Rapid plant movements triggered by action potentials. Bot Mag Tokyo 104:73–95.; Braam, 2005Braam J (2005) In touch: Plant responses to mechanical stimuli. New Phytol 165:373–389.; Volkov et al., 2008Volkov AG, Adesina T, Markin VS and Jovanov E (2008) Kinetics and mechanism of Dionaea muscipula trap closing. Plant Physiol 146:694–702.; Singh et al., 2011Singh AK, Prabhakar S and Sane SP (2011) The biomechanics of fast prey capture in aquatic bladderworts. Biol Lett rsbl.2011.0057.). Still, stamina of Portulaca grandiflora (Portulacaceae), Berberis canadensis(Berberidaceae), Opuntia (Cactaceae) and Loasaceae (Henning and Weigend, 2012Henning T and Weigend M (2012) Total control - pollen presentation and floral longevity in Loasaceae (blazing star family) are modulated by light, temperature and pollinator visitation rates. PloS One 7:e41121.) flowers for example, can also bend in response to a visiting pollinator increasing pollen transfer among the flowers, boosting the cross pollination (Jaffe et al., 1977Jaffe MJ, Gibson C and Biro R (1977) Physiological studies of mechanically stimulated motor responses of flower parts.1. Characterization of thigmotropic stamens of Portulaca grandiflora Hook. Bot Gaz 138:438–447.; Fleurat-Lessard and Millet, 1984Fleurat-Lessard P and Millet B (1984) Ultrastructural features of cortical parenchyma cells (motor cells) in stamen filaments of Berberis canadensis Mill and tertiary pulvini of Mimosa pudica L. J Exp Bot 35:1332–1341.; Schlindwein and Wittmann, 1997Schlindwein C and Wittmann D (1997) Stamen movements in flowers of Opuntia (Cactaceae) favour oligolectic pollinators. Plant Syst Evol 204:179–193.; Scorza and Dornelas, 2011Scorza LCT and Dornelas MC (2011) Plants on the move: Towards common mechanisms governing mechanically-induced plant movements. Plant Signal Behav 6:1979–1986.).
The fact that plants are capable of rapidly moving their structures without having any kind of neurological system is attributed to the capability of specific plant cells to swell or shrink by losing water quickly. The maintenance of a differential turgor pressure among plant tissues provides an energy storage that, when released, is capable of moving plant organs within seconds (Sibaoka, 1991Sibaoka T (1991) Rapid plant movements triggered by action potentials. Bot Mag Tokyo 104:73–95.; Braam, 2005Braam J (2005) In touch: Plant responses to mechanical stimuli. New Phytol 165:373–389.; Volkov et al., 2008Volkov AG, Adesina T, Markin VS and Jovanov E (2008) Kinetics and mechanism of Dionaea muscipula trap closing. Plant Physiol 146:694–702., 2010bVolkov AG, Pinnock MR, Lowe DC, Gay MS, and Markin VS (2010b) Complete hunting cycle of Dionaea muscipula: Consecutive steps and their electrical properties. J Plant Physiol 168:109–120.).
This turgor pressure is maintained by the activity of proton pumps in the plasma membranes (H+-ATPase), which are coupled with K+ and Cl− fluxes (Samejima and Sibaoka, 1980Samejima M and Sibaoka T (1980) Changes in the extracellular ion concentration in the main pulvinus of Mimosa pudica during rapid movement and recovery. Plant Cell Physiol 21:467–479.; Sibaoka, 1991Sibaoka T (1991) Rapid plant movements triggered by action potentials. Bot Mag Tokyo 104:73–95.; Fleurat-lessard et al., 1997Fleurat-Lessard P, Bouché-Pillon S, Leloup C and Bonnemain J-l (1997) Distribution and activity of the plasma membrane H+-ATPase in Mimosa pudica L. in relation to ionic fluxes and leaf movements. Plant Physiol 13:747–754.; Moran, 2007Moran N (2007) Osmoregulation of leaf motor cells. FEBS Lett 581:2337–2347.). When the cells get turgid the H+-ATPase proton pumps is acting extruding H+ protons outwards of the cell. In order to balance the proton gradient across the membrane, K+ ions are pumped inwards, increasing the cell’s osmotic potential, and therefore, there is a water in-flux through aquaporins which causes swelling of the cells. When a mechanical or electrical stimulus is applied, an action potential triggers a rapid turgor loss associated with an efflux of ions in the cells that is followed by water (Campbell and Garber, 1980Campbell NA and Garber RC (1980) Vacuolar reorganization in the motor cells of Albizzia during leaf movement. Planta 148:251–255.; Moran, 2007Moran N (2007) Osmoregulation of leaf motor cells. FEBS Lett 581:2337–2347.; Volkov et al., 2010aVolkov AG, Foster JC, Ashby TA, Walker RK, Johnson JONA and Markin VS (2010a) Mimosa pudica: Electrical and mechanical stimulation of plant movements. Plant Cell Environ 33:163–173.).
Auxin is an important coordinator of plant growth and development, and one of the mechanisms where this hormone is involved is the regulation of water and ion permeability to cells (Blatt and Thiel, 1994Blatt MR and Thiel G (1994) K+ channels of stomatal guard cells: Bimodal control of the K+ inward-rectifier evoked by auxin. Plant J 5:55–68.; Takahashi et al., 2012Takahashi K, Hayashi K and Kinoshita T (2012) Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Plant Physiol 159:632–641.). In order to clarify how this hormone affects cell turgor regulation, experiments related to the pulvinar movements of Cassia fasciculata, Phaseolus vulgaris and M. pudica have been reported (Watanabe and Sibaoka, 1983Watanabe S and Sibaoka T (1983) Light-Induced and auxin-induced leaflet opening in detached pinnae of Mimosa pudica. Plant Cell Physiol 24:641–647.; Bourbouloux et al., 1992Bourbouloux A, Roblin G and Fleuratlessard P (1992) Calcium involvement in the IAA-induced leaflet opening of Cassia fasciculata. J Exp Bot 43:63–71.; Bonmort and Roblin, 1996Bonmort J and Roblin G (1996) Effect of 2,4-dichlorophenoxyacetic acid on the dark- and light-induced pulvinar movements in Cassia fasciculata Michx. Plant Growth Regul 19:61–65.; Iino et al., 2001Iino M, Long C and Wang X (2001) Auxin- and abscisic acid-dependent osmoregulation in protoplasts of Phaseolus vulgaris pulvini. Plant Cell Physiol 42:1219–1227.; Moyen et al., 2007Moyen C, Bonmort J and Roblin G (2007) Membrane effects of 2,4-dichlorophenoxyacetic acid in motor cells of Mimosa pudicaL. Plant Physiol Biochem 45:420–426.). Applying indol-3-acetic acid (IAA) to protoplasts of pulvinus of P. vulgariscaused swelling of the cells. This was interpreted as enhanced effluxes of K+ and Cl− (Iino et al., 2001Iino M, Long C and Wang X (2001) Auxin- and abscisic acid-dependent osmoregulation in protoplasts of Phaseolus vulgaris pulvini. Plant Cell Physiol 42:1219–1227.). When exogenously applied in M. pudica and C. fasciculata, IAA (indol-acetic acid) increased the angles during the folding of the leaflets (Bourbouloux et al., 1992Bourbouloux A, Roblin G and Fleuratlessard P (1992) Calcium involvement in the IAA-induced leaflet opening of Cassia fasciculata. J Exp Bot 43:63–71.). 2,4-D, a synthetic auxin, when applied, inhibited the leaflet folding by dark stimulus (Bonmort and Roblin, 1996Bonmort J and Roblin G (1996) Effect of 2,4-dichlorophenoxyacetic acid on the dark- and light-induced pulvinar movements in Cassia fasciculata Michx. Plant Growth Regul 19:61–65.; Moyen et al., 2007Moyen C, Bonmort J and Roblin G (2007) Membrane effects of 2,4-dichlorophenoxyacetic acid in motor cells of Mimosa pudicaL. Plant Physiol Biochem 45:420–426.). In all cases the auxin effects seem to be directed towards maintaining a high turgor pressure in the cells (Bourbouloux et al., 1992Bourbouloux A, Roblin G and Fleuratlessard P (1992) Calcium involvement in the IAA-induced leaflet opening of Cassia fasciculata. J Exp Bot 43:63–71.; Bonmort and Roblin, 1996Bonmort J and Roblin G (1996) Effect of 2,4-dichlorophenoxyacetic acid on the dark- and light-induced pulvinar movements in Cassia fasciculata Michx. Plant Growth Regul 19:61–65.). These experiments further contributed to the evidence that auxins stimulate the proton extrusion driven by H+-ATPases in the plasma membrane. Although it was known that auxins have an effect on proton pumps, the mechanisms by which it acts became clear only recently, when Takahashi et al. (2012)Takahashi K, Hayashi K and Kinoshita T (2012) Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Plant Physiol 159:632–641. showed that auxins mediate H+-ATPase activation by phosphorylation of the penultimate threonine of the H+-ATPase during hypocotyl elongation in Arabidopsis (Takahashi et al., 2012Takahashi K, Hayashi K and Kinoshita T (2012) Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Plant Physiol 159:632–641.).
The P. sanguinolenta androgynophore is less sensitive to touch than thigmonastic Fabaceae leaflets and also does not respond to a dark or light stimulus (Scorza and Dornelas, 2014Scorza LC and Dornelas MC (2014) Rapid touch-stimulated movement in the androgynophore of Passiflora flowers (subgen. Decaloba; Sect. Xerogona): An adaptation to enhance cross-pollination? Plant Signal Behav 9:e27932.). Therefore, we tested the effects of auxin on the movement of the androgynophore of the model species, Passiflora sanguinolenta. Among the Passiflora species that present a motile androgynophore, we chose P. sanguinolenta because this species shows a more conspicuous movement than the others, thus being easier to observe. Additionally, as this species is evolutionary derived in relation to other Decalobaspecies, and adapted to hummingbird pollination (Scorza and Dornelas, 2014Scorza LC and Dornelas MC (2014) Rapid touch-stimulated movement in the androgynophore of Passiflora flowers (subgen. Decaloba; Sect. Xerogona): An adaptation to enhance cross-pollination? Plant Signal Behav 9:e27932.), we tested whether the flowers of the interspecific hybrid between P. sanguinolenta and the more basal, insect-pollinated P. capsularis inherited thigmotropic androgynophore features. We also tested the effects of a specific inhibitor of the auxin efflux, 1-N-naphthylphthalamicacid (NPA) on androgynophore movement. The effect of NPA has not been tested before in the context of active plant movements.
Material and Methods
Plants
Plants of Passiflora sanguinolenta Mast., Passiflora capsularis L. and their ornamental interspecific hybrid named Passiflora ‘capsang’ (Ulmer and MacDougal 2004Ulmer T and MacDougal JM (eds) (2004) Passiflora: Passion-flowers of the World. Timber Press, Inc., Portland, 430 pp.) were grown under greenhouse conditions at the Universidade Estadual de Campinas, Instituto de Biologia, Campinas, SP, Brazil.
Auxin and NPA treatments
To test whether the movement is influenced by exogenous application of auxin and an auxin polar transport inhibitor we prepared treatment solutions using indol-3-acetic-acid (IAA, SIGMA), and N-1-napthyl-phtalamic acid (NPA, Chem Service, West Chester, PA). IAA solution was made at a final concentration of 1 mM. The concentration of IAA was chosen based on Moyen et al. (2007)Moyen C, Bonmort J and Roblin G (2007) Membrane effects of 2,4-dichlorophenoxyacetic acid in motor cells of Mimosa pudicaL. Plant Physiol Biochem 45:420–426., where pulvinar movements were inhibited using 2,4-D, a synthetic auxin, at a final concentration of 0.1 mM. They argued that 2,4-D is about 10-fold more effective than IAA, so we used IAA for our experiments at 1 mM. The NPA final concentration was 0.1 mM, based on Petrasèk (2003)Petrásèk J, Cerna A, Schwarzerova K, Elckner M, Morris DA and Zazimalova E (2003) Do phytotropins inhibit auxin efflux by impairing vesicle traffic? Plant Physiol 131:254–263..
A preliminary test was made spraying NPA at 0.1 mM on the opened flowers still attached to the plant, and the movement tests were made 3 h after spraying. Alternatively, recently opened flowers of P. sanguinolenta were removed from the plant and directly transferred to water to avoid desiccation. Subsequently, the tips of the flower pedicels were cut with a sharp knife and immediately dipped in the treatment solutions (IAA 1 mM; NPA 0.1 mM and water as the control solution). These solutions were in Petri dishes covered with Parafilm, where tiny holes were made to fit the pedicels and keep the flowers in an upright position. The flowers were kept in the treatment solutions for about 3 h, as this was described as a period when, in general, the maximum effect was achieved in pulvinar movement experiments (Moyen et al., 2007Moyen C, Bonmort J and Roblin G (2007) Membrane effects of 2,4-dichlorophenoxyacetic acid in motor cells of Mimosa pudicaL. Plant Physiol Biochem 45:420–426.).
Recordings and calculations of the duration, angulation and speed of the movement
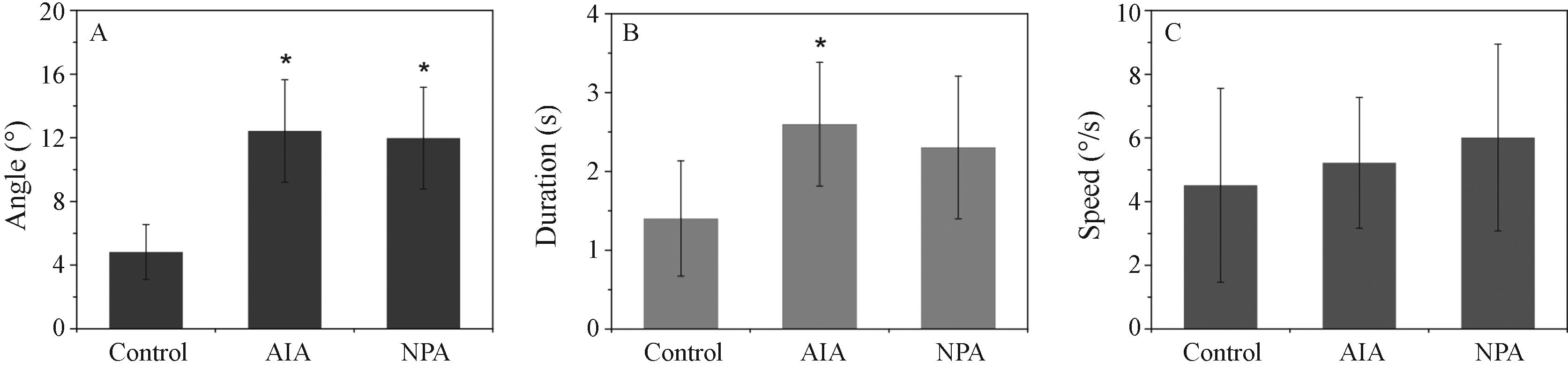
After treatment with hormonal solutions we transferred the flowers to wet floral foam and removed part of the perianth to visualize the entire androgynophore (Figure 1). The flowers were kept untouched for another 15 min as sometimes we touched the androgynophore when cutting off the perianth, inducing the thigmotropic movement. Finally, we recorded the mechanically induced movement of the androgynophore and calculated the duration, the angle formed between the steady state (before the movement) and the final state (after the movement) and speed as described previously (Scorza and Dornelas, 2014Scorza LC and Dornelas MC (2014) Rapid touch-stimulated movement in the androgynophore of Passiflora flowers (subgen. Decaloba; Sect. Xerogona): An adaptation to enhance cross-pollination? Plant Signal Behav 9:e27932.). The same protocol was used to evaluate untreated flowers of the parental species (P. capsularis and P. sanguinolenta) and their interspecific hybrid (P. ‘capsang’). Ten flowers from different individuals were used as replicates for each treatment. The results of the measurements presented in Figures 2 –4 are shown as mean values ± SD and asterisks indicate significant differences at p < 0.05 according to Kruskal-Wallis test.
Images of flowers of P. sanguinolenta showing the androgynophore before (A, B, C) and after (D, E, and F) mechano-stimulation, after being treated with water (control, A and D), IAA 1 mM (B and E) and NPA 0.1 mM (C and F). The images of the androgynophore before and after the movement were put exactly in the same position related to the X-axis to clearly illustrate the change in position. The movement amplitude can be measured by α1− α0, where α0 is the angle formed by the androgynophore axis before the movement (t0) and a line traced perpendicularly to the image; and α1 is the angle formed between the androgynophore axis after the movement (t1) and the same perpendicular line. Note that when IAA or NPA is applied the amplitude of the movement is clearly greater than in the control.
Effect of the IAA and NPA treatments on the movement dynamics of the P. sanguinolenta androgynophore. (A) Movement amplitude measured by the angle formed between the initial state (before the movement) and the final state (after the movement) in degrees. (B) Duration of the movement in seconds. (C) Angular speed of the movement in deg/s. Data represent means ± SD. Asterisks indicate statistical differences from the control (according to Kruskal-Wallis test).
Effect of spraying NPA on opened flowers. (A) Movement amplitude measured by the angle formed between the initial state (before the movement) and the final state (after the movement) in degrees. (B) Duration of the movement in seconds. (C) Angular speed of the movement in deg/s. The data shows that only spraying NPA is not sufficient to cause alteration in the androgynophore movement.
Movement dynamics of the androgynophore of P. capsularis, P. sanguinolenta and their interspecific hybrid P. ‘capsang’. (A) Movement amplitude measured by the angle formed between the initial state (before the movement) and the final state (after the movement) in degrees. (B) Duration of the movement in seconds. (C) Angular speed of the movement in deg/s. Data represent means ± SD. Asterisks indicate statistical differences between genotypes (Kruskal-Wallis test).
Results
When the flowers of P. sanguinolenta were immersed in IAA at a final concentration of 1 mM for 3 h the mean of the angle formed by the androgynophore trajectory before and after the movement was significantly higher than in the control, where flowers were immersed only in water (Figures 1 and 2). The time that the androgynophore took to bend after the mechanical stimulus was also increased in plants treated with IAA (Figure 2). Therefore, we did not find any difference when comparing the speed of the movement in flowers treated with IAA and the water control treatment (Figure 2).
Flowers that were immersed in the auxin polar transport inhibitor NPA also showed a significant increase in the values of the angle formed between the steady state and after the movement (Figures.1 and 2). When analyzing the duration of the movement we did not find a statistical difference between the NPA treated and the water control, albeit there was a tendency to an increase in time (Figure 2). Opened flowers of P. sanguinolenta sprayed with the NPA test solution did not show any difference in the dynamics of the movement compared to control flowers that were not sprayed (Figure 3).
As it has been previously suggested that the hummingbird-pollinated P. sanguinolenta is derived in relation to other insect-pollinated Decaloba species (Scorza and Dornelas, 2014Scorza LC and Dornelas MC (2014) Rapid touch-stimulated movement in the androgynophore of Passiflora flowers (subgen. Decaloba; Sect. Xerogona): An adaptation to enhance cross-pollination? Plant Signal Behav 9:e27932.), we tested whether the flowers of the interspecific hybrid between P. sanguinolenta and the insect-pollinated P. capsularis inherited thigmotropic androgynophore features. We observed that both P. capsularis and P. sanguinolenta presented similar angles formed by the androgynophore trajectory, but P. ‘capsang’ showed a much wider movement when compared to the parental species (Figure 4A). While the duration of the androgynophore movement was significantly shorter in P. capsularis when compared to P. sanguinolenta, it was even shorter in the interspecific hybrid (Figure 4B). Therefore, the speed that the P. ‘capsang’ androgynophore took to bend after the mechanical stimulus was more than twice the one observed for the parental species (Figure 4C).
Discussion
We tested whether exogenous application of auxin (IAA) or an inhibitor of its polar transport, NPA, would alter the thigmotropic movement pattern of androgynophores of P. sanguinolenta.
In a preliminary test we wanted to see whether spraying a solution containing NPA at 0.1 mM would generate any response on the movement. Our results showed that only spraying opened flowers was not sufficient to induce any effect on the movement, probably because the epidermis, together with the cuticle, was a barrier difficult to pass through. We did not intend to spray or treat young flower buds continuously with the "petiole-feeding" technique (Lin et al., 2011Lin YH, Lin MH, Gresshoff PM and Ferguson BJ (2011) An efficient petiole-feeding bioassay for introducing aqueous solutions into dicotyledonous plants. Nat Protoc 6:36–45.; Rocha et al., 2015Rocha DI, Monte-Bello C, Sobol S, Smach A and Dornelas MC (2015) Auxin and physical constraint exerted by the perianth promote the androgynophore bending in Passiflora mucronata L. (Passifloraceae). Plant Biol 17:639–643.) as this could alter the normal development of the flower organs (Benkova et al., 2003Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jürgens G and Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115:591–602.).
When we adopted a treatment procedure of dipping the flower pedicels at anthesis in the treatment solutions for 3 h we were able to detect effects of IAA and NPA on the androgynophore movement. We observed that flowers treated witha1mMIAA solution showed a significant increase in the amplitude of their androgynophore bending movement (Figures 1 and 2). This then caused an increase in the duration of the movement, as the androgynophore took more time to make the longer trajectory while the speed of the movement was unaltered when compared to the water control (Figure 2).
As we had previously shown that Passiflora androgynophore movement presents certain mechanistic similarities with pulvinar movements (Scorza and Dornelas, 2014Scorza LC and Dornelas MC (2014) Rapid touch-stimulated movement in the androgynophore of Passiflora flowers (subgen. Decaloba; Sect. Xerogona): An adaptation to enhance cross-pollination? Plant Signal Behav 9:e27932.; Scorza et al., 2014Scorza LC and Dornelas MC (2014) Rapid touch-stimulated movement in the androgynophore of Passiflora flowers (subgen. Decaloba; Sect. Xerogona): An adaptation to enhance cross-pollination? Plant Signal Behav 9:e27932.), it is worthy of note that when IAA or 2,4-D were applied to detached leaves of C. fasciculata, the amplitude of the leaflet opening induced by light increased, whereas the dark-induced closure of the leaflets was inhibited (Bourbouloux et al., 1992Bourbouloux A, Roblin G and Fleuratlessard P (1992) Calcium involvement in the IAA-induced leaflet opening of Cassia fasciculata. J Exp Bot 43:63–71.; Bonmort and Roblin, 1996Bonmort J and Roblin G (1996) Effect of 2,4-dichlorophenoxyacetic acid on the dark- and light-induced pulvinar movements in Cassia fasciculata Michx. Plant Growth Regul 19:61–65.). When M. pudica leaflets were mechanically stimulated in darkness after application of IAA, rapid closure and opening were observed, similarly to what happens in daylight (Watanabe and Sibaoka, 1983Watanabe S and Sibaoka T (1983) Light-Induced and auxin-induced leaflet opening in detached pinnae of Mimosa pudica. Plant Cell Physiol 24:641–647.). 2,4-D had a more drastic effect and inhibited touch-induced leaflet closure in M. pudica (Moyen et al., 2007Moyen C, Bonmort J and Roblin G (2007) Membrane effects of 2,4-dichlorophenoxyacetic acid in motor cells of Mimosa pudicaL. Plant Physiol Biochem 45:420–426.). The mechanism by which auxins influence the pulvinar movements is the activation of H+-ATPase proton pumps in the plasma membrane, leading to ion and water influx to the cells. This process leads to an increase in turgor pressure and the pulvinar cells are kept constantly turgid, preventing the leaflets to fold up during the dark induction period, and increasing the amplitude of the leaflet opening during the light induction. In P. sanguinolenta a similar effect of increased turgidity might have occurred, but the turgidity was not refractory to touch as sometimes also happened with Fabaceae thigmonastic leaflets treated with auxins, and where the androgynophore was still inclined in response to a mechanical stimulus (Samejima and Sibaoka, 1980Samejima M and Sibaoka T (1980) Changes in the extracellular ion concentration in the main pulvinus of Mimosa pudica during rapid movement and recovery. Plant Cell Physiol 21:467–479.; Moran, 2007Moran N (2007) Osmoregulation of leaf motor cells. FEBS Lett 581:2337–2347.; Volkov et al., 2010aVolkov AG, Foster JC, Ashby TA, Walker RK, Johnson JONA and Markin VS (2010a) Mimosa pudica: Electrical and mechanical stimulation of plant movements. Plant Cell Environ 33:163–173.). We have already shown that the basis of the movement of Passiflora motile androgynophores is the swelling and shrinking of the androgynophore epidermis and parenchyma cells (Scorza et al., 2014Scorza LC and Dornelas MC (2014) Rapid touch-stimulated movement in the androgynophore of Passiflora flowers (subgen. Decaloba; Sect. Xerogona): An adaptation to enhance cross-pollination? Plant Signal Behav 9:e27932.). If the cells get more turgid it is more likely that they also have the potential to lose more water when plasmolysed after being touch-stimulated. This would explain the increase in the amplitude of the P. sanguinolenta androgynophore movement, as seen after IAA treatment.
Auxin is a weak organic acid that enters the cell easily through diffusion across the plasma membrane (Zazímalová et al., 2014Zazímalová E, Petrásek J and Benková E (2014) Auxin and its role in plant development. Springer, Wien, 442 pp.). When inside the cells, most of the auxin dissociates in the anionic form, which makes it more difficult to be transported out of the cells. Auxin efflux carrier proteins promote the transport of auxin from cell to cell in a directional manner (Benkova et al., 2003Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jürgens G and Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115:591–602.). This process is commonly referred to as auxin polar transport, and it has been related to various aspects of plant development. The polar transport inhibitor NPA is probably the most effective inhibitor of auxin polar transport (Petrásèk et al., 2003Petrásèk J, Cerna A, Schwarzerova K, Elckner M, Morris DA and Zazimalova E (2003) Do phytotropins inhibit auxin efflux by impairing vesicle traffic? Plant Physiol 131:254–263.). NPA impairs the auxin polar efflux, but has no influence on decreasing IAA concentration or activity; on the contrary, it can increase auxin accumulation in the cells (Morris et al., 2005Morrys SE, Cox MCH, Ross JJ, Krisantini S and Beveridge CA (2005) Auxin dynamics after decapitation are not correlated with the initial growth of axillary buds. Plant Physiol 138:1665–1672.; Petrásèk et al., 2003Petrásèk J, Cerna A, Schwarzerova K, Elckner M, Morris DA and Zazimalova E (2003) Do phytotropins inhibit auxin efflux by impairing vesicle traffic? Plant Physiol 131:254–263.). In our experiments, when NPA was applied to P. sanguinolenta flowers, a very similar effect to IAA treatments was observed, as the amplitude of the androgynophore movement was also increased.
In Arabidopsis, stamina are major sites of IAA accumulation during flower development (Aloni et al., 2006Aloni R, Aloni E, Langhans M and Ullrich CI (2006) Role of auxin in regulating Arabidopsis flower development. Planta 223:315–328.). As well as in many other species, Arabidopsispetals only develop after stamina have almost fully developed. It has been shown that the high IAA production in young organs, especially in stamina, inhibits the development of other organs, such as petal elongation (Aloni et al., 2006Aloni R, Aloni E, Langhans M and Ullrich CI (2006) Role of auxin in regulating Arabidopsis flower development. Planta 223:315–328.). The androgynophore column only develops at later stages of P. sanguinolentadevelopment, after the stamina and the gynoecium have already developed (our observations). The androgynophore column elongates concomitantly with petals and corona filaments, which also develop later during flower bud formation, suggesting that a mechanism similar to stamen inhibition by auxin might be involved in P. sanguinolenta flower development. Under the hypothesis that the tip of each floral organ is a primary site of auxin production that can induce its own development and differentiation, and sometimes inhibit the growth of neighboring organs (Aloni et al., 2006Aloni R, Aloni E, Langhans M and Ullrich CI (2006) Role of auxin in regulating Arabidopsis flower development. Planta 223:315–328.), it is reasonable to assume that the androgynophore, including the column, the stigma and the gynoecium, also produce auxin, which might be transported basipetally. When NPA is applied, the auxin that might be produced by the P. sanguinolenta androgynophore would not be transported and, thus, accumulate at its base, generating a similar response as when exogenous IAA is applied.
In Passiflora there are many examples of flowers in which selective pressure has driven the evolution of novel mechanisms that impact on the reproduction and survival (Lindberg and Olesen, 2001Lindberg AB and Olesen JM (2001) The fragility of extreme specialization: Passiflora mixta and its pollinating hummingbird Ensifera ensifera. J Trop Ecol 17:323–329.; Aizza and Dornelas, 2011Aizza LCB and Dornelas MC (2011) A genomic approach to study anthocyanin synthesis and flower pigmentation in passionflowers. J Nucleic Acids 2011:1–17.; Rocha et al., 2015Rocha DI, Monte-Bello C, Sobol S, Smach A and Dornelas MC (2015) Auxin and physical constraint exerted by the perianth promote the androgynophore bending in Passiflora mucronata L. (Passifloraceae). Plant Biol 17:639–643.). In the flowers of P. sanguinolenta, P. citrina, P. capsularis and P. rubra, the motile androgynophore seems to be a novel feature that maximizes pollen deposition onto pollinators - hummingbirds in species with tubular flowers such as P. sanguinolenta and P. citrina and insects in bowl shaped flowers, such as those of P. capsularis and P. rubra. As we mentioned, the cellular basis of the movement is a subtle loss of turgor in cells at the stimulated side of the androgynophore, which is capable of turgor recovery within minutes, a mechanism that enables the organ to respond to a new stimulus, i.e., other pollinators visiting the flower (Scorza and Dornelas, 2014Scorza LC and Dornelas MC (2014) Rapid touch-stimulated movement in the androgynophore of Passiflora flowers (subgen. Decaloba; Sect. Xerogona): An adaptation to enhance cross-pollination? Plant Signal Behav 9:e27932.; Scorza et al., 2014Scorza LC and Dornelas MC (2014) Rapid touch-stimulated movement in the androgynophore of Passiflora flowers (subgen. Decaloba; Sect. Xerogona): An adaptation to enhance cross-pollination? Plant Signal Behav 9:e27932.). Auxins are implied in maintaining the cell turgor. Accordingly, the cells of the androgynophore in our study were sensitive to the application of this hormone, showing as a phenotypic response an increase in the amplitude of the movement, which, in turn, has a potential role in the process of pollen transfer onto pollinators. This process seems to be especially decisive on the reproduction of self-incompatible species, as P. sanguinolenta and P. citrina (Scorza and Dornelas, 2014Scorza LC and Dornelas MC (2014) Rapid touch-stimulated movement in the androgynophore of Passiflora flowers (subgen. Decaloba; Sect. Xerogona): An adaptation to enhance cross-pollination? Plant Signal Behav 9:e27932.). Taken together, these data put in evidence a probable role of auxin in modulating the fitness of these Decaloba species to different pollinators.
Another issue is whether the thigmotropic androgynophore features are adaptively inherited for their potential role in the evolution of flower-pollinator relationships in Passiflora. It has been suggested that hummingbird-pollinated Xerogona species, such as P. citrina and P. sanguinolenta, are derived in relation to other insect-pollinated Decaloba species (Milward-de-Azevedo et al.,, 2014Milward-de-Azevedo MA, Freitas LB and Kinoshita LS (2014) Taxonomy and evolutionary relationships of Passiflora subg. Decaloba supersect. Decaloba sect. Xerogona (Passifloraceae): Contributions of palynological, morphological and molecular studies. Acta Bot Bras 28:301–308.). We therefore infer that the wide-moving androgynophores, characteristic of hummingbird-pollinated species (such as P. sanguinolenta) are derived with respect to insect-pollinated species with more ‘restricted’ movement (such as P. capsularis). As artificial interspecific hybrids, used as ornamental plants, are widely available among Passiflora species (Ulmer and MacDougal, 2004Ulmer T and MacDougal JM (eds) (2004) Passiflora: Passion-flowers of the World. Timber Press, Inc., Portland, 430 pp.), we assessed the thigmotropic androgynophore features of P. ‘capsang’ and its parental species, P. capsularis and P. sanguinolenta. We observed that the androgynophore movement of the hybrid is two-times faster than that of the parental species. Although we cannot discard the hypothesis that this particular phenomenon is of epigenetic nature, it seems more likely that it can be explained as a ‘hybrid heterosis effect’ (Birchler et al., 2003Birchler JA, Auger DL and Riddle NC (2003) In search of the molecular basis of heterosis. Plant Cell 15:2236–2239.). Future studies on the gene expression profiles of the parental species and the interspecific hybrid should shed light on this unresolved issue. Although the genetic nature of this heterosis remains to be determined, our results suggest that the characteristic features (such as speed) of the androgynophore movement are prone to be under evolutionary pressure. Taken together and assuming that faster androgynophore movement can provide a greater fitness to flowers that are pollinated by hummingbirds, our results suggest general mechanisms by which hummingbird-pollinated flowers can arise from insect-pollinated ancestors.
Acknowledgments
We acknowledge funding by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo, Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes, Brazil) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil).
-
Associate Editor: Igor Schneider
References
- Aizza LCB and Dornelas MC (2011) A genomic approach to study anthocyanin synthesis and flower pigmentation in passionflowers. J Nucleic Acids 2011:1–17.
- Aloni R, Aloni E, Langhans M and Ullrich CI (2006) Role of auxin in regulating Arabidopsis flower development. Planta 223:315–328.
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jürgens G and Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115:591–602.
- Birchler JA, Auger DL and Riddle NC (2003) In search of the molecular basis of heterosis. Plant Cell 15:2236–2239.
- Blatt MR and Thiel G (1994) K+ channels of stomatal guard cells: Bimodal control of the K+ inward-rectifier evoked by auxin. Plant J 5:55–68.
- Bonmort J and Roblin G (1996) Effect of 2,4-dichlorophenoxyacetic acid on the dark- and light-induced pulvinar movements in Cassia fasciculata Michx. Plant Growth Regul 19:61–65.
- Bourbouloux A, Roblin G and Fleuratlessard P (1992) Calcium involvement in the IAA-induced leaflet opening of Cassia fasciculata J Exp Bot 43:63–71.
- Braam J (2005) In touch: Plant responses to mechanical stimuli. New Phytol 165:373–389.
- Campbell NA and Garber RC (1980) Vacuolar reorganization in the motor cells of Albizzia during leaf movement. Planta 148:251–255.
- Fleurat-Lessard P and Millet B (1984) Ultrastructural features of cortical parenchyma cells (motor cells) in stamen filaments of Berberis canadensis Mill and tertiary pulvini of Mimosa pudica L. J Exp Bot 35:1332–1341.
- Fleurat-Lessard P, Bouché-Pillon S, Leloup C and Bonnemain J-l (1997) Distribution and activity of the plasma membrane H+-ATPase in Mimosa pudica L. in relation to ionic fluxes and leaf movements. Plant Physiol 13:747–754.
- Henning T and Weigend M (2012) Total control - pollen presentation and floral longevity in Loasaceae (blazing star family) are modulated by light, temperature and pollinator visitation rates. PloS One 7:e41121.
- Iino M, Long C and Wang X (2001) Auxin- and abscisic acid-dependent osmoregulation in protoplasts of Phaseolus vulgaris pulvini. Plant Cell Physiol 42:1219–1227.
- Jaffe MJ, Gibson C and Biro R (1977) Physiological studies of mechanically stimulated motor responses of flower parts.1. Characterization of thigmotropic stamens of Portulaca grandiflora Hook. Bot Gaz 138:438–447.
- Lin YH, Lin MH, Gresshoff PM and Ferguson BJ (2011) An efficient petiole-feeding bioassay for introducing aqueous solutions into dicotyledonous plants. Nat Protoc 6:36–45.
- Lindberg AB and Olesen JM (2001) The fragility of extreme specialization: Passiflora mixta and its pollinating hummingbird Ensifera ensifera J Trop Ecol 17:323–329.
- Milward-de-Azevedo MA, Freitas LB and Kinoshita LS (2014) Taxonomy and evolutionary relationships of Passiflora subg. Decaloba supersect. Decaloba sect. Xerogona (Passifloraceae): Contributions of palynological, morphological and molecular studies. Acta Bot Bras 28:301–308.
- Moran N (2007) Osmoregulation of leaf motor cells. FEBS Lett 581:2337–2347.
- Morrys SE, Cox MCH, Ross JJ, Krisantini S and Beveridge CA (2005) Auxin dynamics after decapitation are not correlated with the initial growth of axillary buds. Plant Physiol 138:1665–1672.
- Moyen C, Bonmort J and Roblin G (2007) Membrane effects of 2,4-dichlorophenoxyacetic acid in motor cells of Mimosa pudicaL. Plant Physiol Biochem 45:420–426.
- Petrásèk J, Cerna A, Schwarzerova K, Elckner M, Morris DA and Zazimalova E (2003) Do phytotropins inhibit auxin efflux by impairing vesicle traffic? Plant Physiol 131:254–263.
- Rocha DI, Monte-Bello C, Sobol S, Smach A and Dornelas MC (2015) Auxin and physical constraint exerted by the perianth promote the androgynophore bending in Passiflora mucronata L. (Passifloraceae). Plant Biol 17:639–643.
- Samejima M and Sibaoka T (1980) Changes in the extracellular ion concentration in the main pulvinus of Mimosa pudica during rapid movement and recovery. Plant Cell Physiol 21:467–479.
- Schlindwein C and Wittmann D (1997) Stamen movements in flowers of Opuntia (Cactaceae) favour oligolectic pollinators. Plant Syst Evol 204:179–193.
- Scorza LCT and Dornelas MC (2011) Plants on the move: Towards common mechanisms governing mechanically-induced plant movements. Plant Signal Behav 6:1979–1986.
- Scorza LC and Dornelas MC (2014) Rapid touch-stimulated movement in the androgynophore of Passiflora flowers (subgen. Decaloba; Sect. Xerogona): An adaptation to enhance cross-pollination? Plant Signal Behav 9:e27932.
- Scorza LCT, Rossi ML and Dornelas MC (2014) Vacuolar remodelling mediates touch-induced androgynophore movement in Passiflora(Subg. Decaloba, Sect. Xerogona) flowers. Flora 209:613–619.
- Sibaoka T (1991) Rapid plant movements triggered by action potentials. Bot Mag Tokyo 104:73–95.
- Singh AK, Prabhakar S and Sane SP (2011) The biomechanics of fast prey capture in aquatic bladderworts. Biol Lett rsbl.2011.0057.
- Takahashi K, Hayashi K and Kinoshita T (2012) Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis Plant Physiol 159:632–641.
- Ulmer T and MacDougal JM (eds) (2004) Passiflora: Passion-flowers of the World. Timber Press, Inc., Portland, 430 pp.
- Volkov AG, Adesina T, Markin VS and Jovanov E (2008) Kinetics and mechanism of Dionaea muscipula trap closing. Plant Physiol 146:694–702.
- Volkov AG, Foster JC, Ashby TA, Walker RK, Johnson JONA and Markin VS (2010a) Mimosa pudica: Electrical and mechanical stimulation of plant movements. Plant Cell Environ 33:163–173.
- Volkov AG, Pinnock MR, Lowe DC, Gay MS, and Markin VS (2010b) Complete hunting cycle of Dionaea muscipula: Consecutive steps and their electrical properties. J Plant Physiol 168:109–120.
- Watanabe S and Sibaoka T (1983) Light-Induced and auxin-induced leaflet opening in detached pinnae of Mimosa pudica Plant Cell Physiol 24:641–647.
- Zazímalová E, Petrásek J and Benková E (2014) Auxin and its role in plant development. Springer, Wien, 442 pp.
Publication Dates
-
Publication in this collection
21 Aug 2015 -
Date of issue
July-Sept 2015
History
-
Received
18 Dec 2014 -
Accepted
15 Apr 2015