Abstracts
A medium was prepared from brewery waste yeast with and without mineral salts to study growth and yellow-green fluorescent pigment production (YGFP) by Pseudomonas fluorescens. The King's medium used for detection of siderophore production were expressively weaker inductors of YGFP formation when compared to FYE medium. Although FYE and CYE could be used for growth of P. fluorescens, only FYE was an attractive medium for detection of YGFP strain producers.
Pseudomonas fluorescens; siderophore; yeast extract; yellow-green fluorescent pigment
Diversos microrganismos secretam substâncias com alta afinidade por ferro. Estas moléculas, sideróforos, transportam ferro para o interior das células. Como a produção destas moléculas depende da composição do meio, foi avaliada a influência do extrato de levedura (FYE), proveniente de resíduo de cervejaria, com e sem adição de sais minerais, sobre o crescimento de Pseudomonas fluorescens e sobre a formação de pigmento fluorescente verde-amarelado (YGFP). Observou-se que (i) FYE com sacarose (G7) e o extrato de levedura comercial (CYE) possuem um pico bem definido próximo a 260 nm; (ii) FYE, mas não CYE, promove alta formação de YGFP. Os meios de King's, usados para detectar a formação de sideróforo, se comportaram como indutores expressivamente mais fracos de YGFP que o meio FYE. Embora FYE e CYE possam ser usados para o crescimento de P. fluorescens, apenas FYE pode ser usado como meio para a detecção de linhagens formadoras de YGFP.
BIOLOGICAL AND APPLIED SCIENCES
Production of yellow-green fluorescent pigment by Pseudomonas fluorescens
Gildo Almeida da Silva* * Author for correspondence ; Erik Amazonas de Almeida
Embrapa Uva e Vinho; gildo@cnpuv.embrapa.br; erik.almeida@gmail.com; 95700-000, Bento Gonçalves - RS - Brasil
ABSTRACT
A medium was prepared from brewery waste yeast with and without mineral salts to study growth and yellow-green fluorescent pigment production (YGFP) by Pseudomonas fluorescens. The King's medium used for detection of siderophore production were expressively weaker inductors of YGFP formation when compared to FYE medium. Although FYE and CYE could be used for growth of P. fluorescens, only FYE was an attractive medium for detection of YGFP strain producers.
Key words: Pseudomonas fluorescens, siderophore, yeast extract, yellow-green fluorescent pigment
RESUMO
Diversos microrganismos secretam substâncias com alta afinidade por ferro. Estas moléculas, sideróforos, transportam ferro para o interior das células. Como a produção destas moléculas depende da composição do meio, foi avaliada a influência do extrato de levedura (FYE), proveniente de resíduo de cervejaria, com e sem adição de sais minerais, sobre o crescimento de Pseudomonas fluorescens e sobre a formação de pigmento fluorescente verde-amarelado (YGFP). Observou-se que (i) FYE com sacarose (G7) e o extrato de levedura comercial (CYE) possuem um pico bem definido próximo a 260 nm; (ii) FYE, mas não CYE, promove alta formação de YGFP. Os meios de King's, usados para detectar a formação de sideróforo, se comportaram como indutores expressivamente mais fracos de YGFP que o meio FYE. Embora FYE e CYE possam ser usados para o crescimento de P. fluorescens, apenas FYE pode ser usado como meio para a detecção de linhagens formadoras de YGFP.
INTRODUCTION
Siderophores are metabolites produced by microorganisms. These compounds bind ferric iron, promote the rate of Fe3+ transport, and thus alleviate the problem of iron unavailability. Iron is the fourth most common element in the earth's crust. It is present in soil, with rare exceptions, in the form of oxide hydrates, which have small dissociation constants. Complexation by peptides can also make iron unavailable (Budzikiewicz, 1997). These facts explain why iron is not readily available to microorganisms. Microorganisms other than Pseudomonas like Legionella pneumophila (Liles et al., 2000), Halomonas. Marinobacter (Martinez et al., 2000), and Rhizobium (Guerinot et al., 1990; Barsomian et al., 1992; Persmark et al., 1993; Roy et al., 1994), can produce iron-scavenging molecules.
As microorganisms are in competition for available nutrients, including iron, it is not uncommon to link iron-binding molecules to microbial virulence (Sokol, 1986; Takase et al., 2000; Lamont et al., 2002). Siderophores involved in virulence must have a greater affinity for iron than other iron binding compound in order to be effective (Telford and Raymond, 1998). The relationship between virulence and biological control has been investigated by Genthner et al. (2004). If the virulence of a microorganism is related to the ability of producing siderophore, altering the requirement for iron can also change the general activity of the microorganism in regard to biological control. The success of this kind of control depends, among others, on how aggressive the microorganism is. In fact, it was demonstrated that substrate in which the microorganism was grown could increase virulence (Genthner et al., 2004).
The siderophore production has also been shown to be a medium composition-dependent process. King et al. (1948) demonstrated that fluorescin production was dependent primarily upon the concentration of sulphate, iron, and magnesium in the medium. King et al. (1954) prepared two different media containing as common ground potassium and magnesium salts and glycerol. The medium with proteose peptone was denominated medium B. The other one with peptone was called medium A. The feature of the media defined by King et al. (1948) and King et al. (1954) was the absence of yeast extract. Yeast extract stimulates growth and is an important component of several culture media. One of the benefits of yeast extract should probably be related in some way to the presence of compounds that have the ability to complex iron and thus make it more readily available to microorganisms. In that case, the microorganism does not need to synthesise iron-binding compounds and therefore yeast extract could not be used for siderophore production. The components of yeast extract required by Thermoplasma acidophilum for growth were one or more polypeptides with an affinity for metal ions (Smith et al.,1975). Duffy and Défago(2000) showed that the spontaneous mutant competitiveness was favoured in a nutrient broth containing yeast extract. These mutants reduced the efficacy of wild-type strain for biological control. The problem was solved by using certain mineral amendments or by diluting media. It is well established that the culture media composition has great impact on the siderophore production and on microorganism general behaviour. The aim of this work was to use the fresh yeast extract (FYE) medium, with or without Mg2+ and phosphate addition, prepared from brewery waste for the fluorescent siderophore production by Pseudomonas fluorescens.
MATERIALS AND METHODS
Fresh Yeast Extract (FYE) and medium composition
P. fluorescens was grown in the fresh yeast extract (FYE) medium according to Silva (2002), containing the following, per litre of distilled water: G7 - 114 mL of FYE and 10 g of commercial sucrose; G7a - 114 mL of FYE, 10 g of commercial sucrose and 6.8 g of KH2PO4; G7b - 114 mL of FYE, 10 g of commercial sucrose and 1.5 g of MgSO4.7H2O; G7ab - 114 mL of FYE, 10 g of commercial sucrose, 1.5 g of MgSO4.7H2O, and 6.8 g of KH2PO4; G7c - 114 mL of FYE. The growth and the YGFP production was measured in capped test tubes containing 10 mL of media inoculated with 100 mL of an inoculum (OD600nm 0.15). Cultures were grown at 25 °C with constant agitation (200 rpm) on a platform shaker (New Brunswick Scientific Co. G27). Changes in optical density were followed with a Perkin-Elmer Lambda Bio over 48 h. The samples were diluted in the respective sterile media to give an OD600 between 0.1 and 0.2.
Evaluation of G7c, commercial yeast extract (CYE) and King's media on YGFP production:
The concentration of yeast extract used in CYE was equivalent to amount of total solid matter contained in 114 ml of FYE (2.74 g). The CYE medium was composed of 3 gL-1 yeast extract (Difco). King's A and B media were prepared as described by King et al., (1954). Cultures were grown at 25 °C with constant agitation (150 rpm) for 16 h. The fluorescence of the supernatants was detected under UV light (312nm) using an UV/White light TFP-M\WL table transilluminator (Vilber Loumart- France).
Measurement of growth and pigment formation
Growth was measured as described by Silva (1996). Siderophore assays were performed on supernatants diluted with 0.1 M Na-phosphate buffer pH 7.0 (100 µL sample: 900 µL buffer) by scanning with a Perkin-Elmer Lambda Bio (A600 to A190). The spectrum of salicylic acid (Merck) was also measured to compare its data with G7 medium measurements, since this organic acid is part of some small secreted iron-scavenger molecules (Cox et al., 1981; Ankenbauer and Cox, 1988; Budzikiewicz, 1993; Cornelis and Matthijs, 2002; Crosa and Wlash, 2002). Stock solution of 1 g salicylic acid /100 mL CHCl3 was prepared and further dilutions were made to give a final concentration of 50 µg salicylic acid mL-1.
The absorbance of the supernatant was measured as proposed by Meyer and Abdallah (1978). Determination of the iron-chelating reaction of the pigment-containing supernatant was done by adding successively 5 µL - 35 µL of a freshly prepared 3.25 mM Fe2(SO4)3 solution (Meyer and Abdallah, 1978). Solutions of Fe2(SO4)3 (3.25 mM) and FeCl3 (3.25 mM) were used to determine the spectrogram of Fe+3 salts.
To detect the siderophore inside the cells, a suspension of cells was centrifuged at 10 000 x g (Eppendorf 5416) and the cells were washed 5 times with distilled water. The cells were resuspended in water, transferrerd to a microscope slide, and overnight dryed at 50ºC in a Lab-Line incubator (Imperial II-LabLine Instruments Inc.). The fluorescence of the cells was visually detected under UV light (312nm).
Aerobic growth of bacteria
The initial pH of the culture was 6.8 (±0.2). The growth was monitored in a Biolafitte bioreactor (Biolafitte, France) was described elsewhere (Silva, 1996).
Statistical analysis
The treatments performed in flasks were made in an entirely randomised experiment with three repetitions. Results obtained under different experimental conditions were evaluated by analysis of variance (one-way Anova) and the difference between means was compared by the Tukey test. In each case, results were considered significant at P<0.05.
Chemicals
All chemicals, except sucrose (analytical reagent grade), were purchased from Merck. Commercial sucrose was acquired from local trade.
RESULTS AND DISCUSSION
Spectrogram of prepared media (FYE), commercial yeast extract (CYE) and Fe3+ salts
The absorption spectrum of the diluted G7 medium (100 µL mL-1) showed a single well-defined peak in the 232 nm-392 nm region, with maximum near 260 nm (Fig 1a). This peak almost coincided with one of those peaks for pyochelin at 252 nm found in supernatant of P. aeruginosa (Meyer et al., 1992) or at 250 nm for P. cepacia (Sokol et al., 1992). The commercial salicylic acid solution (50 µg mL-1) spectrum gave two peaks, with maximum at 242 nm and 308 nm (Fig 1) and exhibited a completely different spectrum compared to those obtained with the G7 medium.
It is well known that some biological molecules give peaks at 260 nm. The heterocyclic ring structures in DNA and RNA, for instance, absorb light with a maximum absorbance near 260 nm. Aromatic amino acids of proteins and peptides absorb light with a maximum near 280 nm. Spectrograms made with FeCl3 showed a maximum absorbance near 287 nm in water and near 280 nm in Na-phosphate buffer (pH 7.0), with no peak around 400 nm (Table 1). The successive addition of fresh FeCl3 to G7 medium also resulted in a linear increase in absorbance at 260 nm y=0.0161(µL Fe solution) + 0.3904 (r2 = 0.999). Ferric sulfate solution gave maximum absorption at 296 nm in water. It has been shown that iron-chelator complex formation increases the absorbance at 400 nm (Meyer and Abdallah, 1978; Misaghi et al., 1982; Roy and Chakrabartty, 2000). The results here obtained suggested that the increase of absorbance due to addition of iron solution might mean a chelation process provoked by G7 medium.
The crescent addition of FeCl3 solution to supernatants of P. fluorescens increased the absorbances at 265 nm and at 400 nm. At 265 nm, there was a linear increase (y = 0.0143µL(FeCl3) + 0.399; r2 = 0.9987) and at 400 nm, a logistic dose response increase was observed (y= a+b/(1+(µL(FeCl3)/c)d; r2= 0.984) (Fig 1b). Although there was no clear evidence of the extent of the increase in the absorbance at 265 nm with FeIII addition that could be attributable to the iron-chelator complex formation, the fact was that FEY induced YGFP. These inductors were autoclave temperature-resistant compounds. It was observed that components of cell wall of Saccharomyces cerevisiae bind iron (Lesuisse et al., 1987). It was likely that these cell wall components were extracted and were present in FYE. Amino acids are the usual components of iron-binding compounds.
Siderophores, such as pyoverdine and pyochelin, are biologically active derivatives of small peptides or peptide-like molecules (Byers and Arceneaux, 1998). Viable cells of S. cerevisiae possess other compounds heme molecules that are connected with inducible ferri-reductase activity and iron uptake (Lesuisse and Labbe, 1989).
Pyochelin is a small molecule formed by condensation of one molecule of salicylic acid and two cyclized molecules of cysteine (Cox et al., 1981; Ankenbauer and Cox, 1988). Pyoverdine is a more complex molecule in that it contains a quinoline chromophore, a peptide chain, and a dicarboxylic acid (Budzikiewicz, 1993). Meyer et al. (1992) showed that the spectrum of salicylic acid from P. fluorescens and from a commercial source had the same graphic behaviour but differed totally from the pyochelin spectrum formed by P. aeruginosa. ;
The presence of thiazolidines linked to salicylic acid in the pyochelin molecule changes the spectral behaviour of linked salicylic acid. It is possible that the peak near 260 nm represents a chelating agent. Alkaline hydrolysis made the peak to disappear (data not shown), corresponding probably to the ring opening of a thiazolidine-related compound. Another salicylic acid-based siderophore, pseudomonine, has been identified in P. fluorescens with potential role in plant disease suppression (Mercado-Blanco et al., 2001; Cornelis and Matthijs, 2002; Crosa and Walsh, 2002).
This siderophore included a histamine moiety. One hypothesis could be that the iron depletion could be associated with some chelating agents of FYE. If P. fluorescens cells promptly assimilated the iron bound to chelating agents of FYE, these bacteria would not produce YGFP, otherwise scavenging-molecules (YGFP) should be formed. The transference of iron from chelating agent of FYE to YGFP molecules should decrease the absorption near 260 nm with concomitant absorption increase at 400 nm.
Behaviour of P. fluorescens grown in G7, G7a, G7b, G7c, and G7ab media
Changes in spectrum of media before inoculation as a function of salts and commercial sucrose additions are depicted in Figure 2. The highest peaks near 260 nm were obtained with G7ab medium, which contained commercial sucrose, magnesium, and potassium salts (Fig 2e). The lowest peaks were achieved with G7c in which no commercial sucrose and no mineral salts were added (Fig 2d). In agreement with the evidence already presented above that the presence of iron increased the absorbance near 260 nm (Fig 1b), these results suggested that the commercial sucrose and the salts of magnesium and potassium used could be the sources of Fe+3. If P. fluorescens produced siderophore, the iron of the medium should be unavailable and some FYE compounds should induce this iron unavailability.
To determine whether compounds of FYE could induce YGFP formation, P. fluorescens was grown in these different liquid media. Changes in absorption spectrum of media and their respective culture supernatants are depicted in Figure 2. All peaks near 260 nm of culture media were higher than those of corresponding supernatants (Figs 2a, 2b, 2c, 2d and 2e) and at 400 nm the results were the opposite, suggesting that FYE induced siderophore production and, consequently, good use of iron and significant growth (Fig 2f). These results supported the hypothesis that chelating agents of FYE simulated iron depletion. It was found that there were no peaks at 400 nm and at 450 nm in all the media tested before inoculation (Figs 2a, 2b, 2c, 2d and 2e), showing the absence of YGFP in these media. The highest production of YGFP was achieved with G7ab medium (Fig 2e). YGFP should correspond to the fluorescent pigment found by Meyer and Abdallah (1978). It has been observed that in the presence of inorganic phosphate, salicylate cannot hold iron and the influence of salicylate depends on the prevailing concentrations of phosphate and Mg2+. In that case, the iron solubilization must be made by another compound (Ratledge et al., 1974). G7ab is a phosphate and magnesium-containing medium. If the lack of a certain functional chelating agent leads to enhance the production another chelating molecules, phosphate and magnesium seem to be indirectly involved with the constitutive production of YGFP. The bacterial growth in G7ab medium was highly significant over the other media (P<0.01). The growth in G7c was significantly lower than in G7a and in G7b (P<0.05). The growth in G7, G7a, G7b, and G7c was not significantly different (P>0.05) (Fig 2f).
Comparison between G7c and CYE
Because G7c corresponded to 3 gL-1 yeast extract, equivalent concentration of commercial yeast extract from Difco (CYE) was used to see whether the yeast extract itself accounted for biological production of YGFP. The absorption spectrum of the diluted G7c and CYE media (100 µLmL-1 Na-Phosphate buffer) showed the same peaks in the 232 nm-392 nm region, with maximum near 260 nm (Fig 2d). Both media supported good growth, with significant superiority for growth in G7c (P<0.05). The effect of medium composition (G7c, CYE, King's A and King's B) on YGFP by P. fluorescens was evident both in crude culture media and inside the cells after several washings and overnight drying at 50ºC (Fig 3). The amount of CYE was increased to 10 gL-1 to see whether the synthesis of YGFP was dependent upon the yeast extract powder concentration. The results were the same as those depicted in Figure 3.
The components of the medium that gave peak near 260 nm could not be directly correlated with YGFP production but the CYE seemed to be involved in some way with the ability of making iron more promptly available to microorganism. Since there was higher absorption near 260 nm in CYE medium than in the supernatant without YGFP production, and, at the same time, a good growth, it could be assumed that the iron chelated by CYE components was readily available to the microorganism. The difference found between G7c and CYE in regard to the production of YGFP by P. fluorescens was probably due to the loss of chemical components during the processing for finished product. These results showed that the component responsible for the induction of siderophore production by P. fluorescens was present in FYE but not in the commercial yeast extract (CYE). Even without knowing the specific compounds involved, the results clearly demonstrated a differential ability of FYE to induce YGFP production.
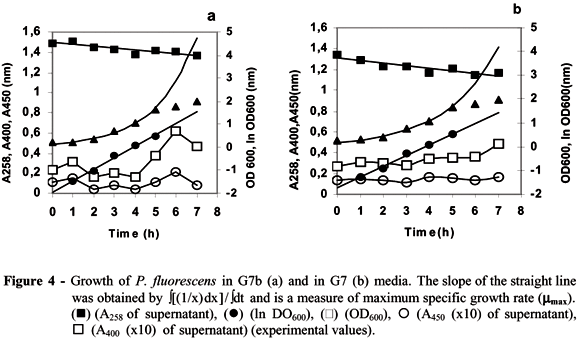
Growth of P. fluorescens and YGFP production in stirred-tank bioreactor
Growth curves generated in stirred-tank bioreactor vessel indicated that in G7b medium (Fig 4a) the microorganism showed a higher maximum specific growth (0.502 h-1) than in G7 (0.451 h-1) medium (Fig 4b). The exponential growth of P. fluorescens lasted 4 h in both media and the microorganism presented a short lag-phase. The absorbance at 258 nm decreased gradually in both media. The production of pyoverdine (400 nm) displayed oscillations and the effectiveness in production began to increase after 4 h. The Fe3+-pyoverdine complex (450 nm) exhibited less changing production in both media. It seemed that P. fluorescens needed more time to start an effective production of pyoverdine in G7 medium.
The oscillation observed at 400 nm in both media when P. fluorescens was grown in the stirred-tank bioreactor could be due to formation of a Fe3+- complex and subsequent use of iron, and thus breaking down the complex decreased the absorbance. This hypothesis was supported by the observations that the A450 followed the same oscillation. The concentration of YGFP at time zero was due to a 15 hour-old prepared inoculum. The results suggested that YGFP started late in the exponential phase of growth and achieved its maximum during the stationary phase. Similar results were found for L. pneumophila (Goldoni et al., 1991). The results provided evidences that Mg2+ curtailed the length of lag-phase for YGFP formation.
ACKNOWLEDGEMENTS
The authors thank to L.M.R. de Mello for critical reading of this manuscript and to M.A.L. Morini for valuable technical assistance.
Received: October 14, 2004;
Revised: July 01, 2005;
Accepted: February 20, 2006.
- Ankenbauer, R. G. and Cox, C. D. (1988), Isolation and characterization of Pseudomonas aeruginosa mutants requiring salicylic acid for pyochelin biosynthesis. J. Bacteriol, 170, 5364-5367.
- Barsomian, G. D.; Urzainqui, A.; Lohman, K. and Walker, G. C. (1992), Rhizobium meliloti mutants unable to synthetize anthranilate display a novel symbiotic phenotype. J. Bacteriol, 174, 4416-4426.
- Budzikiewicz, H. (1993), Secondary metabolites from fluorescent pseudomonads. FEMS Microbiol. Rev, 104, 209-228.
- Budzikiewicz, H. (1997), Siderophores of fluorescent pseudomonads. Z. Naturforsch, 52c, 713-720.
- Byers, B. R. and Arceneaux, J. E. L. (1998), Microbial iron transport: iron acquisition by pathogenic microorganisms. Met. Ions Biol. Syst, 35, 37-66.
- Cornelis, P. and Matthijs, S. (2002), Diversity of siderophore-mediated iron uptake systems in fluorescent pseudomonads: not only pyoverdines. Environ. Microbiol, 4, 787-798.
- Cox, C. D.; Rinehart, K. L.; Moore, M. L. and Cook, J. C. (1981), Pyochelin: novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA, 78, 4256-4260.
- Crosa, J. H. and Walsh, C. T. (2002), Genetics and Assembly Line Enzymology of Siderophore Biosynthesis in Bacteria. Microbiol. Mol. Biol. Rev, 66, 223-249.
- Duffy, B. K. and Défago, G. (2000), Controlling instability in gacS-gacA regulatory genes during inoculant production of Pseudomonas fluorescens biocontrol strains. Appl. Environ. Microbiol, 66, 3142-3150.
- Genthner, F. J.; Foss, S. S. and Glas, P. S. (2004) Virulence of Metarhizium anisopliae to embryos of the grass shrimp Palaemonetes pugio Available in: http://www.isb.vt.edu/brarg/brasym95/genthner95.htm Access on: 8 Sept. 2004.
- Goldoni, P.; Visca, P.; Pastoris, M. C.; Valenti, P. and Orsi, N. (1991), Growth of Legionella spp. under conditions of iron restriction. J. Med. Microbiol, 34, 113-118.
- Guerinot, M. L.; Meidl, E. J. and Plessner, O. (1990), Citrate siderophore in Bradyrhizobium japonicum J. Bacteriol, 172, 3298-3303.
- King, J. V.; Campbell, J. R. and Eagles, B. A. (1948), The mineral requirements for fluorescin production. Can. J. Res, 26, 514-519.
- King, E. O.; Ward, M. K. and Raney, D. E. (1954), Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. and Med, 44, 301-307.
- Lamont, I. L.; Beare, P. A.; Ochsner, U.; Vasil, A. I. and Vasil, M. L. (2002), Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA, 99, 7072-7077.
- Lesuisse, E. and Labbe, P. (1989), Reductive and non-reductive mechanisms of iron assimilation by yeast Saccharomyces cerevisiae J. Gen. Microbiol, 135, 257-263.
- Lesuisse, E.; Raguzzi, F. and Crichton, R. R. (1987), Iron uptake by the yeast Saccharomyces cerevisiae: Involvement of a reduction step. J. Gen. Microbiol, 133, 3229-3236.
- Liles, M. R.; Schee, T. A. and Cianciotto, N. P. (2000), Discovery of a nonclassical siderophore, legiobactin, produced by strains of Legionella pneumophila, J. Bacteriol, 182, 749-757.
- Martinez, J. S.; Zhang, C. P.; Hott, P. D.; Jung, H.-T.; Carrano, C. J.; Haygood, M. G. and Butler, A. (2000), Self-assembling amphiphilic siderophores from marine bacteria. Science, 287, 1245-1247.
- Mercado-Blanco, J.; Drift, K. M. G. M. van der; Olsson, P.E.; Thomas-Oates, J.E.; Loon, L. C. van and Bakker, P. A. H. M. (2001), Analysis of the pmsCEAB gene cluster involved in biosynthesis of salicylic acid and the siderophore pseudomonine in the biocontrol strain Pseudomonas fluorescens WCS374. J. Bacteriol, 183, 1909-1920.
- Meyer, J. M.; Azelvandre, P. and Georges, C. (1992), Iron metabolism in Pseudomonas: salicylic acid, a siderophore of Pseudomonas fluorescens CHAO. BioFactors, 4, 25-27.
- Meyer, J. M. and Abdallah, M. A. (1978), The fluorescent pigment of Pseudomonas fluorescens: biosynthesis, purification and physicochemical properties. J. Gen. Microbiol, 107, 319-328.
- Misaghi, I. J.; Stowell, L. J.; Grogan, R. G. and Spearman, L. C. (1982), Fungistatic activity of water-soluble fluorescent pigments of fluorescent pseudomonads. Phytopathol, 72, 33-36.
- Persmark, M.; Pittman, P.; Buyer, J. S.; Schym, B.; Gill, P. R. and Neilands J. B. (1993), Isolation and structure of rhizobactin 1021, a siderophore from alfafa symbiont Rhizobium meliloti 1021. J. Am. Chem. Soc., 115, 3950-3956.
- Ratledge, C.; Macham, L. P.; Brown, K. A. and Marshall, B. J. (1974), Iron transport in Mycobacterium smegmatis: a restricted role for salicylic acid in the extracellular environment. Biochim. Biophys. Acta, 372, 39-51.
- Roy, N.; Bhattachryya, P. and Chakrabartty, P. K. (1994), Iron acquisition during growth in an iron deficient medium by Rhizobium sp. isolated from Cicer arietium Microbiology, 140, 2811-2820.
- Roy, N. and Chakrabartty, P. K. (2000), Effect of Aluminum on the production of siderophore by Rhizobium sp. (Cicer arietinum) Curr. Microbiol., 41, 5-10.
- Silva G. A. and Embrapa. (2002), Composição para o controle biológico de fitopatógenos, processo de sua obtenção e seus usos. Brazilian Patent Application.
- Silva, G. A. (1996), Occurrence of killer, sensitive, and neutral yeasts in Brazilian Riesling Italico grape must and the effect of neutral strains on killing behaviour. Appl. Microbiol. Biotechnol., 46, 112-121.
- Smith, P. F.; Langworthy, T. A. and Smith, M.R. (1975), Polypeptide nature of growth requirement in yeast extract for Thermoplasma acidophilum. J. Bacteriol., 124, 884-892.
- Sokol, P. A.; Lewis, C. J. and Dennis, J. J. (1992), Isolation of a novel siderophore from Pseudomonas cepacia J. Med. Microbiol., 36, 184-189.
- Sokol, P. A. (1986), Production and utilization of pyochelin by clinical isolates of Pseudomonas cepacia J. Clin. Microbiol., 23, 560-562.
- Takase, H.; Nitanai, H.; Hoshino, K. and Otani, T. (2000), Impact of siderophore production on Pseudomonas aeruginosa infections in immunosuppressed mice. Infect. Immun., 68, 1834-1839.
- Telford, J. R. and Raymond, K. N. (1998), Coordination chemistry of the amonabactins, bis (catecholate) siderophores from Aeromonas hydrophila. Inorg. Chem., 37, 4578-4583.
Publication Dates
-
Publication in this collection
21 June 2006 -
Date of issue
May 2006
History
-
Reviewed
01 July 2005 -
Received
14 Oct 2004 -
Accepted
20 Feb 2006