Abstract
Two methods were used to make crude preparations of surface-active compounds (SACs) produced by Gordonia amicalis grown on the medium containing 1% diesel oil. Using a 2:1 (v/v) solution of chloroform:methanol for extraction, Type I SACs were isolated and shown to produce oil in water (O/W) emulsions. Type II SACs were isolated by precipitation with ammonium sulfate and produced predominantly water in oil emulsions (W/O). The crude Type I and II preparations were able to produce a significant reduction in the surface tension of water; however, the crude Type II preparation had 10-25 fold higher emulsification activity than the Type I preparation. Both SAC preparations were analyzed by the TLC and each produced two distinct bands with Rf 0.44 and 0.62 and Rf 0.52 and 0.62, respectively. The partially purified SACs were characterized by the ESI(+)-MS, FT-IR and NMR. In each one of these fractions, a mixture of 10 oligomers was found consisting of a series of compounds, with masses from 502 to 899, differing in molecular mass by a repeating unit of 44 Daltons. The mass spectra of these compounds did not appear to match other known biosurfactants and could represent a novel class of these compounds.
Bioemulsifier; bioremediation; biosurfactant; Gordonia amicalis; industrial chemicals; microbial surfactant production
ENVIRONMENTAL SCIENCES
Production and characterization of surface-active compounds from Gordonia amicalis
Ani Beatriz Jackisch-MatsuuraI,* * Author for correspondence: ani@amazonia.fiocruz.br ; Leonardo Silva SantosII; Marcos Nogueira EberlinIII; Andréia Fonseca de FariaVI; Takeshi MatsuuraIV; Matthew James GrossmanV; Lucia Regina DurrantVI
ICentro de Pesquisa Leônidas e Maria Deane/Fiocruz Amazônia, Manaus - AM - Brasil
IIInstituto de Química de Recursos Naturales; Universidad de Talca; Talca - Chile
IIILaboratório Thomson de Espectrometria de Massas; Instituto de Química; Universidade Estadual de Campinas; Campinas - SP - Brasil
IVInstituto de Ciências Biológicas; Universidade Federal do Amazonas; Manaus - AM - Brasil
VBioSage; Lawrenceville, New Jersey - USA
VIFaculdade de Ciências de Alimentos; Universidade Estadual de Campinas Campinas - SP - Brasil
ABSTRACT
Two methods were used to make crude preparations of surface-active compounds (SACs) produced by Gordonia amicalis grown on the medium containing 1% diesel oil. Using a 2:1 (v/v) solution of chloroform:methanol for extraction, Type I SACs were isolated and shown to produce oil in water (O/W) emulsions. Type II SACs were isolated by precipitation with ammonium sulfate and produced predominantly water in oil emulsions (W/O). The crude Type I and II preparations were able to produce a significant reduction in the surface tension of water; however, the crude Type II preparation had 10-25 fold higher emulsification activity than the Type I preparation. Both SAC preparations were analyzed by the TLC and each produced two distinct bands with Rf 0.44 and 0.62 and Rf 0.52 and 0.62, respectively. The partially purified SACs were characterized by the ESI(+)-MS, FT-IR and NMR. In each one of these fractions, a mixture of 10 oligomers was found consisting of a series of compounds, with masses from 502 to 899, differing in molecular mass by a repeating unit of 44 Daltons. The mass spectra of these compounds did not appear to match other known biosurfactants and could represent a novel class of these compounds.
Key words: Bioemulsifier, bioremediation, biosurfactant, Gordonia amicalis, industrial chemicals, microbial surfactant production
INTRODUCTION
Gordonia strains have received considerable attention recently due to their ability to degrade a wide variety of xenobiotic and environmental pollutants such as alkanes, polyisoprenes and aromatic hydrocarbons as well as for their ability to desulfurize dibenzothiophene and benzothiophene. The production of surface-active compounds (SACs) by Gordonia sp. has been associated with their ability to degrade the hydrophobic compounds (Arenskötter et al. 2004). However, very little information is currently available about the SACs produced by the Gordonia members. In biomedicine, biosurfactants, or their derivatives are used as detergents, disinfectants, biocides, anti-adhesive coatings, and anti-thrombotic agents. In the food industry, they are used as emulsifiers for the processing of raw materials whereas in agriculture, biosurfactants are used for biological control of plant pathogens. These versatile molecules are also of interest in the cosmetic industry because of their moisturizing properties and skin compatibility (Brown 1991; Stanghellini and Miller 1997; Banat et al. 2000; Singh et al. 2007).
This work aimed to study the production and isolation of two different preparations of SAC's using a Gordonia amicalis strain isolated from diesel-contaminated soil and the partial characterization of the structures of the compounds.
MATERIALS AND METHODS
16s Sequence Analysis of the Biosurfactant Producing Isolate
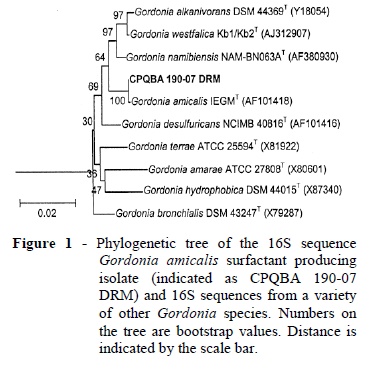
G. amicalis strain DRM 190-07 was isolated from the soil contaminated with diesel oil collected near the REPLAN petroleum refinery in Campinas, São Paulo, Brazil. This strain was originally identified by morphological and biochemical analysis as Planococcus citreus strain CCT 4018 (Jacobucci et al. 2001; Jacobucci et al. 2009). The isolate has been subsequently reclassified as G. amicalis based on 16S rDNA sequence analysis. Total DNA from an isolated colony was purified with the Qiagen genomic DNA extraction kit (Qiagen, CA, USA) according to the manufacturer's instructions. Primers for amplification of 16S rDNA were p 27F (5′-AGA GTT TGA TCM TGG CTC AG-3′ (′M = A or C)) and p 1401R (5'-GCG TGT GTA CAA GAC CC-3'), homologous to the conservative ends of bacterial 16S rDNA. Amplicons obtained from the 16S rDNA were purified (GFX PCR DNA and Gel Band Purification kit GE Health Care, USA) and sequenced with a MegaBACE automated sequencing system (1000 GE Health Care, USA). The sequence (1293 bp) was compared to the Ribosomal Database Project (RDP) data base and a phylogenetic tree was produced using a range of Gordonia 16S sequences obtained from the GeneBank database using the RDP online tools "Seqmatch" and "Tree Builder" (Cole et al. 2007; Cole et al. 2009). Comparison of the sequence against the GeneBank database revealed that it was 100% identical to that of a G. amicalis isolated previously and identified as a dibenzothiophene-desulphurizing actinomycete (11, 16S Genebank sequence ID AF101418.1, RDP identifier S000428902). Figure 1 show a phylogenetic tree based on alignment of the 16S sequence of the surfactant producing isolate and other Gordonia sequences obtained from the RDP (http://rdp.cme.msu.edu/) database.
Biosurfactant Production and Isolation
The bacterium was grown on GYP medium (2% glucose, 0.5% yeast extract; 1% peptone; 2% agar) at 30oC for 72 h, after which the cells were harvested and cell suspensions with OD610nm = 2.0 were prepared and used to inoculate 7 L of liquid medium (1mL/50mL medium) in a BioFlo III fermenter (New Brunswick Scientific) with 14 L capacity. The medium contained (%) 0.05 MgSO4, 0.3 NaNO3, 0.1 KH2PO4, 0.1 yeast extract and 0.03 peptone, and supplemented with 1% diesel oil. The inoculated medium was incubated at 30oC with 250 rpm agitation and 0.3 vvm aeration for 72 h (Rapp and Backhaus 1992). Culture broth was made cell free by centrifugation at 16,192 xg for 15 minutes, followed by filtration through a Whatman 1 filter. Crude extracts of Type I SACs were obtained by extraction with chloroform/methanol (2:1, v/v) (Rocha et al. 1992) while Type II SACs were obtained by precipitation with ammonium sulfate (Navon-Venezia et al. 1995). Crude extracts of both Type I and II SACs were lyophilized and maintained at 4oC until used.
Surface Tension Measurement
The surface tension of the culture broth (20 mL), raw extracts of Type I (1 to 5 mg/mL) and Type II (0.2 mg/mL) and partially purified Type I SACs (1.5 mg/mL) were evaluated. Lyophilized crude extracts and partially purified SACs were re-dissolved in deionized water and the surface tension of these preparations and the culture broth was measured with a tensiometer (K10ST; Krüss, Hamburg, Germany) at 20oC by the Du Nouy method using a platinum-iridium ring.
Emulsification Test
Emulsification was evaluated using SAC preparation solutions as described for surface tension measurements in 1.0 cm diameter test tubes containing 3.5 mL of the Type I, or Type II solutions and 2.0 mL of toluene as emulsification substrate. Optical density was measured at 610 nm with a spectrophotometer before and after vigorous mixing for one minute on a vortex mixer. The ∆OD was reported as oil-in-water (O/W) emulsification activity. After 24 h, the height of the emulsion layer that formed above the aqueous phase was measured and reported as water-in-oil (W/O) emulsification activity and expressed in cm. Water-in-oil emulsification activities were classified based on the emulsion height formed as high (≥ 1.8 cm), moderate (1 to 1.7 cm) and low (<1 cm). Oil-in-water emulsions were classified as high OD610nm≥1.2, moderate OD610nm 0.7 to 1.1 and low OD610nm 0.1 to 0.6.
Comparison of G. amicalis's Sacs with Commercial Surfactants
Surface tension measurements and emulsification activities of the SACs isolated from G. amicalis's growth media were compared to those obtained with the synthetic surfactants such as Triton X-100®(t-octylphenoxypoly-ethoxyethanol), Niaproof®(Sodium 7-ethyl-2-methyl-4-undecyl sulfate) and Span 20®(Sorbitan monolaurate), and the biosurfactant Surfactin (all obtained from Sigma) and used at the concentration of 106 µg/mL, 415 µg/mL and 516 µg/mL, and 200 µg/mL, respectively.
Antimicrobial activity
Type I and II extracts were assayed for antimicrobial activity by the diffusion method in agar at concentrations of 10, 100, 1000 and 10000 mg/L. Ten µL of each SAC solution were added to a 6.0 mm paper filter disk and tested against Staphylococcus aureus, Bacillus cereus, Pseudomonas aeruginosa, Escherichia coli, Listeria monocytogenes, Mycobacterium smegmatis, Candida albicans and Aspergillus flavus.
Thin-Layer Chromatography
Thin-layer chromatography (TLC) was carried out on silica gel 60 F 254 plates (Merck). Crude extracts (2.5 mg) of the SACs were dissolved in 100 µL of methanol. Ten microliter aliquots of the methanol solutions were developed with chloroform/MeOH/NH4OH (65:25:4). After air-drying, the SACs were identified by spraying the plates with Rhodamine 6G (0.005%) and visualizing under UV light and by UV visualization alone. After TLC treatment, the partially purified SAC's were obtained by scraping the bands off the plates and re-suspended in the TLC eluting phase solution. The solubilized SAC's were transferred to a fresh tube to remove silica gel and evaporated under reduced pressure to remove the solvent. The partially purified SACs were used for subsequent analysis.
Electrospray Ionization Mass Spectrometry (ESI(+)-MS), FT-IR AND NMR
The fractions of the crude extracts separated by thin layer chromatography were identified and characterized by ESI(+)-MS, FT-IR and NMR. ESI(+)-MS analysis used a high-resolution Q-TOF mass spectrometer (Micromass, UK). Fourier Transform Infrared spectroscopy (FT-IR) was performed in a Nicolet Impact 410 using NaCl, or KBr cell films. The absorbance bands were expressed in cm-1. Nuclear Magnetic Resonance analyses were performed in a Varian Gemini-2000 (300 MHz, 7.9 Tesla) equipment and chemical shifts were expressed in (δ) ppm values using tetramethylsilane (TMS) as the internal standard for 1H NMR.
RESULTS AND DISCUSSION
The surface tension of the G. amicalis cell-free culture broth was reduced to 37 mN/m after 72 h of cultivation and formed O/W emulsions. For comparison, water was used, which had a surface tension of 72 mN/m at 25 ºC. Crude Type I SACs (isolated by chloroform-methanol extraction) were obtained with a yield of 0.53 g/L. As is shown in Table 1, 5.0 mg/mL of the crude Type I preparation reduced the surface tension of water to 37 mN/m and had high oil in water (O/W) emulsion activity (OD610nm = 2). Crude extracts of Type II SACs (isolated by ammonium sulfate precipitation) were isolated with a yield of 0.11 g/L. At a concentration of 0.2 mg/mL the Type II crude extract demonstrated excellent water in oil (W/O) emulsification activity, with an emulsion phase of 2.3 cm height after 24 h. However, there was no significant production of an O/W emulsion. The surface tension of water was reduced to 55.6 mN/m by the Type II preparation at 0.2 mg/L (Table 1).
Triton X-100, Span 20, Niaproof and Surfactin showed similar W/O emulsification abilities as the Type II extract, totally emulsifying all the oil substrate, with the exception of Span 20, which emulsified only 50% of the substrate at a concentration of 516 µg/mL. However, it lowered the surface tension of water to 29.3 mN/m. None of the commercial surfactants produced significant O/W emulsification activity at the concentrations used here.
Two distinct spots were detected by the TLC from the Type I and II crude extracts. Extract Type I produced spots with Rf 0.44 and 0.62; Type II produced spots with Rf 0.52 and 0.62. The SACs recovered from the Type I band with Rf 0.62 were tested for their ability to reduce the surface tension of water and a decrease to 35 mN/m was observed at a concentration of 1.5 mg/mL. In contrast, the Type I SACs from the TLC band with Rf 0.44 were less effective than those from band Rf 0.62 at reducing the surface tension of water. Both the Rf 0.44 and 0.62 SAC's produced O/W emulsions; however those from Rf 0.44 were more effective.
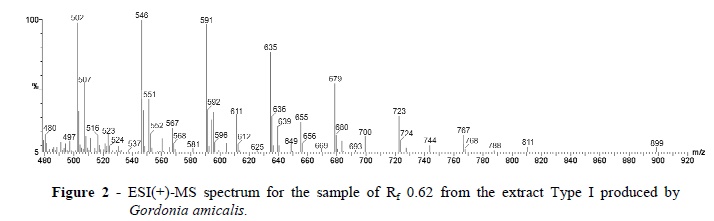
The partially purified SAC from the Type I Rf 0.62 band was further characterized by ESI(+)-MS and FT-IR and 1H NMR spectroscopy. In the ESI(+)-MS of the Type I Rf 0.62 band, a homologue series of 10 ions separated by 44 m/z units dominated from m/z 502 to m/z 899 (Fig. 2).
The results suggested that these were oligomers containing a repeating unit of 44 Da. The 13C isotopologue ions with its m/z value 1 unit higher (m/z 591 and 592 for instance) showed that these species were singly charged protonated molecules, that is [M + H]+ions. To our knowledge a biosurfactant with a similar ESI(+)-MS subunit pattern for which -(CH2CH2O)n- or an isomeric unit such as -(CH2CH(OH))n- seems likely and has not previously been reported (Nitschke et al. 2004; Nitschke et al. 2005; Jacques et al. 2007; de Araújo et al. 2011).
FT-IR analysis of the Type I SACs from the Rf 0.62 band (Fig. 3) showed a fairly broad band at 3403 cm-1, suggesting a OH functional group from an alcohol, not to a carboxylic acid, which have been typically broader and centered around 3000 cm-1. The bands at 1022 and 1092 cm-1 were consistent with the C-O stretch bands observed for the alcohols. The sharp bands at 2961, 2919 and 2850 cm-1 were indicative of the presence of C-H bands. No C-C aromatic bands (1530-1560 cm-1) were detected. The bands at 1709 and 1639 cm-1 suggested the presence of C=O carbonyl bond and the band in 1639 cm-1was consistent with the C=O stretch band of an amide.
1H NMR (300 MHz, CDCl3) analysis of the Type I SACs from the Rf 0.62 band showed the presence of unsaturated carbon bonds =CH (δ 5.39), saturated carbon bonds -CH (δ 0.4 -2,5) and amide bonds (δ 8.60).A structure consistent with the observed data and a 44 Da repeated was, therefore, indeed [-(CH2-CHOH)-]n. Alternatively, the 44 Da repeating unit could result from acetylation (CH3-CO-, 43 Da) of a carbon double bound, which would be expected to also result in the addition of a hydrogen atom to one of the other carbon atom of the double band, resulting in an increase in mass of 44 Da.
Two possible structures for the [M + H]+ ion of m/z 502, based on the combined analytical data discussed above, were:
1) CH3-(CH2)14-(CH=CH-CH2-CH2)3-CH=CH-CH2-CHOH-CH2-CONH2
2) CH3-(CH2)7-CH=CH-(CH2)2- CH=CH-(CH2-CHOH)6-CONH2
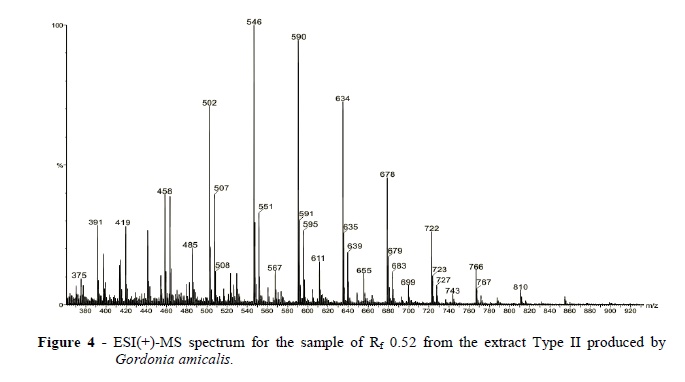
ESI(+)-MS analysis of the TLC Type II band with Rf0.62 produced a similar spectrum as that from the Type I band with Rf0.62. Figure 4 shows the spectrum of the Type II SAC's from the TLC band with Rf 0.52. Again, a homologous mixture of 10 oligomers with a m/z separation of 44 units was observed. However, the SACs from the Rf 0.52 band showed relevant changes in relative abundances with, for instance, more abundant ions of m/z 546 and 590. 1H NMR analysis of this sample (Rf 0.52) showed similar characteristics as that obtained in the spectra of band with Rf 0.62, suggesting the same basic structure.
The crude extracts of the Type I and Type II SACs did not present antimicrobial activity at any of the concentrations assayed. The absence of antimicrobial activity is desirable for in-situ bioremediation to avoid the disruption of indigenous microbial communities that typically provide most of the biodegradation activity in contaminated sites.
In the genus Gordonia, the most extensively studied species for the production of SACs is G. amarae (Iwahori et al. 2001; Pagilla et al. 2002; Dogan et al. 2006). Franzettiet al. (2008) reported the production of at least two types of SACs byG. amicalis; however, structural characterization of these compounds was not performed. The present study showed that, depending on the isolation technique used, the G. amicalis used in this study yielded two types of SACs (Type I and II) with different activities, one which reduced the surface tension of water and produced O/W emulsions (Type I) and one that primarily produced W/O emulsions (Type II). The analysis by ESI(+)-MS, FT-IR and 1H NMR indicated that the structure of the Type I and II SAC's were very similar. Both the preparations contained a homologous series of oligomers, differing by a structural repeating unit of 44 Da. The data indicated that the compounds contained C, N and O, and carbonyl, hydroxyl and amide bonds. The spectroscopic and spectrometric data of these compounds did not to match with other known biosurfactants and could represent a novel class of these interesting compounds. To elucidate further the structure of the identified SACs, the results showed that modified sugars and amino acids were possibly attached via the amide group. Lipids containing amide linkages to polar head groups are common in the cell membranes and lipopolysaccharides of Gram-negative bacteria in the form of ornithine-containing lipids and lipid A. They are present in the biosurfactant emulsion produced by Acinetobacter calcoaceticus RAG-1(Gautam et al. 2006). Ornithine lipids with amide linkages have also been found in Mycobacterium (Lanéelle et al. 1990).
CONCLUSIONS
The specific activity of the crude SAC preparations was similar to the commercial surfactants tested in this study in terms of emulsification activity. It was likely that the superficial activity could be significantly increased with further purification. The ability to produce two different types of SAC activities by G. amicalis could be advantageous in applications in the chemical, cosmetic, food, biomedical applications and bioremediation industries.
ACKNOWLEDGMENTS
This work was supported by FAPESP (00/05092-0 and 98/11906-9).
Received: October 31, 2012;
Accepted: August 14, 2013.
- Arenskötter M, Broker D, Steinbuchel A. Biology of the metabolically diverse genus Gordonia. Appl Environ Microbiol 2004; 70: 3195-3204.
- Banat IM, Makkar RS, Cameotra SS. Potential commercial applications of microbial surfactants. Appl Microbiol Biotechnol 2000; 53: 495-508.
- Brown MJ. Biosurfactants for cosmetic applications. Int J Cosmet Sci 1991; 3: 61-64.
- Cole JR, Chai B, Farris RJ, Wang Q, Kulam-Syed-Mohideen AS, McGarrell D, et al. The ribosomal database project (RDP-II): introducing myRDP space and quality controlled public data. Nucleic Acids Res. 2007; 35: D169-D172.
- Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 2009; 37: D141-D145.
- de Araújo MEMB, Campos PRB, Noso TM, Alberici RM, Cunha IBS, Simas RC, et al. Response surface modeling of the production of structured lipids from soybean oil using Rhizomucor miehei lipase. Food Chem. 2011; 127: 28-33.
- Dogan I, Pagilla KR, Webster DA, Stark BC. Expression of Vitreoscilla hemoglobin in Gordonia amarae enhances biosurfactant production. J Ind Microbiol Biotechnol 2006; 33: 693-700.
- Franzetti A, Bestetti G, Caredda P, La Colla P, Tamburini E. Surface-active compounds and their role in the access to hydrocarbons in Gordonia strains. FEMS Microbiol Ecol. 2008; 63: 238-248.
- Gautam KK, Tyagi VK. Microbial surfactants: A review. J Olea Sci 2006; 55: 155-166.
- Iwahori K, Tokutomi T, Miyata N, Fujita M. Formation of stable foam by the cells and culture supernatant of Gordonia (Nocardia) amarae J Biosci Bioeng 2001; 92: 77-79.
- Jacobucci DFC, Oriani MRG, Durrant LR. Reducing COD level on oily effluent by utilizing biosurfactant-producing bacteria. Braz Arch Biol Technol 2009; 52:1037-1042.
- Jacobucci DFC, Vasconcelos CK, Matsuura ABJ, Falconi FA, Durrant LR. Degradation of diesel oil by biosurfactant-producing bacterial strains. AEHS Contaminated Soil, Sediment and Water 2001; 8: 31-34.
- Jacques RJS, Santos EC, Haddad R, Catharino RR, Eberlin MN, Bento FM, Camargo FAD. Mass spectrometry analysis of surface tension reducing substances produced by a path-degrading Pseudomonas citronellolis strain. Braz J Microbiol. 2007; 39: 352-353.
- Lanéelle MA, Promé D, Lanéelle G,Promé JC. Ornithine lipid of Mycobacterium tuberculosis: its distribution in some slow- and fast-growing mycobacteria. J Gen Microbiol 1990; 136: 773-778.
- Navon-Venezia S, Zosim Z, Gottlieb A, Legmann R, Carmeli S, Ron EZ, Rosenberg E. Alasan, a new bioemulsifier from Acinetobacter radioresistens Appl Environ Microbiol 1995; 61: 3240-3244.
- Nitschke M, Costa SGVAO, Haddad R, Gonçalves LAG, Eberlin MN, Contiero J. Oil Wastes as Unconventional Substrates for Rhamnolipid Biosurfactant Production by Pseudomonas aeruginosa LBI. Biotechnol Prog. 2005; 21: 1562-1566.
- Nitschke M, Haddad R, Costa GN, Gilioli R, Meurer EC, Gatti MSV, Eberlin MN, Höehr NF, Pastore GM. Structural Characterization and Biological Properties of a Lipopeptide Surfactant Produced by Bacillus subtilis on Cassava Wastewater Medium. Food Sci Biotechnol. 2004; 13: 591-596.
- Pagilla KR, Sood A, Kim H. Gordonia (Nocardia) amarae foaming due to biosurfactant production. Water Sci Technol 2002; 46: 519-24.
- Rapp P, Backhaus S. Formation of extracellular lipases by filamentous fungi, yeast and bacteria. Enzyme Microb Technol 1992; 14: 938-943.
- Rocha C, San-Blas F, San-Blas GE, Vierma L. Biosurfactant production by two isolates of Pseudomonas aeruginosa World J Microbiol Biotechnol 1992; 8: 125-128.
- Singh A, Van Hamme JD, Ward OP. Surfactants in microbiology and biotechnology: Part 2 - Application aspects. Biotechnol Adv 2007; 25: 99-121.
- Stanghellini ME, Miller RM. Biosurfactants - Their identity and potential efficacy in the biological control of zoosporic plant pathogens. Plant Dis 1997; 81: 4-12.
Publication Dates
-
Publication in this collection
24 Feb 2014 -
Date of issue
Feb 2014
History
-
Received
31 Oct 2012 -
Accepted
14 Aug 2013