SPECIAL ARTICLE
Guidelines of the Brazilian society of bone Marrow transplantation on hematopoietic stem cell transplantation as a treatment for the autoimmune diseases systemic sclerosis and multiple sclerosis
Maria Carolina de Oliveira RodriguesI; Nelson HamerschlakII; Daniela Aparecida de MoraesI; Belinda Pinto SimõesI; Morgani RodriguesII; Andreza Alice Feitosa RibeiroII; Júlio César VoltarelliI, (in memoriam)
IHospital das Clínicas de Ribeirão Preto, Universidade de São Paulo - USP, Ribeirão Preto, SP, Brazil
IIHospital Israelita Albert Einstein - HIAE, São Paulo, SP, Brazil
Corresponding author Corresponding author: Nelson Hamerschlak Hospital Israelita Albert Einstein Av Albert Einstein 627, sala 1203 Morumbi 05651-901 - São Paulo, SP, Brazil Phone 55 11 3747-3203 hamer@einstein.br
Introduction
Hematopoietic stem cell transplantation has been used with increasing frequency over the last 16 years, albeit still experimentally. Data is available from the International Bone Marrow Transplant Registry (IBMTR) totaling more than 300 transplant recipients by 2008(1). In 2009, a Consensus Meeting was held to review available evidence on the subject of autoimmune diseases(2). The procedure has improved and the incidence of complications and mortality decreased over time. There are still many points of discussion, some of which should be clarified by ongoing randomized studies.
This consensus paper aims to gather data available in the international literature and to compare it to the Brazilian experience in order to establish evidence-based recommendations for the application of hematopoietic stem cell transplantation (HSCT) in the treatments of systemic and multiple sclerosis in Brazil.
Systemic sclerosis
Systemic sclerosis is an autoimmune disease in which the skin and, in most cases, internal organs are involved. The clinical evolution usually begins from vascular hyperreactivity and endothelial changes associated with inflammatory phenomena that result in progressive tissue damage, leading to fibrosis. The etiology is still unknown, but it certainly comes from the interaction between genetic predisposition and environmental stimuli that cause an immune imbalance and tissue lesions. Cardiopulmonary involvement is common and affects up to 80% of patients(1). The response to conventional immunosuppression is usually poor and the mortality rate among patients with diffuse cutaneous and/or visceral involvement ranges from 30 to 50% in 5 years(1,3). The most frequent causes of death are cardiac involvement and, secondly, pulmonary involvement. A recently published meta-analysis showed that the mortality rate has not decreased in recent years despite the introduction of new therapeutic options(4).
Conventional treatments
Systemic sclerosis is a challenge to the available conventional therapies. Corticosteroids and immunosuppressants commonly used and successful in the treatment of rheumatic diseases have little influence on the course of systemic sclerosis. More recently, antifibrotic drugs such as penicillamine, and endothelin receptor antagonists, including bosentan, have also been prescribed but with limited responses. Other drugs, such as mycophenolate mofetil, imatinib and dasatinib have also been used, but with no conclusive results(5).
Randomized studies conducted in recent years have shown conflicting results. In 1996, van der Hoogen et al. published a study comparing methotrexate with placebo in the treatment of early stage systemic sclerosis(6). There was significant cutaneous improvement in the group treated with low dose methotrexate (15 mg/week), but the follow-up time was short (between 24 and 48 weeks) and only 11/29 (38%) of the patients had the diffuse type of disease. Besides, there were more patients with the limited disease in the placebo group than in the group treated with methotrexate (12 versus six, respectively). A later, double blind, randomized study, evaluated 71 patients with the early form of systemic sclerosis, with a follow-up time of one year. In this study, unlike the previous one, there were no differences between the groups, suggesting a lack of efficacy of methotrexate to treat systemic sclerosis(7).
In 2002, an English study evaluated 14 patients with interstitial lung involvement by systemic sclerosis, who underwent monthly pulses of cyclophosphamide and methylprednisolone(8). High-resolution computed tomography (CT) scans and evaluations of the diffusion of carbon monoxide (CO) showed improvement in pulmonary symptoms after six months of pulses, a response that remained after six months of observation, once the pulses were suspended. However, at a mean period of 26 months, 67% of patients again presented progression of the pulmonary involvement. In 2006, a multicenter, prospective, double-blind study, also from England, evaluated 45 patients with pulmonary involvement, divided into two groups. The first group received low-dose corticosteroids plus six monthly pulses of cyclophosphamide followed by oral azathioprine for maintenance, and the second group received placebo. Patients were followed for around 12 months, but there were no differences between groups(9). In the same year, a U.S. group evaluated 145 patients with pulmonary involvement in a randomized, double-blind and prospective study. There was a slight, but significant improvement of pulmonary function, skin thickening and increase in quality of life of patients treated with oral cyclophosphamide for one year(10,11).
In recent years, tyrosine kinase inhibitors, particularly imatinib mesylate, have been experimentally applied in systemic sclerosis, but with no conclusive results. A North American phase I/IIA study showed only a tendency of the drug to promote increased forced vital capacity of the lung and improvements in the Rodnan score(12). The results were hampered by poor compliance of the patients to the high doses of medication prescribed. Similarly, a study published in 2011, with a small number of patients, was interrupted early due to poor tolerance to the drug. Efficacy of treatment was not, therefore, observed(13). More recently, a Chinese case series described cutaneous improvement and stabilization of pulmonary involvement in patients treated with low doses of imatinib(14). Furthermore, a review gathered data from 108 patients previously reported in small clinical studies, concluding that, in selected cases, imatinib mesylate may be beneficial(15). Further investigations are needed.
Although not recognized by the European Society of Rheumatology (EULAR) as a therapeutic approach to systemic sclerosis, extracorporeal photopheresis (ECP) has been applied in some centers as a treatment for the cutaneous forms of systemic sclerosis(6,9,16-19). Case reports show stabilization or even improvement in patients with milder and initial forms of the skin disease, but a randomized trial comparing ECP sessions with placebo failed to show a significant difference between groups(20). However, as there was a trend to better results in the ECP group, new studies with a larger number of patients investigating this will probably be started soon. Additionally, none of the studies demonstrated improvement or stabilization of the visceral involvement of the disease.
Finally, biologic products have been applied to the treatment of systemic sclerosis. Studies with infliximab and etanercept, both inhibitors of the tumor necrosis factor (TNF) pathway, suggest improvement of joint inflammation and quality of life. Other agents, such as rituximab, antithymocyte globulin, and others, failed to demonstrate significant results(21).
Autologous hematopoietic stem cell transplantation: international experience
Much of the information on HSCT for systemic sclerosis comes from multicenter, non-randomized, North American and European studies, which have been published since 2001. The European record of transplants reported in 2004 experience with 57 patients receiving HSCT(21). Of these, 50 had the diffuse cutaneous form of the disease and 40 had some type of pulmonary involvement, such as interstitial lung disease or pulmonary hypertension. There was a wide variety of schemes used, but the predominant conditioning regimen was high-dose cyclophosphamide with or without antithymocyte globulin (ATG). Approximately 60 to 70% of the patients had a significant and lasting improvement of the skin, and the pulmonary involvement stabilized. Five (8.6%) patients died from causes associated with transplantation and eight (14%) due to disease progression in an average follow up of 22 months. About 35% of patients showed disease progression on average 10 months post-transplant. When the data were compared with those of the first publication of the same group, in 2001, improved survival and decreased transplant-related mortality (TRM) were seen(21,22).
In 2007 a US multicenter group, led by Dr. Nash, published a series of 34 cases of patients with diffuse cutaneous involvement, who underwent conditioning with total body irradiation (TBI), cyclophosphamide and ATG(17). This myeloablative regimen has brought some criticism from other transplant groups, who advocate less toxic regimens(23). Significant skin improvement and stabilization of pulmonary, kidney and heart function were observed. Unprecedentedly in this study, the improvement of skin involvement was confirmed by comparative skin biopsies performed before and six months after transplantation. There were 12 deaths during the study, eight related to transplantation and four due to disease progression. The progression-free survival and overall survival were estimated at 64%.
The transplant center at Northwestern University, in Chicago, presented a small case series published in 2007(24). Their results are of great relevance due to the experience of Dr. Burt with HSCT for autoimmune diseases, especially for the low rate of deaths related to the procedure. Ten cases with the diffuse type of the disease were described, impairment of at least one organ (lungs, heart or gastrointestinal tract). The patients underwent non-myeloablative conditioning with cyclophosphamide (120 mg/kg) and rabbit ATG (7.5 mg/kg) followed by the infusion of unselected in vitro autologous cells. All patients had gastrointestinal involvement and interstitial lung disease, but none had pulmonary hypertension or significant renal changes. All patients had initial skin improvement but two had progression of the disease after transplantation. Moreover, all patients had their lung, heart and kidney function stabilized. There was only one death, unrelated to the transplant. Overall survival was 90% and progression-free survival was 70%.
The longest follow-up period in studies was reported in 2008 by the consortium of one French and two Dutch centers(25). In that study, 26 patients underwent autologous transplantation, all conditioned with 200 mg/kg of cyclophosphamide and rescued with cells previously selected for CD34+ concentration. Twelve patients had diffuse cutaneous involvement and 14 visceral involvement, particularly of the lungs. In an average follow up of 5.3 years, 81% of patients showed clinical improvement with transplantation. There was also significant improvement of skin condition in 94% of patients and stabilization of pulmonary, renal and cardiac functions. The estimated overall survival (calculated by the Kaplan-Meier method) was 96.2% at five years and 86.8% in seven years. Six (28%) patients had disease reactivation after transplantation, requiring additional immunosuppressive treatment. Of these, only one developed disease progression.
The recent publication from a German group brought the results of 26 patients submitted to autologous HSCT, who had been conditioned with cyclophosphamide and ATG and received infusions of selected CD34+ cells(26). A significant improvement in skin and lung function was seen in 78.3% of patients during six months of follow up. Three patients died between mobilization and conditioning, two due to progression to severe disease and one for treatment-related toxicity. During the 4.4 years of follow-up, seven patients relapsed. The progression-free survival was 74%. Four patients died during follow-up with the most frequent causes being cardiac and pulmonary complications of systemic sclerosis.
Although autologous HSCT has consolidated as an alternative therapy for the treatment of systemic sclerosis, high rates of disease progression stimulate the search for more aggressive treatment regimens. In Seattle, two patients with severe lung disease received myeloablative allogeneic HSCT. Both presented clinical responses, but one of them evolved with fatal sepsis due to Pseudomonas, 18 months after transplantation(27). In Chicago, one patient received allogeneic non-myeloablative HSCT with a progressive improvement of skin disease, complete donor chimerism and without graft versus host disease (GVHD) or infectious complications(28). The same occurred in Houston, Texas(27). In Japan, the case of a female patient with interstitial lung disease who underwent non-myeloablative allogeneic transplantation, together with TBI and fludarabine, was recently reported. One year after transplantation, she developed membranous glomerulonephritis due to GVHD which remitted with corticosteroids. The skin score improved dramatically and lung function remained stable for four years after the procedure(29). Overall, individual reports of allogeneic transplants have described encouraging results, but they are still insufficient to prove safety and superiority to autologous transplants.
Three randomized studies were designed, with similar inclusion criteria and control groups treated with cyclophosphamide to comparatively assess the effects of autologous transplantation for systemic sclerosis. The ASTIS (European Multicenter Autologous Stem Cell Transplantation International Scleroderma) study(30), with a non-myeloablative conditioning regimen (cyclophosphamide and rabbit ATG), selected CD34+ cells positively, while the US study (ASSIST)(28) infused not selected cells. In the third research, the multicenter North American Scleroderma: Cyclophosphamide or Transplantation (SCOT) study, cyclophosphamide pulses were compared to myeloablative autologous transplantation performed with TBI plus 120 mg/kg of cyclophosphamide and horse ATG(3).
The ASSIST study was the first to publish results. Held at Northwestern University, Chicago, this phase II study compared patients receiving cyclophosphamide (1 g/m² intravenous monthly for six months) with transplant performed with cyclophosphamide (200 mg/kg) and rabbit ATG (6.5 mg/kg) without selection of the infused CD34+ cells. From 2006 to 2009, 19 patients were screened, and of these, ten were randomized to undergo transplant. All ten patients had improved Rodnan scores and forced vital capacity scores within 12 months of follow up. Of the nine patients receiving monthly cyclophosphamide, eight evolved with disease progression, and seven were transplanted. Improvements in Rodnan score and forced vital capacity persisted in a two-year post-transplant follow-up of 11 patients(31).
For the ASTIS study, patient recruitment was closed in 2009 and the results of the 156 patients included will be released in the coming months. These studies bring answers regarding the real efficacy and safety of autologous HSCT in the treatment of systemic sclerosis.
Experience with HSCT for systemic sclerosis since 1996, when it was first used, has shown some difficulties in the management of the disease during the procedure. Among autoimmune diseases, systemic sclerosis has the highest rates of TRM most likely due to visceral involvement and the poor clinical condition of these patients, including malnutrition, skin ulcers and prior chronic use of immunosuppressive drugs. Cardiopulmonary involvement, which is often subclinical and not evidenced by echocardiography, is worrisome, because it contributes to the poor outcome of transplantation. Currently, there is great concern in selecting appropriate patients to be transplanted, and detecting subtle cardiac dysfunction, which may worsen during and after the procedure.
Non-myeloablative conditioning regimens show remission rates similar to myeloablative regimens, but with lower TRM. In the multicenter, North American study, all patients received myeloablative conditioning with TBI and horse ATG(17). This regimen was criticized by Dr. Burt, who, in his experience, considered the non-myeloablative regimen equally effective and less toxic, taking into account the cardiac lability of these patients(23). In the European multicenter group study, there was greater variation between conditioning regimens, but among the 57 patients included in the study, at least 47 were conditioned with high-dose cyclophosphamide (non-myeloablative), with or without ATG. The three studies showed similar results as to the control of the disease, but the study with the myeloablative regimen showed a TRM of 23.4%, while with the non-myeloablative regimens the TRM was 8.6% (European Group), and zero (Chicago Group).
Among myeloablative conditioning regimens, HSCT for autoimmune diseases mostly employs high-dose cyclophosphamide. The vast experience with the drug has overcome the learning curve, which contributes to the reduction of complications related to the transplant. Cyclophosphamide, however, is known to be toxic for the heart and it can generate dose-dependent myocardial hemorrhage and edema which manifest by acute, severe, potentially fatal heart failure(16). Patients with cardiac involvement should therefore be assessed individually for drug substitution or suspension of transplantation. Melphalan and fludarabine-containing regimens may also be associated with cardiac toxicity, although there are no descriptions of such toxic events in transplantation for autoimmune diseases(32). Komatsuda et al. described HSCT applied to a patient with cardiac involvement using thiotepa and cyclophosphamide in small doses (100 mg/kg) as a conditioning regimen. There was no toxicity during transplantation and the patient was in disease remission(33).
In systemic sclerosis, there may be involvement of the electrical impulse conduction system of cardiac contractility, besides pulmonary hypertension and, less commonly, coronary disease(16,34). Conventional cardiac tests used in the pre-transplant evaluation, such as an electrocardiogram or echocardiogram may fail to detect myocardial involvement, especially diastolic dysfunction. Recently, some publications have addressed the issue by proposing algorithms for pre-transplant evaluation in order to detect patients at high risk(16,35). In 2007 Miniati et al. proposed a sequence of steps to be followed in the investigation of cardiac dysfunction in patients with systemic sclerosis(16). The first step would be a careful clinical cardiac evaluation, followed by electrocardiography (ECG) and chest radiography. Then, echocardiogram, serum atrial natriuretic peptide dosage and the troponin curve would be used to assess myocardial involvement and Holter monitoring to evaluate conduction disturbances. In case of abnormalities found in the previous steps, stress ventriculography and even cardiac catheterization should follow, the latter if ischemic areas are detected. Burt et al. also suggest detailed cardiac evaluations, including invasive and costly tests, such as right heart catheterization and magnetic resonance imaging (MRI)(36).
Many centers select the cells infused into the patient by removing T cells and concentrating CD34+ cells. Not all researchers, however, agree with this procedure(37,38). In addition to increasing the cost of transplant, the selection reduces the number of stem cells available for infusion and predisposes the material to potential contamination by handling(18,39). The benefits, moreover, are questionable. Snowden et al. found that patients with rheumatoid arthritis undergoing transplant with or without T-cell depletion had the same remission rate(37,38). Other centers, including Chicago, do not use the depletion of T cells in their most recent protocols(5,24). This issue is not yet clarified and a randomized study, selecting CD34+ or not, is being planned in Europe.
HSCT for systemic sclerosis has shown great impact on the skin of patients with significant reduction of skin fibrosis, which is associated with recovery of joint range of motion, decreased incidence of extremity ulcers and improvement in quality of life(17,21,24,25). Additionally, patients with diffuse skin disease, even if isolated, without visceral involvement, have poor long-term survival, thus justifying the risks of transplantation(40).
Deficiencies in the perfusion of extremities and digital ulcers are frequent and debilitating manifestations in patients with systemic sclerosis. Miniati et al. describe recovery of impaired nail bed capillaries, showing angiogenesis and revascularization of ischemic areas in six patients after transplant(41). The angiogenic effect of stem cells was also demonstrated by Nevskaya et al. in 2009, in a study of local injections of cells from marrow or peripheral blood in the palms and calves of patients with severe impairment of circulation and ulcers of the extremities(42). The patients had significant improvement in irrigation of treated limbs and healing of much of the ischemic ulcers.
Autologous hematopoietic stem cell transplantation: national experience
In Brazil, the first autologous HSCT for systemic sclerosis was performed in 1999 at the Pontifícia Universidade Católica do Rio Grande do Sul (PUC-RS) in Porto Alegre, in a patient with cutaneous, pulmonary and digestive tract involvement, who received low dose conditioning (1.5 g/m2 of cyclophosphamide and 105 mg/m2 of fludarabine) followed by infusion of 2 x 106 of autologous hematopoietic stem cells (HSC) per kg. There was a transient improvement of the intestinal problem, but three months after transplantation, he presented a hypertensive crisis and intestinal relapse, when once more the immunosuppressive regimen was administered (three and five months post-transplant). However, one year after transplantation, there was a bowel obstruction, probably due to recurrence of the underlying disease, aspiration pneumonia and death(43).
In an International Workshop held in Ribeirão Preto in October 2000, attended by transplant specialists from Europe, the United States and the main groups in Brazil, together with specialists in autoimmune diseases, it was decided to start a nationwide cooperative pilot project (phases I/II) of HSCT for autoimmune diseases coordinated by the Center for Cellular Therapy, Blood Center of Ribeirão Preto and the Bone Marrow Transplant (BMT) Unit of the Hospital das Clínicas, FMRPUSP. The transplants were started in June 2001, primarily in severe forms of systemic lupus erythematosus (SLE), systemic sclerosis and multiple sclerosis refractory to conventional therapy, using autologous unmanipulated HSC with in vivo depletion of T cells and ATG(2) .
Until May 2012, 36 patients with systemic sclerosis were included in the Brazilian protocol, 32 from the University Hospital of the Universidade de São Paulo (USP) in Ribeirão Preto and four from other services. Three were not transplanted: one patient died before transplantation due to disease reactivation and post-mobilization infectious complications and two had great improvement of the skin after mobilization with cyclophosphamide and granulocyte colony-stimulating factor (G-CSF) and opted not to pursue the conditioning and transplantation. Of the 33 transplants performed, all patients had diffuse cutaneous involvement. Twenty-three also had pulmonary interstitial type involvement and pulmonary hypertension. In 22 patients, esophageal involvement by systemic sclerosis was identified, and in four, cardiac involvement. The mean time with the disease prior to the HSCT was 2.8 years. There was one death due to sepsis 23 days after transplantation. All other patients presented with large initial improvement in skin elasticity and posterior stabilization, but three of them again presented worsening of the skin after transplant. The others kept the skin elasticity and stabilization of pulmonary involvement with a median follow-up time of 27 months (range: 2-90 months). Of the three patients who presented recurrence, the first has digital involvement even with prescribed bosentan. The second has progress of the skin and pulmonary involvement, even on receiving monthly pulses of cyclophosphamide; this patient began to take mofetil mycophenolate. While the third, also with progression of the skin condition, is taking methotrexate with partial response.
Systemic sclerosis: conclusions
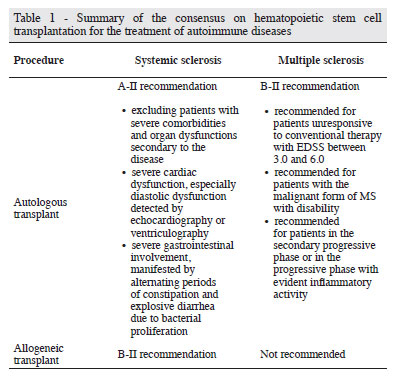
The international publications reveal that autologous HSCT has been consolidated as an alternative therapy for systemic sclerosis. The results, especially regarding the control of pulmonary involvement, are encouraging, since it attains responses so far not obtained with other treatments. Additionally, the mortality and frequency of adverse events related to the procedure have been decreasing as the experience and the number of transplanted patients grow. It is believed that this is due to more careful selection of patients with the exclusion of those with serious comorbidities and organ dysfunctions secondary to the disease. Patients with severe cardiac dysfunctions, especially diastolic dysfunction detected by echocardiography or ventriculography, as well as those with severe gastrointestinal involvement, manifested by alternating periods of constipation and explosive diarrhea due to bacterial proliferation should be excluded. In Brazil, the results, although more recent, are similar to those of the international literature; the doubts and disagreements regarding the procedure are also similar.
Additionally, during the Second Consensus Meeting in Bone Marrow Transplantation held in 2012, there was an agreement in the assembly that Brazilian transplant centers treating patients with systemic sclerosis should have the support of a rheumatologist with experience in the management of such patients who is able to evaluate and judge the severity of disease manifestations that would contraindicate transplantation.
Based on available published evidence, which consist of case studies and in one randomized trial, we conclude that, for the treatment of systemic sclerosis, autologous transplants are classified as level A-II recommendation. The other ongoing randomized studies (ASTIS and SCOT) should reinforce the recommendation of autologous HSCT. The recommendation is still level B-II for allogeneic transplants. Table 1 summarizes the recommendations.
Multiple sclerosis
Multiple sclerosis (MS) is a chronic inflammatory disease that causes destruction of central nervous system (CNS) myelin, with varying degrees of axonal damage. It mainly affects young adults (the symptoms appear around 30 years old), and is twice as common in women as in men. Mortality in patients with MS is not very different from that found in the normal population, but the progression of neurological deficits occurs in all patients with the disease. It is estimated that 20 years after disease onset, approximately 60% of patients are unable to walk(44,45).
MS leads to incapacitation through impairment of sensory, motor, autonomic and neurocognitive functions. Clinical manifestations are nonspecific and include visual loss, extraocular movement disorders, paresthesis, sensory loss, weakness, dysarthria, spasticity, ataxia and bladder dysfunction. The majority of patients present with alternating clinical outbreaks and remissions. Outbreaks are considered as neurological dysfunction symptoms found in anamnesis data over 24 hours, in the absence of fever or infections and with impairment of at least two long fiber systems (white matter)(46). The most used disability scale in neurological diagnosis is the Expanded Disability Status Scale (EDSS) described by Kurtzke(47).
The etiology of multiple sclerosis is not fully understood; it is believed that the disease is triggered by environmental factors in genetically susceptible individuals. It is considered an autoimmune disease because some studies have suggested an auto reactivity role of T lymphocytes that enter the CNS through smaller veins, triggering an immunological cascade, which induces and sustains other immune and inflammatory events causing typical changes in the white mass. The histopathological picture of MS is characterized by the presence of demyelinating plates normally located around the ventricles, optic nerves and brain. In outbreaks, perivascular infiltrates of lymphocytes are observed in the plaques(48).
Multiple sclerosis is present in three main types with different clinical approaches. The relapsing-remitting type is characterized by episodic outbreaks followed by partial or complete recovery of the dysfunction, interspersed with periods of remission of at least three days. After an average period of 10 years, outbreaks become less frequent, with less evident recovery, more sequelae and gradual worsening of neurological symptoms, characterizing the secondary progressive form. The primary progressive form is characterized by continuous functional decline since the onset of the disease. A "malignant" form of MS has also been recognized: these are serious cases, progressing quickly to a severe form of neurological disability or even death in a short period of time. The secondary progressive form, as mentioned above, starts with the relapsing-remitting form, which, at some point, starts a continuous neurological deterioration with or without overlapping outbreaks(49).
Conventional treatment
The introduction of immunomodulatory therapy in MS since 1993 has changed the disease scenario. The first-line treatments of MS are immunomodulators such as glatiramer acetate(50) and interferon-beta (IFN-β)(51) which delay the progression of disability, acting in the long term. Therapy with high doses of corticosteroids is used for acute relapses (attacks). After this, immunosuppressants (cyclosporine, cyclophosphamide, azathioprine, mitoxantrone etc.) can be used. Recently, the emergence of an oral medication, sphingosine 1 phosphate receptor agonist, has been considered more effective than IFN-β and glatiramer acetate(52) and is also approved as first-line therapy. Second line treatments are mitoxantrone(53) and the monoclonal natalizumab(54). Both first- and second-line treatments aim to treat the early stage of inflammation and modify disease evolution, but virtually none stop the development of long-term disabilities. In addition, administration of these agents may be complicated by adverse events that are infrequent, but can be severe, such as progressive multifocal leukoencephalopathy with natalizumab, severe infections and other effects with the sphingosine 1 phosphate receptor agonist, and cardiomyopathy and secondary leukemia with mitoxantrone(55). Overall, a subset of unresponsive patients was observed in clinical studies with older and newer therapies. Treatment alternatives are especially needed for this group of patients, i.e., those who have failed multiple treatments and evolve with progressive clinical disability.
Hematopoietic progenitor cell transplantation in multiple sclerosis
Studies published from the 1990s brought animal models and theoretical considerations of HSCT in the prevention and treatment of autoimmune diseases(56-59), with clinical responses in some patients with autoimmune diseases who received HSCT(56,60,61), suggesting that high-dose chemotherapy followed by HSCT rescue could "reset" the immunological changes through the control of autoreactive clones, followed by immunological tolerance after immune reconstitution. This led to the conclusion that HSCT may be a viable therapeutic option for MS(62,63).
Since 1996, HSCT has been extensively described around the world as a tool to induce a prolonged restoration of self-tolerance in patients(64-67). Since its first use in the second half of the 90s, more than 700 HSCTs have been performed in patients with MS around the world and its use has been frequently revised(59,68-70).
Most patients were treated in small, phase I-II clinical trials(63,71-83) or in multicenter studies(62,84-87). In retrospective analyzes of two centers, there was a progression-free survival of more than five years after transplant(77,85). The neurological outcomes were considerably more favorable in patients with the relapsing-remitting type and/or those who showed an inflammatory pattern in MRI during the pre-transplant screening(77).
In fact, reports of excellent results, particularly in the aggressive forms of MS(86,87) reinforce the effectiveness HSCT in MS patients with prominent inflammatory activity. The risk of TRM in HSCTwas conventionally considered very high but has declined since 2001 to 1.3%, according to the European Group for Blood and Marrow Transplantation (EBMT, www.ebmt.org) analysis(59,88). This probably resulted from the suspension of the use of the more aggressive conditioning, thus reducing toxicity. In particular, the use of busulfan was significantly associated with greater TRM in a multivariate analysis(76,89). Besides, high doses of ATG were associated with depletion of T cells ex vivo, and the use of a more aggressive conditioning regimen resulted in the unexpected occurrence of posttransplant lymphomas associated with the Epstein-Barr virus (EBV)(90). It is also worth mentioning the evolution of the learning curve for each center with decreasing mortality, a fact also noted in the review of North American transplants in autoimmune diseases(91).
Most patients worldwide have been conditioned with a carmustine, etoposide, cytarabine, melphalan regimen (BEAM) and ATG, showing an acceptable toxicity relative to the effectiveness(89). Conditioning with cyclophosphamide and ATG was used for autologous transplantations in 21 patients with the relapsing-remitting form of MS, with low toxicity, although not insignificant. After a 24-48 months follow-up period, patients had no worsening of the EDSS expanded scale, and 16 of 21 patients were free of relapses and significant improvements in EDSS(5). The Brazilian group also used a reduced-intensity regimen with cyclophosphamide, with no deaths(92).
More experience is needed to evaluate the role of reducedintensity regimens, as well as comparative studies with the best standard treatment(93). Some randomized clinical trials are already ongoing. In 2004, the EBMT launched a prospective, randomized phase II study, Autologous Stem Cell Transplantation International Multliple Sclerosis (ASTIMS)(94), comparing HSCT with mitoxantrone; the endpoint was the appearance of new lesions on MRI (number of new lesions on T2* in the first and second year after randomization). The study was discontinued due to difficulties including patients, but other phase II, single-arm studies are ongoing in the United States(95,96) and Canada(97). Preliminary analyzes of the Canadian study showed complete suppression of relapses and new MRI lesions in 23 patients assessed over a mean follow-up of five years. The US study (HALT-MS), also with preliminary results, showed that in 25 patients with high inflammatory activity refractory to conventional treatments, 77% had a progression-free survival at two years post-HSCT, with an endpoint that included relapses and new lesions on MRI and progression of neurological disability(98).
Studies published with the highest number of cases were from the European Group, the EBMT. Initially, the group performed a retrospective study that showed that 74% of 85 patients were free of disease progression for up to three years after transplantation. In this study, patients with relapsing-remitting and secondary-progressive MS, who had inflammatory characteristics, showed progression-free survival of 78% ± 13%, while for those with the primary-progressive form (more degenerative) the result was 66% ± 23% in three years(85). In 2006 the update of the analysis of 143 cases, with follow-up of 41.7 months, was published: the disease remained stable or improved in 63% of cases and worsened in 37%(93).
In 2010, EBMT published a review of all cases of autoimmune diseases studied, and showed 345 treatments in MS with mortality due to the procedure of 2%, three-year survival of 93%, and absence of disease progression in 55% of cases(88).
More recent data, with almost 500 autologous transplants for MS in Europe, showed an overall survival of 92% in five years and progression-free survival of 46%(99). The main cause of mortality and morbidity is the recurrence of the autoimmune disease.
The experience with all the studies strongly suggests that the effect of HSCT occurs mainly in patients with inflammatory manifestations of MS. The efficacy and safety of transplantation compared to conventional therapies has not been established. In November 2008, a meeting of experts in MS and BMT was held in Minneapolis, under the auspices of Center for International Blood and Marrow Transplant Research (CIBMT), the National Institute of Allergy and Infectious Diseases (NIAID) and EBMT, with the goal of establishing common criteria for evaluating the effectiveness of HSCT in MS and to evaluate the possibility of further prospective studies(100). These studies are already underway.
Brazilian experience
The study by the Brazilian group of transplantation in autoimmune diseases reported experience with the use of autologous transplantation in MS in 2010(92). The group studied patients not responsive to conventional therapy and who had disease progression. Two types of conditioning were studies: BEAM/ATG and cyclophosphamide/ATG(101). In this protocol, 41 autologous transplants were performed. The results showed overall event-free survival of 58.54%, and when analyzed separately, 47% in the BEAM/ATG and 70% in the cyclophosphamide/ATG; no statistically significant difference was found (p-value = 0288). There were three deaths in the BEAM group and none in the cyclophosphamide group.
After this study(98), other patients underwent HSCT using cyclophosphamide/rabbit ATG for conditioning. By 2010, when the first consensus(102) was held, a total of 46 patients had been treated in University Hospital of the Universidade de São Paulo (USP) in Ribeirão Preto, with similar results in the evolution and no mortality. The neurological evaluation, with a mean of 26 months (range: 3-52 months) in the 38 patients with a follow-up longer than six months, showed improvement in 30% of patients, stabilization in 47% and worsening in 23%. In the Hospital Albert Einstein, a total of 29 HSCTs were performed in MS by 2010, also with no deaths. The 2012 assessment showed a loss of follow up in nine patients. Of those evaluated, there was worsening of the EDSS over time in approximately 53% and stability or improvement with stability in 46% of patients. The importance of this work was to demonstrate that conditioning with high-dose cyclophosphamide and ATG was safer than the BEAM (carmustine 300 mg/m2 on D-7, etoposide 200 mg/m2, 200 mg/m2 of Aracytin in D-6 to D-3 and melphalan 140 mg/ m2 on D-2) and ATG regimen in our experience and population. The BEAM protocol showed a mortality of 15%, which was much higher than acceptable for patients with MS. The conditioning regimen cyclophosphamide/ATG showed zero mortality(92).
Some groups dispute this conclusion, arguing that patients transplanted in Brazil had very advanced disease, with approximately 80% of patients having an EDSS greater than or equal to 6. Examples of this were the results of the European Group, showing progression-free survival of 57% for patients with low-intensity conditioning and 46% and 49% for patients with intermediate to high intensity regimens respectively(84,88,89,93).
Although the type of conditioning remains controversial, most transplant groups agree that the manipulation of the graft for in vitro depletion of T cells is not necessary. The best results in disease remission are achieved in patients with the relapsing-remitting form and up to five years of diagnosis, reaching 70% of progression-free survival in this group(59).
The use of cell therapy (e.g., mesenchymal cells) in MS has been developed in recent years(103-107) but enough data for recommendation about effectiveness and safety are not yet available.
Multiple sclerosis: conclusions
The consensus on HSCT in autoimmune diseases provides an indication of HSCT in patients with progressive MS unresponsive to conventional therapy and EDSS between 3.0 and 6.0. The forms of the disease that might benefit from transplantation are: relapsing-remitting, primary or secondary progressive, and the "malignant" form, provided there is evidence of inflammatory activity at the time of transplant indication. The patients should be transplanted preferably in centers with experience in this procedure. Table 1 summarizes the recommendations.
Indications: IIB
- Patients in the relapsing-remitting phase with high inflammatory activity (clinically and by imaging), with progressive worsening despite the use of one or two lines of treatment;
- Patients with the "malignant" form of MS, who developed severe disability in the previous year;
- Patients in the secondary progressive phase when they show evident inflammatory activity (clinical relapse or new lesions or worsening of lesions in the imaging exam) with a significant and sustained clinical deterioration over the last year, despite treatment;
- Primary progressive phase (with evidence of inflammatory activity);
- Patients with EDSS 6.0 (patients who have lost the ability to walk, usually with a score of 6.5, must be excluded) with the exception of the "malignant" form.
References
1. Farge D, Nash R, Laar JM. Autologous stem cell transplantation for systemic sclerosis. Autoimmunity. 2008;41(8):616-24.
2. Voltarelli JC. Hematopoietic stem cell transplantation for autoimmune diseases in Brazil: current status and future prospectives. Rev Bras Hematol Hemoter. 2002;24(3):206-11.
3. Tyndall A, Furst DE. Adult stem cell treatment of scleroderma. Curr Opin Rheumatol. 2007;19(6):604-10.
4. Elhai M, Meune C, Avouac J, Kahan A, Allanore Y. Trends in mortality in patients with systemic sclerosis over 40 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford). 2012;51(6):1017-26. Comment in: Rheumatology (Oxford). 2012;51(6):959-61.
5. Burt RK, Loh Y, Cohen B, Stefoski D, Balabanov R, Katsamakis G, et al. Autologous non-myeloablative haemopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: a phase I/II study. Lancet Neurol. 2009;8(3):244-53. Erratum in: Lancet Neurol. 2009;8(4):309. Stefosky, Dusan [corrected to Stefoski, Dusan]. Comment in: Lancet Neurol. 2009;8(3):219-21; Nat Rev Neurol. 2009;5(6):300-2.
6. van der Hoogen FH, Boerbooms AM, Swaak AJ, Rasker JJ, van Lier HJ, van de Putte LB. Comparison of methotrexate with placebo in the treatment of systemic sclerosis: a 24 week randomized double-blind trial, followed by a 24 week observational trial. Br J Rheumatol. 1996;35(4):364-72.
7. Pope JE, Bellamy N, Seibold JR, Baron M, Ellman M, Carette S, et al. A randomized, controlled trial of methotrexate versus placebo in early diffuse scleroderma. Arthritis Rheum. 2001;44(6):1351-8. Comment in: Arthritis Rheum. 2002;46(3):845.
8. Griffiths B, Miles S, Moss H, Robertson R, Veale D, Emery P. Systemic sclerosis and intersticial lung disease: a pilot study using pulse intravenous methylprednisolone and cyclophosphamide to assess the effect on high resolution computed tomography scan and lung function. J Rheumatol. 2002;29(11):2371-8.
9. Hoyles RK, Ellis RW, Wellsbury J, Lees B, Newlands P, Goh NS, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 2006;54(12):3962-70. Comment in: Nat Clin Pract Rheumatol. 2007;3(7):372-3.
10. Khanna D, Yan X, Tashkin DP, Furst DE, Elashoff R, Roth MD, Silver R, Strange C, Bolster M, Seibold JR, Riley DJ, Hsu VM, Varga J, Schraufnagel DE, Theodore A, Simms R, Wise R, Wigley F, White B, Steen V, Read C, Mayes M, Parsley E, Mubarak K, Connolly MK, Golden J, Olman M, Fessler B, Rothfield N, Metersky M, Clements PJ; Scleroderma Lung Study Group. Impact of oral cyclophosphamide on health-related quality of life in patients with active scleroderma lung disease: results from the scleroderma lung study. Arthritis Rheum. 2007;56(5):1676-84.
11. Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, Arriola E, Silver R, Strange C, Bolster M, Seibold JR, Riley DJ, Hsu VM, Varga J, Schraufnagel DE, Theodore A, Simms R, Wise R, Wigley F, White B, Steen V, Read C, Mayes M, Parsley E, Mubarak K, Connolly MK, Golden J, Olman M, Fessler B, Rothfield N, Metersky M; Scleroderma Lung Study Research Group. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655-66. Comment in: Expert Rev Clin Immunol. 2006;2(6):849-52; N Engl J Med. 2006;354(25):2707-9; N Engl J Med. 2006;355(11):1173; author reply 1174; N Engl J Med. 2006;354(25):2655-6; Expert Opin Investig Drugs. 2007;16(3):393-5; N Engl J Med. 2006;355(11):1174-4; author reply 1174.
12. Khanna D, Saggar R, Mayes MD, Abtin F, Clements PJ, Maranian P, et al. A one-year, phase I/IIa, open-label pilot trial of imatinib mesylate in the treatment of systemic sclerosis-associated active interstitial lung disease. Arthritis Rheum. 2011;63(11):3540-6. Comment in: Arthritis Rheum. 2011;63(11):3199-203.
13. Pope J, McBain D, Petrlich L, Watson S, Vanderhoek L, de Leon F, et al. Imatinib in active diffuse cutaneous systemic sclerosis: Results of a six-month, randomized, double-blind, placebo-controlled, proof-of-concept pilot study at a single center. Arthritis Rheum. 2011;63(11):3547-51. Comment in: Arthritis Rheum. 2011;63(11):3199-203.
14. Guo L, Chen XX, Gu YY, Zou HJ, Ye S. Low-dose imatinib in the treatment of severe systemic sclerosis: a case series of six Chinese patients and literature review. Clin Rheumatol. 2012;31(9):1395-400. Comment in: Clin Rheumatol. 2013;32(1):149-50.
15. Bournia VK, Evangelou K, Sfikakis PP. Therapeutic inhibition of tyrosine kinases in systemic sclerosis: a review of published experience on the first 108 patients treated with imatinib. Semin Arthritis Rheum. 2013;42(4):377-90.
16. Miniati I, Conforti ML, Bernardo P, Tyndall A, Gensini GF, Matucci- Cerinic M. Hematopoietic stem cell transplantation in autoimmune diseases: algorithm for cardiovascular assessment. Herz. 2007;32(1):43-50.
17. Nash RA, McSweeney PA, Crofford LJ, Abidi M, Chen CS, Godwin JD, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for severe systemic sclerosis: long-term follow-up of the US multicenter pilot study. Blood. 2007;110(4):1388-96.
18. Slaper-Cortenbach IC, Wijngaarden-du Bois MJ, de Vries-van Rossen A, Borst HP, van der Lelie H, van Heugten HG, et al. The depletion of T cells from haematopoietic stem cell transplants. Rheumatology (Oxford). 1999;38(8):751-4.
19. van Laar JM, Farge D, Tyndall A. Stem cell transplantation: a treatment option for severe systemic sclerosis? Ann Rheum Dis. 2008;67 Suppl 3:iii35-8.
20. Loh Y, Oyama Y, Statkute L, Verda L, Quigley K, Yaung K, et al. Non-myeloablative allogeneic hematopoietic stem cell transplantation for severe systemic sclerosis: graft-versus-autoimmunity without graftversus-host disease? Bone Marrow Transplant. 2007;39(7):435-7.
21. Phumethum V, Jamal S, Johnson SR. Biologic therapy for systemic sclerosis: a systematic review. J Rheumatol. 2011;38(2):289-96.
22. Binks M, Passweg JR, Furst D, McSweeney P, Sullivan K, Besenthal C, et al. Phase I/II trial of autologous stem cell transplantation in systemic sclerosis: procedure related mortality and impact on skin disease. Ann Rheum Dis. 2001;60(6):577-84. Comment in: Ann Rheum Dis. 2001;60(6):548-9.
23. Burt RK, Patel D, Thomas J, Yeager, A, Traynor A, Heipe F, et al. The rationale behind autologous autoimmune hematopoietic stem cell transplant conditioning regimens: concerns over the use of total-body irradiation in systemic sclerosis. Bone Marrow Transplant. 2004;34(9):745-51. Erratum in: Bone Marrow Transplant. 2005;35(1):105.
24. Oyama Y, Barr WG, Statkute L, Corbridge T, Gonda EA, Jovanovic B, et al. Autologous non-myeloablative hematopoietic stem cell transplantation in patients with systemic sclerosis. Bone Marrow Transplant. 2007;40(6):549-55.
25. Vonk MC, Marjanovic Z, van den Hoogen FH, Zohar S, Schattenberg AV, Fibbe WE, et al. Long-term follow-up results after autologous haematopoietic stem cell transplantation for severe systemic sclerosis. Ann Rheum Dis. 2008;67(1):98-104. Erratum in: Ann Rheum Dis. 2008;67(2):280.
26. Henes JC, Schmalzing M, Vogel W, Riemekasten G, Fend F, Kanz L, et al. Optimization of autologous stem cell transplantation for systemic sclerosis--a single-center longterm experience in 26 patients with severe organ manifestations. J Rheumatol. 2012;39(2):269-75. Comment in: J Rheumatol. 2012;39(2):206-9.
27. Nash RA, McSweeney PA, Nelson JL, Wener M, Georges GE, Langston AA, et al. Allogeneic marrow transplantation in patients with severe systemic sclerosis: resolution of dermal fibrosis. Arthritis Rheum. 2006;54(6):1982-6.
28. Burt RK, Oyama Y, Verda L, Quigley K, Brush M, Yaung K, et al. Induction of remission of severe and refractory rheumatoid arthritis by allogeneic mixed chimerism. Arthritis Rheum. 2004;50(8):2466-70. Comment in: Arthritis Rheum. 2004;50(8):2387-90.
29. Shiratsuchi M, Motomura S, Abe Y, Shiokawa S, Nishimura J. Long-term follow-up after nonmyeloablative allogeneic hematopoietic stem cell transplantation for systemic sclerosis. Clin Rheumatol. 2008;27(9):1207-9.
30. Farge D, van Laar JM, Tyndall A. The European randomized HSCT trial for scleroderma. Blood Marrow Transpl. 2004;14(2):7-9.
31. Burt RK, Shah S, Dill K, Grant T, Gheorghiade M, Schroeder J, et al. Autologous non-myeloabaltive haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet. 2011;378(9790):498-506. Comment in: Lancet. 2011; 378(9790):460-2. Lancet. 2012;379(9812):219; author reply 219-20.
32. Morandi P, Ruffini PA, Benvenuto GM, Raimondi R, Fosser V. Cardiac toxicity of high-dose chemotherapy. Bone Marrow Transplant. 2005;35(4):323-34.
33. Komatsuda A, Kawabata Y, Horiuchi T, Motegi M, Ozawa M, Fujishima N, et al. Successful autologous peripheral blood stem cell transplantation using thiotepa in a patient with systemic sclerosis and cardiac involvement. Tohoku J Exp Med. 2006;209(1):61-7.
34. Allanore Y, Meune C, Kahan A. Systemic sclerosis and cardiac dysfunction: evolving concepts and diagnostic methodologies. Curr Opin Rheumatol. 2008;20(6):697-702.
35. Coghlan JG, Handler CE, Kottaridis PD. Cardiac assessment of patients for haematopoietic stem cell transplantation. Best Pract Res Clin Haematol. 2007;20(2):247-63.
36. Burt RK, Shah SJ, Gheorghiade M, Ruderman E, Schroeder J. Hematopoietic stem cell transplantation for systemic sclerosis: if you are confused, remember: "it is a matter of the heart". J Rheumatol. 2012;39(2):206-9. Comment in: J Rheumatol. 2012;39(2):269-75.
37. Snowden JA, Passweg J, Moore JJ, Milliken S, Cannell P, Van Laar J, et al. Autologous hemopoietic stem cell transplantation in severe rheumatoid arthritis: a report from the EBMT and ABMTR. J Rheumatol. 2004;31(3):482-8.
38. Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabeles mellitus. JAMA. 2007;297(14):1568-76. Comment in: JAMA. 2007;297(14):1599-600; JAMA. 2007;298(3):285; author reply 285-6.
39. Webb IJ, Coral FS, Andersen JW, Elias AD, Finberg RW, Nadler LM, et al. Sources and sequelae of bacterial contamination of hematopoietic stem cell components: implications for the safety of hematotherapy and graft engineering. Transfusion. 1996;36(9):782-8.
40. Czirják L, Foeldvari I, Müller-Ladner U. Skin involvement in systemic sclerosis. Rheumatology (Oxford). 2008;47 Suppl 5:v44-5.
41. Miniati I, Guiducci S, Conforti ML, Rogai V, Fiori G, Cinelli M, et al. Autologous stem cell transplantation improves microcirculation in systemic sclerosis. Ann Rheum Dis. 2009;68(1):94-8.
42. Nevskaya T, Ananieva L, Bykovskaia S, Eremin I, Karandashov E, Khrennikov J, et al. Autologous progenitor cell implantation as a novel therapeutic intervention for ischaemic digits in systemic sclerosis. Rheumatology (Oxford). 2009;48(1):61-4.
43. Mühlen CA, Hinterholz EL, Garicochea B, Weingrill P, Barrios C, Fernandes MS, et al. Imunossupressão com ciclofosfamida e fludarabina, com suporte de células tronco hematopoéticas autólogas CD-34+, para tratamento de esclerose sistêmica severa. Rev Bras Reumatol. 2004;44(2):169-74.
44. World Health Organization. Atlas multiple sclerosis resources in the world 2008 [Internet]. Geneva: WHO; 2008 [cited 2012 Oct 19] Available from: http://www.who.int/mental_health/neurology/Atlas_MS_WEB.pdf
45. Multiple Sclerosis International Federation. Facts [Internet]. London; 2012 [cited 2012 Oct 19] Available from: http://www.msif.org/pt/quick_facts/index.html
46. Multiple Sclerosis International Federation. Evolution of Multiple Sclerosis [Internet]. London; 2012 [cited 2012 Oct 19] Available from: http://www.msif.org/pt/ms_the_disease/the_course_of_ms.html
47. Kurtzke JF. Clinical definition for multiple sclerosis treatment trials. Ann Neurol. 1994;36 Suppl:S73-9.
48. Oliveira EM, Souza NA. Esclerose múltipla. Rev Neurociên [Internet]. 1998[cited 2012 Oct 19];6(3):114-8. 2012 Available from: http://www.revistaneurociencias.com.br/edicoes/1998/RN%2006%2003/Pages%20 from%20RN%2006%2003-4.pdf
49. Multiple Sclerosis International Federation. Types of multiple sclerosis [Internet]. London: 2012. [cited 2012 Oct 19] Available from: http://www.msif.org/pt/ms_the_disease/types_of_ms.html
50. Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, Myers LW, Panitch HS, Rose JW, Schiffer RB, Vollmer T, Weiner LP, Wolinsky JS; Copolymer 1 Multiple Sclerosis Study Group. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo- controlled trial 1995. Neurology. 2001;57(12 Suppl 5):S16-24.
51. Paty DW, Li DK. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology. 1993;43(4):662-7. Comment in: Neurology. 1993;43(4):641-3.
52. Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Wu S, Aradhye S, Kappos L; TRANSFORMS Study Group. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402-15. Comment in: Curr Neuro Neurosci Rep. 2010;10(5):333-5. N Engl J Med. 2010; 362(5):456-8. N Engl J Med. 2010;362(18):1738; author reply 1739-40. Expert Opin Pharmacother. 2010;11(11):1957-60; Expert Opin Pharmacother. 2010;11(10):1777-81. Ann Intern Med. 2010;152(10):JC5-6, JC5-7, JC5-8.
53. Edan G, Miller D, Clanet M, Confavreux C, Lyon-Caen O, Lubetzki C, et al. Therapeutic effect of mitoxantrone combined with methylprednisolone in multiple sclerosis: a randomised multicentre study of active disease using MRI and clinical criteria. J Neurol Neurosurg Psychiatry. 1997;62(2):112-8. Comment in: ACP J Club. 1997;127(1):12.
54. Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, Libonati MA, Willmer-Hulme AJ, Dalton CM, Miszkiel KA, O'Connor PW; International Natalizumab Multiple Sclerosis Trial Group. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348(1):15-23. Comment in: N Engl J Med. 2003;348(1):68-72; N Engl J Med. 2003;348(16):1598-9; author reply 1598-9.
55. Marriott JJ, Miyasaki JM, Gronseth G, O'Connor PW; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Evidence Report: The efficacy and safety of mitoxantrone (Novantrone) in the treatment of multiple sclerosis: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;74(18):1463-70. Comment in: Neurology. 2010;74(18):1410-1.
56. Snowden JA, Brooks PM, Biggs JC. Haemopoietic stem cell transplantation for autoimmune diseases. Br J Haematol. 1997;99(1):9-22.
57. van Bekkum DW. New opportunities for the treatment of severe autoimmune diseases: bone marrow transplantation. Clin Immunol Immunopathol. 1998;89(1):1-10.
58. Knaan-Shanzer S, Houben P, Kinwel-Bohré EP, van Bekkum DW. Remission induction of adjuvant arthritis in rats by total body irradiation and autologous bone marrow transplantation. Bone Marrow Transplant. 1991;8(5):333-8.
59. Mancardi G, Saccardi R. Autologous haematopoietic stem-cell transplantation in multiple sclerosis. Lancet Neurol. 2008;7(7):626-36.
60. Marmont AM. Stem cell transplantation for severe autoimmune disorders, with special reference to rheumatic diseases. J Rheumatol Suppl. 1997;48:13-8.
61. Fassas A, Anagnostopoulos A, Kazis A, Kapinas K, Sakellari I, Kimiskidis V, et al. Peripheral blood stem cell transplantation in the treatment of progressive multiple sclerosis: first results of pilot study. Bone Marrow Transplant. 1997;20(8):631-8.
62. Nash RA, Bowen JD, McSweeney PA, Pavletic SZ, Maravilla KR, Park MS, et al. High-dose immunosupressive therapy and autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Blood. 2003;102(7):2364-72.
63. Kozák T, Havrdová E, Pit'ha J, Gregora E, Pytlík R, Maaloufová J, et al. High-dose immunosupressive therapy with PBPC support in the treatment of poor risk multiple sclerosis. Bone Marrow Transplant. 2000;25(5):525-31.
64. Breban M, Hammer RE, Richardson JA, Taurog JD. Transfer of the inflammatory disease of HLA-B27 transgenic rats by bone marrow engraftment. J Exp Med. 1993;178(5):1607-16.
65. de Kleer I, Vastert B, Klein M, Teklenburg G, Arkesteijn G, Yung GP, et al. Autologous stem cell transplantation for autoimmunity induces immunologic self-tolerance by reprogramming autoreactive T cells and restoring the CD4+CD25+ immune regulatory network. Blood. 2006;107(4):1696-702.
66. Takayama T, Nishioka Y, Lu L, Lotze MT, Tahara H, Thomson AW. Retroviral delivery of viral interleukin-10 into myeloid dendritic cells markedly inhibits their allostimulatory activity and promotes the induction of T-cell hyporesponsiveness. Transplantation. 1998;66(12):1567-74.
67. Herrmann MM, Gaetner S, Stadelmann C, van den Brandt J, Böscke R, Budach W, et al. Tolerance induction by bone marrow transplantation in a multiple sclerosis model. Blood. 2005;106(5):1875-83.
68. Burt RK, Cohen B, Rose J, Petersen F, Oyama Y, Stefoski D, et al. Hematopoietic stem cell transplantation for multiple sclerosis. Arch Neurol. 2005;62(6):860-4.
69. Good RA, Verjee T. Historical and current perspectives on bone marrow transplantation for prevention and treatment of immunodeficiencies and autoimmunities. Biol Blood Marrow Transplant. 2001;7(3):123-35.
70. Tyndall A. Successes and failures of stem cell transplantation in autoimmune diseases. Hematology Am Soc Hematol Educ Program. 2011;2011:280-4.
71. Burt RK, Cohen BA, Russell E, Spero K, Joshi A, Oyama Y et al. Hematopoietic stem cell transplantation for progressive multiple sclerosis: failure of a total body irradiation-based conditioning regimen to prevent disease progression in patients with high disability scores. Blood. 2003;102(7):2373-8.
72. Carreras E, Saiz A, Marín P, Martínez C, Rovira M, Villamor N, et al. CD34+ selected autologous peripheral blood stem cell transplantation for multiple sclerosis: report of toxicity and treatment results at one year of follow-up in 15 patients. Haematologica. 2003;88(3):306-14. Comment in: Haematologica. 2003;88(3):244-5.
73. Samijn JP, te Boekhorst PA, Mondria T, van Doorn PA, Flach HZ, van der Meché FG, et al. Intense T cell depletion followed by autologous bone marrow transplantation for severe multiple sclerosis. J Neurol Neurosurg Psychiatry. 2006;77(1):46-50.
74. Atkins H, Freedman M. Immune ablation followed by autologous hematopoietic stem cell transplantation for the treatment of poor prognosis multiple sclerosis. Methods Mol Biol. 2009;549:231-46.
75. Su L, Xu J, Ji BX, Wan SG, Lu CY, Dong HQ, et al. Autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Int J Hematol. 2006;84(3):276-81.
76. Openshaw H, Lund BT, Kashyap A, Atkinson R, Sniecinski I, Weiner LP, et al. Peripheral blood stem cell transplantation in multiple sclerosis with busulfan and cyclophosphamide conditioning: report of toxicity and immunological monitoring. Biol Blood Marrow Transplant. 2000;6(5A):563-75.
77. Fassas A, Kimiskidis VK, Sakellari I, Kapinas K, Anagnostopoulos A, Tsimourtou V, et al. Long-term results of stem cell transplantation for MS: a single-center experience. Neurology. 2011;76(12):1066-70.
78. Saiz A, Blanco Y, Berenguer J, Gómez-Choco M, Carreras E, Arbizu T, et al. Resultado clínico a 6 años del trasplante autólogo de progenitores hematopoyéticos en la esclerosis múltiple. Neurologia. 2008;23(7):405-7.
79. Kozák T, Havrdová E, Pit'ha J, Gregora E, Pytlík R, Maaloufová J, et al. Immunoablative therapy with autologous stem cell transplantation in the treatment of poor risk multiple sclerosis. Transplant Proc. 2001;33(3):2179-81.
80. Kozák T, Havrdová E, Pitha J, Mayerova K, Novakova L, Trneny M, et al. Immunoablative therapy with autologous PBPC transplantation in the treatment of poor-risk multiple sclerosis[Internet]. Oral Presentation at: 34th Annual Meeting of the European Group of Blood and Marrow Transplantation; 24 th Meeting of the EBMT Nurses group; 7th Meeting of EBMT Data Management Group; Florence, Italy; 2008. Bone Marrow Transplant. 2008[cited 2012 Sep 26]; 41(Suppl 1):S18 [abstract]. Available from: http://registration.akm.ch/einsicht.php?XNABSTRACT_ID=68025&XNSPRACHE_ID=2&XNKONGRESS_ID=69&XNMASKEN_ID=900
81. Shevchenko YL, Novik AA, Kuznetsov AN, Afanasiev BV, Lisukov IA, Kozlov VA, et al. High-dose immunosuppressive therapy with autologous hematopoietic stem cell transplantation as a treatment option in multiple sclerosis. Exp Hematol. 2008;36(8):922-8.
82. Xu J, Ji BX, Su L, Dong HQ, Sun XJ, Liu CY. Clinical outcomes after autologous haematopoietic stem cell transplantation in patients with progressive multiple sclerosis. Chin Med J (Engl). 2006;119(22):1851-5.
83. Ni XS, Ouyang J, Zhu WH, Wang C, Chen B. Autologous hematopoietic stem cell transplantation for progressive multiple sclerosis: report of efficacy and safety at three yr of follow up in 21 patients. Clin Transplant. 2006;20(4):485-9.
84. Saccardi R, Mancardi GL, Solari A, Bosi A, Bruzzi P, Di Bartolomeo P, et al. Autologous HSCT for severe progressive multiple sclerosis in a multicenter trial: impact on disease activity and quality of life. Blood. 2005;105(6):2601-7.
85. Krasulová E, Trneny M, Kozák T, Vacková B, Pohlreich D, Kemlink D, et al. High-dose immunoablation with autologous haematopoietic stem cell transplantation in aggressive multiple sclerosis: a single-centre 10-year experience. Mult Scler. 2010;16(6):685-93.
86. Mancardi GL, Murialdo A, Rossi P, Gualandi F, Martino G, Marmont A, et al. Autologous stem cell transplantation as rescue therapy in malignant forms of multiple sclerosis. Mult Scler. 2005;11(3):367-71.
87. Fagius J, Lundgren J, Oberg G. Early highly aggressive MS successfully treated by hematopoietic stem cell transplantation. Mult Scler. 2009;15(2):229-37.
88. Farge D, Lapobin M, Tyndall A, Fassas A, Mancardi GL, Van Laar J, et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases: an observational study on 12 years' experience from the European Group for Blood and Marrow Transplantation Working Party on Autoimmune Disease. Haematologica. 2010;95(2):284-92. Comment in: Haematologica. 2010;95(2):185-8.
89. Saccardi R, Kozak T, Bocelli-Tyndall C, Fassas A, Kazis A, Havrdova E, et al. Autologous stem cell transplantation for progressive multiple sclerosis: update of the European Group for Blood and Marrow Transplantation autoimmune diseases working party database. Mult Scler. 2006;12(6):814-23.
90. Nash RA, Dansey R, Storek J, Georges GE, Bowen JD, Holmberg LA, et al. Epstein-Barr virus-associated posttransplantation lymphoproliferative disorder after high-dose immunosuppressive therapy and autologous CD34-selected hematopoietic stem cell transplantation for severe autoimmune diseases. Biol Blood Marrow Transplant. 2003;9(9):583-91.
91. Pasquini MC, Voltarelli J, Atkins HL, Hamerschlak N, Zhong X, Ahn KW, et al. Transplantation for autoimmune disease in north and South America: a report of the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2012;18(10):1471-8.
92. Hamerschlak N, Rodrigues M, Moraes DA, Oliveira MC, Stracieri AB, Pieroni F, et al. Brazilian experience with two conditioning regimens in patients with multiple sclerosis: BEAM/horse ATG and CY/rabbit ATG. Bone Marrow Transplant. 2010;45(2):239-48.
93. Saccardi R, Freedman MS, Sormani MP, Atkins H, Farge D, Griffith LM, et al. A prospective, randomized, controlled trial of autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: a position paper. Mult Scler. 2012;18(6):825-34. Comment in: Mult Scler. 2012;18(6):772-5.
94. ASTIMS: Autologous Stem cell Transplantation International Multiple Sclerosis [Internet]. [cited 2012 Jan 3]. Available from: http://www.astims.org/
95. Halt MS: High-dose immunosuppression and autologous stem cell transplantation for poor prognosis multiple sclerosis [Internet]. Houston; 2008. [cited 2010 Jun 3]. Available from: www.halt-ms.org
96. Nash RA, Bowen JD, Kraft GH, Popat U, Racke MK, Devine SM, Wundes A, Georges GE. High-dose immunosuppression and autologous transplantation for multiple sclerosis (HALT MS) study [Internet]. ClinicalTrials.gov NCT00288626.[cited 2012 Jan 13]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00288626?term=NCT00288626&rank=1
97. Atkins H, Freedman MS. Autologous stem cell transplant for multiple sclerosis (MS/BMT) [Internet]. ClinicalTrials.gov NCT01099930. [cited 2010 Jun 21] Available from: http://www.clinicaltrials.gov/ct2/show/NCT01099930?term=NCT01099930&rank=1
98. Nash RA, Hutton GJ, Racke MK, Popat UR, Devine SM, Griffith LM, et al. Treatment of severe relapsing-remitting multiple sclerosis with high-dose immunosuppressive therapy and autologous hematopoietic cell transplantation: early results of the HALT MS Clinical Trial (Immune tolerance network: ITNO33AI) [Internet]. Paper presented at: 53rd Annual Meeting and Exposition. San Diego; 2011 [abtract 3075]. [cited 2012 Jun 23]. Available from: https://ash.confex.com/ash/2011/webprogram/Paper38504.html
99. Snowden JA, Saccardi R, Allez M, Ardizzone S, Arnold R, Cervera R, et al. Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2012;47(6):770-90.
100. Pasquini MC, Griffith LM, Arnold DL, Atkins HL, Bowen JD, Chen JT, et al. Hematopoietic stem cell transplantation for multiple sclerosis: collaboration of the CIBMTR and EBMT to facilitate international clinical studies. Biol Blood Marrow Transplant. 2010;16(8):1076-83.
101. Carrá A, Macías-Islas MÁ, Gabbai AA, Correale J, Bolaña C, Sotelo ED, et al. Optimizing outcomes in multiple sclerosis: consensus guidelines for the diagnosis and treatment of multiple sclerosis in Latin America. Ther Adv Neurol Disord. 2011;4(6):349-60.
102. Voltarelli JC, Moraes DA, Ribeiro AA, Oliveira MC, Rodrigues M, Brum DG, et al. [Brazilian consensus on hematopoietic stem cell transplantation for autoimmune diseases]. Rev Bras Hematol Hemoter. 2010;32(supl. 1):125-35. Portuguese.
103. Kuwana M. Induction of anergic and regulatory T cells by plasmacytoid dendritic cells and other dendritic cell subsets. Human Immunol. 2002;63(12):1156-63.
104. Le Blanc K, Ringdén O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(5):321-34.
105. Le Blanc K, Ringdén O. Mesenchymal stem cells: properties and role in clinical bone marrow transplantation. Curr Opin Immunol. 2006;18(5):586-91.
106. Lu L, Thomson AW. Manipulation of dendritic cells for tolerance induction in transplantation and autoimmune disease. Transplantation. 2002;73(1 Suppl):S19-22.
107. Lu L, Gambotto A, Lee WC, Qian S, Bonham CA, Robbins PD, et al. Adenoviral delivery of CTLA4Ig into myeloid dendritic cells promotes their in vitro tolerogenicity and survival in allogeneic recipients. Gene Ther. 1999;6(4):554-63.
Submitted: 12/13/2012
Accepted: 2/27/2013
Conflict-of-interest disclosure: The authors declare no competing financial interest
- 1. Farge D, Nash R, Laar JM. Autologous stem cell transplantation for systemic sclerosis. Autoimmunity. 2008;41(8):616-24.
- 2. Voltarelli JC. Hematopoietic stem cell transplantation for autoimmune diseases in Brazil: current status and future prospectives. Rev Bras Hematol Hemoter. 2002;24(3):206-11.
- 3. Tyndall A, Furst DE. Adult stem cell treatment of scleroderma. Curr Opin Rheumatol. 2007;19(6):604-10.
- 4. Elhai M, Meune C, Avouac J, Kahan A, Allanore Y. Trends in mortality in patients with systemic sclerosis over 40 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford). 2012;51(6):1017-26. Comment in: Rheumatology (Oxford). 2012;51(6):959-61.
- 5. Burt RK, Loh Y, Cohen B, Stefoski D, Balabanov R, Katsamakis G, et al. Autologous non-myeloablative haemopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: a phase I/II study. Lancet Neurol. 2009;8(3):244-53. Erratum in: Lancet Neurol. 2009;8(4):309. Stefosky, Dusan [corrected to Stefoski, Dusan]. Comment in: Lancet Neurol. 2009;8(3):219-21; Nat Rev Neurol. 2009;5(6):300-2.
- 6. van der Hoogen FH, Boerbooms AM, Swaak AJ, Rasker JJ, van Lier HJ, van de Putte LB. Comparison of methotrexate with placebo in the treatment of systemic sclerosis: a 24 week randomized double-blind trial, followed by a 24 week observational trial. Br J Rheumatol. 1996;35(4):364-72.
- 7. Pope JE, Bellamy N, Seibold JR, Baron M, Ellman M, Carette S, et al. A randomized, controlled trial of methotrexate versus placebo in early diffuse scleroderma. Arthritis Rheum. 2001;44(6):1351-8. Comment in: Arthritis Rheum. 2002;46(3):845.
- 8. Griffiths B, Miles S, Moss H, Robertson R, Veale D, Emery P. Systemic sclerosis and intersticial lung disease: a pilot study using pulse intravenous methylprednisolone and cyclophosphamide to assess the effect on high resolution computed tomography scan and lung function. J Rheumatol. 2002;29(11):2371-8.
- 9. Hoyles RK, Ellis RW, Wellsbury J, Lees B, Newlands P, Goh NS, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 2006;54(12):3962-70. Comment in: Nat Clin Pract Rheumatol. 2007;3(7):372-3.
- 10. Khanna D, Yan X, Tashkin DP, Furst DE, Elashoff R, Roth MD, Silver R, Strange C, Bolster M, Seibold JR, Riley DJ, Hsu VM, Varga J, Schraufnagel DE, Theodore A, Simms R, Wise R, Wigley F, White B, Steen V, Read C, Mayes M, Parsley E, Mubarak K, Connolly MK, Golden J, Olman M, Fessler B, Rothfield N, Metersky M, Clements PJ; Scleroderma Lung Study Group. Impact of oral cyclophosphamide on health-related quality of life in patients with active scleroderma lung disease: results from the scleroderma lung study. Arthritis Rheum. 2007;56(5):1676-84.
- 11. Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, Arriola E, Silver R, Strange C, Bolster M, Seibold JR, Riley DJ, Hsu VM, Varga J, Schraufnagel DE, Theodore A, Simms R, Wise R, Wigley F, White B, Steen V, Read C, Mayes M, Parsley E, Mubarak K, Connolly MK, Golden J, Olman M, Fessler B, Rothfield N, Metersky M; Scleroderma Lung Study Research Group. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655-66. Comment in: Expert Rev Clin Immunol. 2006;2(6):849-52; N Engl J Med. 2006;354(25):2707-9; N Engl J Med. 2006;355(11):1173; author reply 1174; N Engl J Med. 2006;354(25):2655-6; Expert Opin Investig Drugs. 2007;16(3):393-5; N Engl J Med. 2006;355(11):1174-4; author reply 1174.
- 12. Khanna D, Saggar R, Mayes MD, Abtin F, Clements PJ, Maranian P, et al. A one-year, phase I/IIa, open-label pilot trial of imatinib mesylate in the treatment of systemic sclerosis-associated active interstitial lung disease. Arthritis Rheum. 2011;63(11):3540-6. Comment in: Arthritis Rheum. 2011;63(11):3199-203.
- 13. Pope J, McBain D, Petrlich L, Watson S, Vanderhoek L, de Leon F, et al. Imatinib in active diffuse cutaneous systemic sclerosis: Results of a six-month, randomized, double-blind, placebo-controlled, proof-of-concept pilot study at a single center. Arthritis Rheum. 2011;63(11):3547-51. Comment in: Arthritis Rheum. 2011;63(11):3199-203.
- 14. Guo L, Chen XX, Gu YY, Zou HJ, Ye S. Low-dose imatinib in the treatment of severe systemic sclerosis: a case series of six Chinese patients and literature review. Clin Rheumatol. 2012;31(9):1395-400. Comment in: Clin Rheumatol. 2013;32(1):149-50.
- 15. Bournia VK, Evangelou K, Sfikakis PP. Therapeutic inhibition of tyrosine kinases in systemic sclerosis: a review of published experience on the first 108 patients treated with imatinib. Semin Arthritis Rheum. 2013;42(4):377-90.
- 16. Miniati I, Conforti ML, Bernardo P, Tyndall A, Gensini GF, Matucci- Cerinic M. Hematopoietic stem cell transplantation in autoimmune diseases: algorithm for cardiovascular assessment. Herz. 2007;32(1):43-50.
- 17. Nash RA, McSweeney PA, Crofford LJ, Abidi M, Chen CS, Godwin JD, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for severe systemic sclerosis: long-term follow-up of the US multicenter pilot study. Blood. 2007;110(4):1388-96.
- 18. Slaper-Cortenbach IC, Wijngaarden-du Bois MJ, de Vries-van Rossen A, Borst HP, van der Lelie H, van Heugten HG, et al. The depletion of T cells from haematopoietic stem cell transplants. Rheumatology (Oxford). 1999;38(8):751-4.
- 19. van Laar JM, Farge D, Tyndall A. Stem cell transplantation: a treatment option for severe systemic sclerosis? Ann Rheum Dis. 2008;67 Suppl 3:iii35-8.
- 20. Loh Y, Oyama Y, Statkute L, Verda L, Quigley K, Yaung K, et al. Non-myeloablative allogeneic hematopoietic stem cell transplantation for severe systemic sclerosis: graft-versus-autoimmunity without graftversus-host disease? Bone Marrow Transplant. 2007;39(7):435-7.
- 21. Phumethum V, Jamal S, Johnson SR. Biologic therapy for systemic sclerosis: a systematic review. J Rheumatol. 2011;38(2):289-96.
- 22. Binks M, Passweg JR, Furst D, McSweeney P, Sullivan K, Besenthal C, et al. Phase I/II trial of autologous stem cell transplantation in systemic sclerosis: procedure related mortality and impact on skin disease. Ann Rheum Dis. 2001;60(6):577-84. Comment in: Ann Rheum Dis. 2001;60(6):548-9.
- 23. Burt RK, Patel D, Thomas J, Yeager, A, Traynor A, Heipe F, et al. The rationale behind autologous autoimmune hematopoietic stem cell transplant conditioning regimens: concerns over the use of total-body irradiation in systemic sclerosis. Bone Marrow Transplant. 2004;34(9):745-51. Erratum in: Bone Marrow Transplant. 2005;35(1):105.
- 24. Oyama Y, Barr WG, Statkute L, Corbridge T, Gonda EA, Jovanovic B, et al. Autologous non-myeloablative hematopoietic stem cell transplantation in patients with systemic sclerosis. Bone Marrow Transplant. 2007;40(6):549-55.
- 25. Vonk MC, Marjanovic Z, van den Hoogen FH, Zohar S, Schattenberg AV, Fibbe WE, et al. Long-term follow-up results after autologous haematopoietic stem cell transplantation for severe systemic sclerosis. Ann Rheum Dis. 2008;67(1):98-104. Erratum in: Ann Rheum Dis. 2008;67(2):280.
- 26. Henes JC, Schmalzing M, Vogel W, Riemekasten G, Fend F, Kanz L, et al. Optimization of autologous stem cell transplantation for systemic sclerosis--a single-center longterm experience in 26 patients with severe organ manifestations. J Rheumatol. 2012;39(2):269-75. Comment in: J Rheumatol. 2012;39(2):206-9.
- 27. Nash RA, McSweeney PA, Nelson JL, Wener M, Georges GE, Langston AA, et al. Allogeneic marrow transplantation in patients with severe systemic sclerosis: resolution of dermal fibrosis. Arthritis Rheum. 2006;54(6):1982-6.
- 28. Burt RK, Oyama Y, Verda L, Quigley K, Brush M, Yaung K, et al. Induction of remission of severe and refractory rheumatoid arthritis by allogeneic mixed chimerism. Arthritis Rheum. 2004;50(8):2466-70. Comment in: Arthritis Rheum. 2004;50(8):2387-90.
- 29. Shiratsuchi M, Motomura S, Abe Y, Shiokawa S, Nishimura J. Long-term follow-up after nonmyeloablative allogeneic hematopoietic stem cell transplantation for systemic sclerosis. Clin Rheumatol. 2008;27(9):1207-9.
- 30. Farge D, van Laar JM, Tyndall A. The European randomized HSCT trial for scleroderma. Blood Marrow Transpl. 2004;14(2):7-9.
- 31. Burt RK, Shah S, Dill K, Grant T, Gheorghiade M, Schroeder J, et al. Autologous non-myeloabaltive haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet. 2011;378(9790):498-506. Comment in: Lancet. 2011; 378(9790):460-2. Lancet. 2012;379(9812):219; author reply 219-20.
- 32. Morandi P, Ruffini PA, Benvenuto GM, Raimondi R, Fosser V. Cardiac toxicity of high-dose chemotherapy. Bone Marrow Transplant. 2005;35(4):323-34.
- 33. Komatsuda A, Kawabata Y, Horiuchi T, Motegi M, Ozawa M, Fujishima N, et al. Successful autologous peripheral blood stem cell transplantation using thiotepa in a patient with systemic sclerosis and cardiac involvement. Tohoku J Exp Med. 2006;209(1):61-7.
- 34. Allanore Y, Meune C, Kahan A. Systemic sclerosis and cardiac dysfunction: evolving concepts and diagnostic methodologies. Curr Opin Rheumatol. 2008;20(6):697-702.
- 35. Coghlan JG, Handler CE, Kottaridis PD. Cardiac assessment of patients for haematopoietic stem cell transplantation. Best Pract Res Clin Haematol. 2007;20(2):247-63.
- 36. Burt RK, Shah SJ, Gheorghiade M, Ruderman E, Schroeder J. Hematopoietic stem cell transplantation for systemic sclerosis: if you are confused, remember: "it is a matter of the heart". J Rheumatol. 2012;39(2):206-9. Comment in: J Rheumatol. 2012;39(2):269-75.
- 37. Snowden JA, Passweg J, Moore JJ, Milliken S, Cannell P, Van Laar J, et al. Autologous hemopoietic stem cell transplantation in severe rheumatoid arthritis: a report from the EBMT and ABMTR. J Rheumatol. 2004;31(3):482-8.
- 38. Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabeles mellitus. JAMA. 2007;297(14):1568-76. Comment in: JAMA. 2007;297(14):1599-600; JAMA. 2007;298(3):285; author reply 285-6.
- 39. Webb IJ, Coral FS, Andersen JW, Elias AD, Finberg RW, Nadler LM, et al. Sources and sequelae of bacterial contamination of hematopoietic stem cell components: implications for the safety of hematotherapy and graft engineering. Transfusion. 1996;36(9):782-8.
- 40. Czirják L, Foeldvari I, Müller-Ladner U. Skin involvement in systemic sclerosis. Rheumatology (Oxford). 2008;47 Suppl 5:v44-5.
- 41. Miniati I, Guiducci S, Conforti ML, Rogai V, Fiori G, Cinelli M, et al. Autologous stem cell transplantation improves microcirculation in systemic sclerosis. Ann Rheum Dis. 2009;68(1):94-8.
- 42. Nevskaya T, Ananieva L, Bykovskaia S, Eremin I, Karandashov E, Khrennikov J, et al. Autologous progenitor cell implantation as a novel therapeutic intervention for ischaemic digits in systemic sclerosis. Rheumatology (Oxford). 2009;48(1):61-4.
- 43. Mühlen CA, Hinterholz EL, Garicochea B, Weingrill P, Barrios C, Fernandes MS, et al. Imunossupressão com ciclofosfamida e fludarabina, com suporte de células tronco hematopoéticas autólogas CD-34+, para tratamento de esclerose sistêmica severa. Rev Bras Reumatol. 2004;44(2):169-74.
- 44. World Health Organization. Atlas multiple sclerosis resources in the world 2008 [Internet]. Geneva: WHO; 2008 [cited 2012 Oct 19] Available from: http://www.who.int/mental_health/neurology/Atlas_MS_WEB.pdf
- 45. Multiple Sclerosis International Federation. Facts [Internet]. London; 2012 [cited 2012 Oct 19] Available from: http://www.msif.org/pt/quick_facts/index.html
- 46. Multiple Sclerosis International Federation. Evolution of Multiple Sclerosis [Internet]. London; 2012 [cited 2012 Oct 19] Available from: http://www.msif.org/pt/ms_the_disease/the_course_of_ms.html
- 47. Kurtzke JF. Clinical definition for multiple sclerosis treatment trials. Ann Neurol. 1994;36 Suppl:S73-9.
- 48. Oliveira EM, Souza NA. Esclerose múltipla. Rev Neurociên [Internet]. 1998[cited 2012 Oct 19];6(3):114-8. 2012 Available from: http://www.revistaneurociencias.com.br/edicoes/1998/RN%2006%2003/Pages%20 from%20RN%2006%2003-4.pdf
- 49. Multiple Sclerosis International Federation. Types of multiple sclerosis [Internet]. London: 2012. [cited 2012 Oct 19] Available from: http://www.msif.org/pt/ms_the_disease/types_of_ms.html
- 50. Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, Myers LW, Panitch HS, Rose JW, Schiffer RB, Vollmer T, Weiner LP, Wolinsky JS; Copolymer 1 Multiple Sclerosis Study Group. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo- controlled trial 1995. Neurology. 2001;57(12 Suppl 5):S16-24.
- 51. Paty DW, Li DK. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology. 1993;43(4):662-7. Comment in: Neurology. 1993;43(4):641-3.
- 52. Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Wu S, Aradhye S, Kappos L; TRANSFORMS Study Group. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402-15. Comment in: Curr Neuro Neurosci Rep. 2010;10(5):333-5. N Engl J Med. 2010; 362(5):456-8. N Engl J Med. 2010;362(18):1738; author reply 1739-40. Expert Opin Pharmacother. 2010;11(11):1957-60; Expert Opin Pharmacother. 2010;11(10):1777-81. Ann Intern Med. 2010;152(10):JC5-6, JC5-7, JC5-8.
- 53. Edan G, Miller D, Clanet M, Confavreux C, Lyon-Caen O, Lubetzki C, et al. Therapeutic effect of mitoxantrone combined with methylprednisolone in multiple sclerosis: a randomised multicentre study of active disease using MRI and clinical criteria. J Neurol Neurosurg Psychiatry. 1997;62(2):112-8. Comment in: ACP J Club. 1997;127(1):12.
- 54. Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, Libonati MA, Willmer-Hulme AJ, Dalton CM, Miszkiel KA, O'Connor PW; International Natalizumab Multiple Sclerosis Trial Group. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348(1):15-23. Comment in: N Engl J Med. 2003;348(1):68-72; N Engl J Med. 2003;348(16):1598-9; author reply 1598-9.
- 55. Marriott JJ, Miyasaki JM, Gronseth G, O'Connor PW; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Evidence Report: The efficacy and safety of mitoxantrone (Novantrone) in the treatment of multiple sclerosis: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;74(18):1463-70. Comment in: Neurology. 2010;74(18):1410-1.
- 56. Snowden JA, Brooks PM, Biggs JC. Haemopoietic stem cell transplantation for autoimmune diseases. Br J Haematol. 1997;99(1):9-22.
- 57. van Bekkum DW. New opportunities for the treatment of severe autoimmune diseases: bone marrow transplantation. Clin Immunol Immunopathol. 1998;89(1):1-10.
- 58. Knaan-Shanzer S, Houben P, Kinwel-Bohré EP, van Bekkum DW. Remission induction of adjuvant arthritis in rats by total body irradiation and autologous bone marrow transplantation. Bone Marrow Transplant. 1991;8(5):333-8.
- 59. Mancardi G, Saccardi R. Autologous haematopoietic stem-cell transplantation in multiple sclerosis. Lancet Neurol. 2008;7(7):626-36.
- 60. Marmont AM. Stem cell transplantation for severe autoimmune disorders, with special reference to rheumatic diseases. J Rheumatol Suppl. 1997;48:13-8.
- 61. Fassas A, Anagnostopoulos A, Kazis A, Kapinas K, Sakellari I, Kimiskidis V, et al. Peripheral blood stem cell transplantation in the treatment of progressive multiple sclerosis: first results of pilot study. Bone Marrow Transplant. 1997;20(8):631-8.
- 62. Nash RA, Bowen JD, McSweeney PA, Pavletic SZ, Maravilla KR, Park MS, et al. High-dose immunosupressive therapy and autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Blood. 2003;102(7):2364-72.
- 63. Kozák T, Havrdová E, Pit'ha J, Gregora E, Pytlík R, Maaloufová J, et al. High-dose immunosupressive therapy with PBPC support in the treatment of poor risk multiple sclerosis. Bone Marrow Transplant. 2000;25(5):525-31.
- 64. Breban M, Hammer RE, Richardson JA, Taurog JD. Transfer of the inflammatory disease of HLA-B27 transgenic rats by bone marrow engraftment. J Exp Med. 1993;178(5):1607-16.
- 65. de Kleer I, Vastert B, Klein M, Teklenburg G, Arkesteijn G, Yung GP, et al. Autologous stem cell transplantation for autoimmunity induces immunologic self-tolerance by reprogramming autoreactive T cells and restoring the CD4+CD25+ immune regulatory network. Blood. 2006;107(4):1696-702.
- 66. Takayama T, Nishioka Y, Lu L, Lotze MT, Tahara H, Thomson AW. Retroviral delivery of viral interleukin-10 into myeloid dendritic cells markedly inhibits their allostimulatory activity and promotes the induction of T-cell hyporesponsiveness. Transplantation. 1998;66(12):1567-74.
- 67. Herrmann MM, Gaetner S, Stadelmann C, van den Brandt J, Böscke R, Budach W, et al. Tolerance induction by bone marrow transplantation in a multiple sclerosis model. Blood. 2005;106(5):1875-83.
- 68. Burt RK, Cohen B, Rose J, Petersen F, Oyama Y, Stefoski D, et al. Hematopoietic stem cell transplantation for multiple sclerosis. Arch Neurol. 2005;62(6):860-4.
- 69. Good RA, Verjee T. Historical and current perspectives on bone marrow transplantation for prevention and treatment of immunodeficiencies and autoimmunities. Biol Blood Marrow Transplant. 2001;7(3):123-35.
- 70. Tyndall A. Successes and failures of stem cell transplantation in autoimmune diseases. Hematology Am Soc Hematol Educ Program. 2011;2011:280-4.
- 71. Burt RK, Cohen BA, Russell E, Spero K, Joshi A, Oyama Y et al. Hematopoietic stem cell transplantation for progressive multiple sclerosis: failure of a total body irradiation-based conditioning regimen to prevent disease progression in patients with high disability scores. Blood. 2003;102(7):2373-8.
- 72. Carreras E, Saiz A, Marín P, Martínez C, Rovira M, Villamor N, et al. CD34+ selected autologous peripheral blood stem cell transplantation for multiple sclerosis: report of toxicity and treatment results at one year of follow-up in 15 patients. Haematologica. 2003;88(3):306-14. Comment in: Haematologica. 2003;88(3):244-5.
- 73. Samijn JP, te Boekhorst PA, Mondria T, van Doorn PA, Flach HZ, van der Meché FG, et al. Intense T cell depletion followed by autologous bone marrow transplantation for severe multiple sclerosis. J Neurol Neurosurg Psychiatry. 2006;77(1):46-50.
- 74. Atkins H, Freedman M. Immune ablation followed by autologous hematopoietic stem cell transplantation for the treatment of poor prognosis multiple sclerosis. Methods Mol Biol. 2009;549:231-46.
- 75. Su L, Xu J, Ji BX, Wan SG, Lu CY, Dong HQ, et al. Autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Int J Hematol. 2006;84(3):276-81.
- 76. Openshaw H, Lund BT, Kashyap A, Atkinson R, Sniecinski I, Weiner LP, et al. Peripheral blood stem cell transplantation in multiple sclerosis with busulfan and cyclophosphamide conditioning: report of toxicity and immunological monitoring. Biol Blood Marrow Transplant. 2000;6(5A):563-75.
- 77. Fassas A, Kimiskidis VK, Sakellari I, Kapinas K, Anagnostopoulos A, Tsimourtou V, et al. Long-term results of stem cell transplantation for MS: a single-center experience. Neurology. 2011;76(12):1066-70.
- 78. Saiz A, Blanco Y, Berenguer J, Gómez-Choco M, Carreras E, Arbizu T, et al. Resultado clínico a 6 años del trasplante autólogo de progenitores hematopoyéticos en la esclerosis múltiple. Neurologia. 2008;23(7):405-7.
- 79. Kozák T, Havrdová E, Pit'ha J, Gregora E, Pytlík R, Maaloufová J, et al. Immunoablative therapy with autologous stem cell transplantation in the treatment of poor risk multiple sclerosis. Transplant Proc. 2001;33(3):2179-81.
- 80. Kozák T, Havrdová E, Pitha J, Mayerova K, Novakova L, Trneny M, et al. Immunoablative therapy with autologous PBPC transplantation in the treatment of poor-risk multiple sclerosis[Internet]. Oral Presentation at: 34th Annual Meeting of the European Group of Blood and Marrow Transplantation; 24 th Meeting of the EBMT Nurses group; 7th Meeting of EBMT Data Management Group; Florence, Italy; 2008.
- 81. Shevchenko YL, Novik AA, Kuznetsov AN, Afanasiev BV, Lisukov IA, Kozlov VA, et al. High-dose immunosuppressive therapy with autologous hematopoietic stem cell transplantation as a treatment option in multiple sclerosis. Exp Hematol. 2008;36(8):922-8.
- 82. Xu J, Ji BX, Su L, Dong HQ, Sun XJ, Liu CY. Clinical outcomes after autologous haematopoietic stem cell transplantation in patients with progressive multiple sclerosis. Chin Med J (Engl). 2006;119(22):1851-5.
- 83. Ni XS, Ouyang J, Zhu WH, Wang C, Chen B. Autologous hematopoietic stem cell transplantation for progressive multiple sclerosis: report of efficacy and safety at three yr of follow up in 21 patients. Clin Transplant. 2006;20(4):485-9.
- 84. Saccardi R, Mancardi GL, Solari A, Bosi A, Bruzzi P, Di Bartolomeo P, et al. Autologous HSCT for severe progressive multiple sclerosis in a multicenter trial: impact on disease activity and quality of life. Blood. 2005;105(6):2601-7.
- 85. Krasulová E, Trneny M, Kozák T, Vacková B, Pohlreich D, Kemlink D, et al. High-dose immunoablation with autologous haematopoietic stem cell transplantation in aggressive multiple sclerosis: a single-centre 10-year experience. Mult Scler. 2010;16(6):685-93.
- 86. Mancardi GL, Murialdo A, Rossi P, Gualandi F, Martino G, Marmont A, et al. Autologous stem cell transplantation as rescue therapy in malignant forms of multiple sclerosis. Mult Scler. 2005;11(3):367-71.
- 87. Fagius J, Lundgren J, Oberg G. Early highly aggressive MS successfully treated by hematopoietic stem cell transplantation. Mult Scler. 2009;15(2):229-37.
- 88. Farge D, Lapobin M, Tyndall A, Fassas A, Mancardi GL, Van Laar J, et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases: an observational study on 12 years' experience from the European Group for Blood and Marrow Transplantation Working Party on Autoimmune Disease. Haematologica. 2010;95(2):284-92. Comment in: Haematologica. 2010;95(2):185-8.
- 89. Saccardi R, Kozak T, Bocelli-Tyndall C, Fassas A, Kazis A, Havrdova E, et al. Autologous stem cell transplantation for progressive multiple sclerosis: update of the European Group for Blood and Marrow Transplantation autoimmune diseases working party database. Mult Scler. 2006;12(6):814-23.
- 90. Nash RA, Dansey R, Storek J, Georges GE, Bowen JD, Holmberg LA, et al. Epstein-Barr virus-associated posttransplantation lymphoproliferative disorder after high-dose immunosuppressive therapy and autologous CD34-selected hematopoietic stem cell transplantation for severe autoimmune diseases. Biol Blood Marrow Transplant. 2003;9(9):583-91.
- 91. Pasquini MC, Voltarelli J, Atkins HL, Hamerschlak N, Zhong X, Ahn KW, et al. Transplantation for autoimmune disease in north and South America: a report of the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2012;18(10):1471-8.
- 92. Hamerschlak N, Rodrigues M, Moraes DA, Oliveira MC, Stracieri AB, Pieroni F, et al. Brazilian experience with two conditioning regimens in patients with multiple sclerosis: BEAM/horse ATG and CY/rabbit ATG. Bone Marrow Transplant. 2010;45(2):239-48.
- 93. Saccardi R, Freedman MS, Sormani MP, Atkins H, Farge D, Griffith LM, et al. A prospective, randomized, controlled trial of autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: a position paper. Mult Scler. 2012;18(6):825-34. Comment in: Mult Scler. 2012;18(6):772-5.
- 95. Halt MS: High-dose immunosuppression and autologous stem cell transplantation for poor prognosis multiple sclerosis [Internet]. Houston; 2008. [cited 2010 Jun 3]. Available from: www.halt-ms.org
- 96. Nash RA, Bowen JD, Kraft GH, Popat U, Racke MK, Devine SM, Wundes A, Georges GE. High-dose immunosuppression and autologous transplantation for multiple sclerosis (HALT MS) study [Internet]. ClinicalTrials.gov NCT00288626.[cited 2012 Jan 13]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00288626?term=NCT00288626&rank=1
- 98. Nash RA, Hutton GJ, Racke MK, Popat UR, Devine SM, Griffith LM, et al. Treatment of severe relapsing-remitting multiple sclerosis with high-dose immunosuppressive therapy and autologous hematopoietic cell transplantation: early results of the HALT MS Clinical Trial (Immune tolerance network: ITNO33AI) [Internet]. Paper presented at: 53rd Annual Meeting and Exposition. San Diego; 2011 [abtract 3075]. [cited 2012 Jun 23]. Available from: https://ash.confex.com/ash/2011/webprogram/Paper38504.html
- 99. Snowden JA, Saccardi R, Allez M, Ardizzone S, Arnold R, Cervera R, et al. Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2012;47(6):770-90.
- 100. Pasquini MC, Griffith LM, Arnold DL, Atkins HL, Bowen JD, Chen JT, et al. Hematopoietic stem cell transplantation for multiple sclerosis: collaboration of the CIBMTR and EBMT to facilitate international clinical studies. Biol Blood Marrow Transplant. 2010;16(8):1076-83.
- 101. Carrá A, Macías-Islas MÁ, Gabbai AA, Correale J, Bolaña C, Sotelo ED, et al. Optimizing outcomes in multiple sclerosis: consensus guidelines for the diagnosis and treatment of multiple sclerosis in Latin America. Ther Adv Neurol Disord. 2011;4(6):349-60.
- 102. Voltarelli JC, Moraes DA, Ribeiro AA, Oliveira MC, Rodrigues M, Brum DG, et al. [Brazilian consensus on hematopoietic stem cell transplantation for autoimmune diseases]. Rev Bras Hematol Hemoter. 2010;32(supl. 1):125-35. Portuguese.
- 103. Kuwana M. Induction of anergic and regulatory T cells by plasmacytoid dendritic cells and other dendritic cell subsets. Human Immunol. 2002;63(12):1156-63.
- 104. Le Blanc K, Ringdén O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(5):321-34.
- 105. Le Blanc K, Ringdén O. Mesenchymal stem cells: properties and role in clinical bone marrow transplantation. Curr Opin Immunol. 2006;18(5):586-91.
- 106. Lu L, Thomson AW. Manipulation of dendritic cells for tolerance induction in transplantation and autoimmune disease. Transplantation. 2002;73(1 Suppl):S19-22.
- 107. Lu L, Gambotto A, Lee WC, Qian S, Bonham CA, Robbins PD, et al. Adenoviral delivery of CTLA4Ig into myeloid dendritic cells promotes their in vitro tolerogenicity and survival in allogeneic recipients. Gene Ther. 1999;6(4):554-63.
Publication Dates
-
Publication in this collection
05 June 2013 -
Date of issue
2013