Abstracts
A fungal pathogen Batrachochytrium dendrobatidis (Bd), which can cause morbidity and death of anurans, has affected amphibian populations on a worldwide basis. Availability of pure cultures of Bd isolates is essential for experimental studies to understand the ecology of this pathogen. We evaluated the relationships of body length of Hylodes cf. ornatus and Lithobates catesbeianus tadpoles to depigmentation of mouthparts and determined if dekeratinization indicated an infection by Batrachochytrium dendrobatidis. A strong association existed for both species, one from South America (Brazil: São Paulo) and one from North America (USA: Maine). We believe it prudent not to kill adult amphibians if avoidable, thus obtaining tissue for isolating Bd from tadpoles is reasonable because infected specimens of some species can be selectively collected based on depigmentation of mouthparts.
Batrachochytrium dendrobatidis; depigmentation; Hylodes cf. ornatus; Lithobates catesbeianus; tadpole
O fungo patógeno Batrachochytrium dendrobatidis (Bd) é apontado como o causador de morbidade e morte em anuros, e tem afetado populações de anfíbios em uma base mundial. Avaliar culturas puras de isolados de Bd é essencial para estudos experimentais para o entendimento da ecologia desse patógeno. Avaliou-se a relação entre o comprimento do corpo em girinos de Hylodes cf. ornatus e Lithobates catesbeianus com a despigmentação das peças bucais, para verificar se a desqueratinização indica uma infecção por Batrachochytrium dendrobatidis. Uma forte associação existe para ambas as espécies, uma da América do Sul (Brasil: São Paulo) e uma da América do Norte (USA: Maine). Acredita-se ser prudente este uso, para evitar a morte de anfíbios adultos; dessa forma, obter tecidos para isolar o Bd de girinos é razoável, porque espécimes infectados podem ser coletados seletivamente com base na despigmentação do aparelho bucal.
Batrachochytrium dendrobatidis; despigmentação; Hylodes cf. ornatus; Lithobates catesbeianus; girino
BIOLOGY
Body length of Hylodes cf. ornatus and Lithobates catesbeianus tadpoles, depigmentation of mouthparts, and presence of Batrachochytrium dendrobatidis are related
Tamanho do corpo, despigmentação das partes bucais e presença de Batrachochytrium dendrobatidis estão relacionados em Hylodes cf. ornatus e Lithobates catesbeianus
Vieira, CA.I,II; Toledo, LF.II,* * e-mail: toledolf2@yahoo.com ; Longcore, JE.III; Longcore, JR.IV
ILaboratório de Antígenos Bacterianos II, Departamento Microbiologia e Imunologia, Instituto de Biologia, Universidade Estadual de Campinas UNICAMP, CP 6109, Campinas, SP, Brazil
IIMuseu de Zoologia "Prof. Adão José Cardoso", Instituto de Biologia, Universidade Estadual de Campinas UNICAMP, CP 6109, CEP 13083‑970, Campinas, SP, Brazil
IIISchool of Biology and Ecology, University of Maine, Orono, Maine 04469 USA
IV151 Bennoch Road, Orono, Maine 04473 USA
ABSTRACT
A fungal pathogen Batrachochytrium dendrobatidis (Bd), which can cause morbidity and death of anurans, has affected amphibian populations on a worldwide basis. Availability of pure cultures of Bd isolates is essential for experimental studies to understand the ecology of this pathogen. We evaluated the relationships of body length of Hylodes cf. ornatus and Lithobates catesbeianus tadpoles to depigmentation of mouthparts and determined if dekeratinization indicated an infection by Batrachochytrium dendrobatidis. A strong association existed for both species, one from South America (Brazil: São Paulo) and one from North America (USA: Maine). We believe it prudent not to kill adult amphibians if avoidable, thus obtaining tissue for isolating Bd from tadpoles is reasonable because infected specimens of some species can be selectively collected based on depigmentation of mouthparts.
Keywords:Batrachochytrium dendrobatidis, depigmentation, Hylodes cf. ornatus, Lithobates catesbeianus, tadpole.
RESUMO
O fungo patógeno Batrachochytrium dendrobatidis (Bd) é apontado como o causador de morbidade e morte em anuros, e tem afetado populações de anfíbios em uma base mundial. Avaliar culturas puras de isolados de Bd é essencial para estudos experimentais para o entendimento da ecologia desse patógeno. Avaliou-se a relação entre o comprimento do corpo em girinos de Hylodes cf. ornatus e Lithobates catesbeianus com a despigmentação das peças bucais, para verificar se a desqueratinização indica uma infecção por Batrachochytrium dendrobatidis. Uma forte associação existe para ambas as espécies, uma da América do Sul (Brasil: São Paulo) e uma da América do Norte (USA: Maine). Acredita-se ser prudente este uso, para evitar a morte de anfíbios adultos; dessa forma, obter tecidos para isolar o Bd de girinos é razoável, porque espécimes infectados podem ser coletados seletivamente com base na despigmentação do aparelho bucal.
Palavras-chave:Batrachochytrium dendrobatidis, despigmentação, Hylodes cf. ornatus, Lithobates catesbeianus, girino.
1. Introduction
A chytrid fungus, Batrachochytrium dendrobatidis (Longcore et al., 1999) (hereafter Bd) infects keratinized tissue of amphibians, and can be lethal to post-metamorphic individuals (Berger et al., 1998; Lamirande and Nichols, 2002). This pathogen is associated with worldwide population declines of amphibian species (Berger et al., 1998; Daszak et al., 1999; Stuart et al., 2004; Lips et al., 2006). Although Bd skin infections can kill metamorphs and adults, most larvae (= tadpoles) survive when infected and may serve as reservoirs of infective zoospores (Lips, 1999; Lips et al., 2003, 2004; Daszak et al., 2004). Important research topics for this pathogen are geographic origin and circumstances under which it arose, relative pathogenicity of different strains (Berger et al., 2005), and genetic differences among strains; research of these topics requires pure cultures of Bd (Voyles et al., 2009, 2010). Most Bd isolates now in culture were isolated from haphazardly contributed animals, whereas isolates from specific geographic areas are needed. If Bd, which in adults is not detectable in the field, can be identified in tadpoles in the field, fewer animals will need to be euthanized and examined to obtain infected tissue for isolation (Longcore et al., 1999). Isolation of Bd from freshly euthanized larvae is also relatively easy, because mouthparts are seldom contaminated by other fungi or bacteria (J.E. Longcore, personal observation).
Only mouthparts of the oral disc (i.e., labial tooth rows, papillae, and jaw sheaths; McDiarmid and Altig, 1999) of larval anurans are keratinized (Luckenbill, 1965), and these are the sites of Bd infection (Knapp and Morgan, 2006). Keratinized structures in healthy tadpoles are uniformly metallic black and symmetrical (McDiarmid and Altig, 1999). The oral disc and labial tooth rows begin to differentiate at about Gosner stage 23 (Gosner, 1960), stage 24 (Kuang, 1975), or stage 25 (Thibaudeau and Altig, 1988) depending on species, but are complete and entirely keratinized by Gosner stage 25-26 (Altig and Johnston, 1986). Gosner (1960) noted that the extent of change in pigmentation varies among species, but that the period between stages 30-40 is one of relative stability in "key" traits; after stage 40 mouthparts begin to break down in reverse order of development (Thibaudeau and Altig, 1988). Bd infections of larval anurans have been associated with depigmentation of keratinized mouthparts by several authors (e.g., Berger et al., 1998, Pessier et al., 1999; Fellers et al., 2001; Marantelli et al., 2004; Toledo et al., 2006a, b; Drake et al., 2007; Smith et al., 2007).
2. Material and Methods
While in North America (Orono, Maine) LFT participated in sampling a population of bullfrog tadpoles (Lithobates catesbeianus; Anura, Ranidae) with a history of Bd infections (Longcore et al., 2007). With this effort in mind, LFT returned to Brazil and with CAV sampled a population of torrent frogs (Hylodes cf. ornatus; Anura, Hylodidae) in southeastern Brazil to determine if tadpole mouthparts were depigmented and similarly infected with Bd. Our purpose was to evaluate tadpole mouthpart data regarding pigmentation and status of Bd infection for two anuran species with distant phylogenetic relationships and from different eco-regions. Moreover, we examined tadpoles in the field to confirm the ability to selectively collect infected tadpoles on the basis of depigmentation status of mouthparts.
The site in Maine (Crocker Pond, Oxford County, Albany Township, 70° 49' 27.2" N; 44° 18' 30.6" W) was 11 km west of West Bethel, Maine. There we collected 50 bullfrog tadpoles with long-handled nets and with a hand lens (10×) in the field examined jaw sheaths for depigmentation. We collected tadpoles with body lengths of 14 to 47 mm, which corresponded approximately to Gosner stages 26 to 37. We systematically sub-sampled every 7th specimen (i.e., numbers 7, 14, 21, 28, 35, 42, 49) plus specimen 19, which looked infected by Bd based on JRL's experience.
In Brazil we collected 243 Hylodes cf. ornatus tadpoles from March to May 2010 in the Reserva Biológica Serra do Japi, county of Jundiaí, state of São Paulo (23° 14' 57.8" S; 46° 56' 59.4" W; about 1100 m a.s.l.). We separated these individuals into 5, 5-mm body length classes (i.e., <5.1, 5.1-10, 10.1-15, 15.1-20, and 20.1-25 mm; Table 1). We examined the mouthparts of these specimens in the laboratory with a stereo-microscope (400×) for signs of Bd infections (i.e., lack of keratin in the tooth rows and jaw sheaths). We considered structures as depigmented if mouthparts were brown or pale and lacked the dark melanin of keratin on teeth rows or jaw sheaths and appeared light sepia or nearly clear (Rachowicz and Vredenburg, 2004; Knapp and Morgan, 2006). To confirm that depigmentation was associated with Bd infection, we killed all sub-sampled individuals and observed mouthparts under a microscope (400×).
3. Results
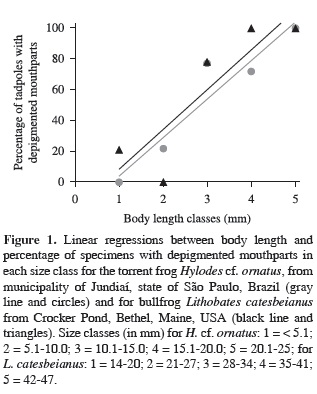
Out of the 8 bullfrog tadpoles in the North American (Maine) sample, jaw sheaths for specimen numbers 7, 19 and 21 were clearly depigmented and specimen 14 was questionable with only slight depigmentation at the base of a jaw sheath; no depigmentation was observed for specimens 28, 35, 42, and 49. Three out of 4 specimens with depigmented jaw sheaths, but not specimen 14, were infected with Bd; none of the 4 specimens with intact jaw sheaths were infected as determined by microscopic examination of the jaw sheaths at 400 and 1000×. We isolated pure cultures from tadpoles 10 and 19. We separated individuals into 5, 5 mm body length classes (i.e., 14-20, 21-27, 28-34, 35-41, and 42-47 mm; Table 1) and regressed the percent depigmented on length class of tadpoles. Presence of Bd was closely associated with depigmentation (r2 = 0.769, df = 4, P = 0.0507) (Figure 1).
For the South American (Brazil) sample of torrent frogs 113 of 243 (46.5%) tadpoles had depigmented mouthparts (Table 1) and the larger tadpole classes had higher percentages of depigmented mouthparts. A linear regression (alpha value of P < 0.05) confirmed this relationship (r2 = 0.903; df = 4; P = 0.013) (Figure 1). Under a microscope we examined 92 specimens with depigmented mouthparts; 87 (94.5%) were positive for Bd infection. From three (3.4%) of these 87 tadpoles, we isolated Bd with standard methods (Longcore and Berger, 2000).
4. Discussion
Although some researchers have reported that mouthpart dekeratinization is not a good predictor of infection by Bd (Padgett-Flohr and Goble, 2007), our data from L. catesbeianus and H. cf. ornatus support findings of Fellers et al. (2001), who reported that 67% of Rana muscosa tadpoles with missing or abnormally dekeratinized mouthparts were infected with Bd; Knapp and Morgan (2006), who reported that depigmentation of R. muscosa tadpole upper jaw sheaths was strongly associated with Bd infections (89%, 50 of 56) and Drake et al. (2007), who reported that 70.3% of tadpoles from 5 frog and 3 toad species in southeastern United States had deformed jaw sheaths with dekeratinization the most common (50.3%) defect. Mouthpart deformations were explained mostly by taxonomic group (family) and presence of Bd.
Because keratinized mouthparts of amphibians atrophy during metamorphosis after Gosner stage 40 (Gosner, 1960) and jaw sheaths fall off after tooth rows are gone (Thibaudeau and Altig, 1988) age class of tadpoles is important in finding Bd. Rachowicz (2002) observed that variability of pigmentation in keratinized cells in tooth rows and jaw sheaths is also related to seasonal changes in temperature. Because loss of labial tooth rows occurred in L. catesbeianus tadpoles at sites polluted by coal ash (Rowe et al., 1996) and jaw sheaths are the last keratinized structures to be shed (Thibaudeau and Altig, 1988), depigmentation of jaw sheaths may be more reliable in indicating Bd infections than depigmentation or loss of other mouthparts. We believe that, at least for some species, larvae can be evaluated with a hand lens and only those with dekeratinized erosions of the upper jaw sheaths, i.e., the ones most likely to be infected with Bd, can then be brought into the laboratory for isolation procedures. We have been successful in finding and isolating Bd from L. catesbeianus and H. cf. ornatus and 10 other Brazilian amphibian species (L.F. Toledo, personal observation) after screening and selecting larvae by this method.
This method may not work for some amphibian species and will be more informative at certain Gosner stages; we found that only the more advanced age classes had dekeratinized structures and were infected with Bd. Amphibians that have 2-year larval stages such as the bullfrog (Lithobates catesbeianus) (Albright, 1999) and Mountain Yellow-Legged Frog (Rana muscosa) (Rachowicz, 2002) are exposed to Bd zoospores for substantially longer periods than species that undergo metamorphosis during their first year. The larger and older an individual is, the longer the period that it would be exposed to infective zoospores in an aquatic environment. These findings are congruent with those from Smith et al. (2007), where infected tadpoles were larger than uninfected ones. However, our data is complementary as we show a gradual increase in the infection prevalence with the increase in body size, similarly as recently reported for Telmatobius jelskii in Peru (Catenazzi et al., 2013). Lower rates of infection of Bd in younger tadpoles also may be related to the smaller area of keratinized cells. Thus, exposure of larger tadpoles (either single or multiple exposure times) is greater than that of younger individuals. This method of selecting larvae for isolation of Bd will miss early infections because recently infected individuals may not have visibly depigmented mouthparts.
Our results expand knowledge for a South American frog species and corroborate observations for a wild population of bullfrogs (L. catesbeianus) in the northern United States of America; both showed a similar depigmentation/infection pattern (Figure 1) of smaller tadpoles being less frequently infected than larger tadpoles and Bd infection corresponding with depigmentation of keratinized mouthparts. This similarity of response to Bd infections between phylogenetically distant species (Ranidae vs. Hylodidae) and between species of different eco-regions (Neotropics vs. Nearctics) suggests a consistent and perhaps a universal pattern. The high association of upper jaw sheath dekeratinization with Bd infection can be used to select tadpoles to examine for Bd infection, which can reduce the number of specimens killed to obtain cultures. This is an important issue as amphibians are the most threatened vertebrates in the world (Barnosky et al., 2011).
Acknowledgements Nelson R. Silva and Thaís C. Postali helped during field samplings. Domingos da Silva Leite allowed our access to the microbiology lab. Sarah Mangia made suggestions in the manuscript. FAPESP provided grants (2008/50325‑5 and 2011/51694-7) and fellowships (2008/52847-9 and 2010/15259-1). ICMBio provided the collecting permit number 27745-1). At the University of Maine, specimens were handled under Animal Care and Use Committee Protocol No. A2009-09-02 (Isolating Batrachochytrium dendrobatidis).
Received November 28, 2011
Accepted March 16, 2012
Distributed February 28, 2013
- ALBRIGHT, J., 1999. Bullfrog Rana catesbeiana In HUNTER JUNIOR, ML., CALHOUN, AJK. and McCOLLOUGH, M. (Eds.). Maine Amphibians and Reptiles Orono: The University of Maine Press.
- ALTIG, R. and JOHNSTON, GF., 1986. Major characteristics of free-living anuran tadpoles. Smithsonian Herpetological Information Service, vol. 67, p.1-75.
- ALTIG, R., McDIARMID, RW., NICHOLS, KA. and USTACH, PC., 1998. Tadpoles of the United States and Canada: A tutorial and Key. Available from: <http://www.pwrc.usga.gov/TADPOLE/>. Access in: 27 Dec. 2010.
- BARNOSKY, AD., MATZKE, N., TOMIYA, S., WOGAN, GOU., SWARTZ, B., QUENTAL, TB., MARSHALL, C., McGUIRE, JL., LINDSEY, EL., MAGUIRE, KC., MERSEY, B. and FERRER, EA., 2011. Has the Earth's sixth mass extinction already arrived? Nature, vol. 471, p. 52-57.
- BERGER, L., SPEARE, R., DASZAK, P., GREEN, DE., CUNNINGHAM, AA., GOGGIN, CL., SLOCOMBE, R., RAGAN, MA., HYATT, AD., McDONALD, KR., HINES, HB., LIPS, KR., MARANTELLI, G. and PARKES, H., 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proceedings of the National Academy of Science, vol. 95, p. 9031-9036.
- BERGER, L., MARANTELLI, G., SKERRATT, LF. and SPEARE, R., 2005. Virulence of the amphibian chytrid fungus Batrachochytrium dendrobatidis varies with the strain. Diseases of Aquatic Organisms, vol. 68, p. 47-50. PMid:16465833. http://dx.doi.org/10.3354/dao068047
- CATENAZZI, A., VON MAY, R., and VREDENBURG, VT., 2013. High prevalence of infection in tadpoles increases vulnerability to fungal pathogen in high-Andean amphibians. Biological Conservation, vol. 159, p. 413-421. http://dx.doi.org/10.1016/j.biocon.2012.11.023
- DASZAK, P., BERGER, L., CUNNINGHAM, AA., HYATT, AD., GREEN, DE. and SPEARE, R., 1999. Emerging infectious diseases and amphibian population declines. Emergent Infectious Diseases, vol. 5, p. 735-748. PMid:10603206 PMCid:2640803. http://dx.doi.org/10.3201/eid0506.990601
- DASZAK, P., STRIEBY, A., CUNNINGHAM, AA., LONGCORE, JE., BROWN, CC. and PORTER, D., 2004. Experimental evidence that the bullfrog (Rana catesbiana) is a potential carrier of chytridomycosis, an emerging fungal disease of amphibians. Herpetological Journal, vol. 14, p. 201-207.
- DRAKE, DL., ALTIG, R., GRACE, JG. and WALLS, SC., 2007. Occurrence of oral deformities in larval anurans. Copeia, vol. 2, p. 449-458.
- FELLERS, GM., GREEN, DE. and LONGCORE, JE., 2001. Oral chytridiomycosis in the Mountain Yellow-Legged Frog (Rana muscosa). Copeia, p. 945-953.
- GOSNER, KL., 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica, vol. 16, p. 183-190.
- KNAPP, RA. and MORGAN, JAT., 2006. Tadpole mouthpart depigmentation as an accurate indicator of chytridiomycosis, an emerging disease of amphibians. Copeia, p. 188-197.
- KUANG, H-LC., 1975. Development of beaks of Rana pipiens larvae. Anatomical Record, vol. 182, p. 401-414. PMid:1080023. http://dx.doi.org/10.1002/ar.1091820402
- LAMIRANDE, EW. and NICHOLS, DK., 2002. Effects of host age on susceptibility to cutaneous chytridiomycosis in blue-and-yellow poison dart frogs (Dendrobates tinctorius). In McKINNELL, RG. and CARLSON, DL. (Eds.). Proceedings 6th International Symposium on the Pathology of Reptiles and Amphibians St. Paul: University of Minnesota Printing Services.
- LIPS, KR., 1999. Mass mortality and population declines of anurans at an upland site in western Panama. Conservation Biology, vol. 13, p. 117-125. http://dx.doi.org/10.1046/j.1523-1739.1999.97185.x
- LIPS, KR., GREEN, DE. and PAPENDICK, R., 2003. Chytridiomycosis in wild frogs from southern Costa Rica. Journal of Herpetology, vol. 37, p. 215-218. http://dx.doi.org/10.1670/0022-1511(2003)037[0215:CIWFFS]2.0.CO;2
- LIPS, KR., MENDELSON III, JR., MUÑOZ-ALONSO, A., CANSECO-MÁRQUEZ, L. and MULCAHY, D., 2004. Amphibian population declines in montane southern Mexico: resurveys of historical localities. Biological Conservation, vol. 119, p. 555-564. http://dx.doi.org/10.1016/j.biocon.2004.01.017
- LIPS, KR., BREM, F., BRENES, R., REEVE, JD., ALFORD, RA., VOYLES, J., CAREY, C., LIVO, L., PESSIER, AP. and COLLINS, JP., 2006. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proceedings of National Academy of Science, vol. 103, p. 3165-3170. PMid:16481617 PMCid:1413869. http://dx.doi.org/10.1073/pnas.0506889103
- LONGCORE, J. and BERGER, L., 2000. Workshop on culturing chytrids: Recognizing, isolating, and culturaling Batrachochytrium dendrobatidis from amphibians. Cains: James Cook University. Proceedings: Getting Jump on the Amphibian Disease, Cains, Australia (26-29 August 2000).
- LONGCORE, JE., PESSIER, AP. and NICHOLS, DK., 1999. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia, vol. 91, p. 219-227.
- LONGCORE, JR., LONGCORE, JE., PESSIER, AP. and HALTEMAN, WA., 2007. Chytridiomycosis widespread in anurans of Northeastern United States. Journal of Wildlife Management, vol. 71, p. 435- 444. http://dx.doi.org/10.2193/2006-345
- LUCKENBILL, LM., 1965. Morphogenesis of the horny jaws of Rana pipiens larvae. Developmental Biology, vol. 11, p. 25-49. http://dx.doi.org/10.1016/0012-1606(65)90036-9
- MARANTELLI, G., BERGER, L., SPEARE, R. and KEEGAN, L., 2004. Distribution of amphibian chytrid Batrachochytrium dendrobatidis and keratin during tadpole development. Pacific Conservation Biology, vol. 10, p. 173-179.
- McDIARMID, RW. and ALTIG, R., 1999. Tadpoles: The Biology of Anuran Larvae. Chicago: University of Chicago Press.
- PADGETT-FLOHR, GE. and GOBLE, ME., 2007. Evaluation of tadpole mouthpart depigmentation as a diagnostic test for infection by Batrachochytrium dendrobatidis for four California anurans. Journal of Wildlife Diseases, vol. 43, p. 690-699.
- PESSIER, AP., NICHOLS, DK., LONGCORE, JE. and FULLER, MS., 1999. Cutaneous chytridiomycosis in poison dart frogs (Dendrobates spp.) and White's tree frogs (Litoria caerulea). Journal of Veterinary Diagnostic Investigation, vol. 11, p. 194-199. PMid:10098698. http://dx.doi.org/10.1177/104063879901100219
- RACHOWICZ, LJ., 2002. Mouthpart pigmentation in Rana muscosa tadpoles: seasonal changes without chytridiomycosis. Herpetological Review, vol. 33, p. 263-265.
- RACHOWICZ, LJ. and VREDENBURG, VT., 2004. Transmission of Batrachochytrium dendrobatidis within and between amphibian life stages. Diseases of Aquatic Organisms, vol. 61, p.75-83. PMid:15584413. http://dx.doi.org/10.3354/dao061075
- ROWE, CL., KINNEY, OM., FIORI, AP. and CONGDON, JD., 1996. Oral deformities in tadpoles (Rana catesbeiana) associated with coal ash deposition: effects on grazing ability and Growth. Freshwater Biology, vol. 36, p.723-730. http://dx.doi.org/10.1046/j.1365-2427.1996.00123.x
- SMITH, KG., WELDON, C., CONRADIE, W. and DU PREEZ, LH., 2007. Relationships among size, development, and Batrachochytrium dendrobatidis infection in African tadpoles. Diseases of Aquatic Organisms, vol. 74, p. 159-164. PMid:17432045. http://dx.doi.org/10.3354/dao074159
- STUART, SN., CHANSON, JS., COX, NA., YOUNG, BE., RODRIGUES, ASL., FISHMAN, DL. and WALLER, RW., 2004 Status and trends of amphibian declines and extinctions worldwide. Science, vol. 306, p. 1783-1786. PMid:15486254. http://dx.doi.org/10.1126/science.1103538
- THIBAUDEAU, DG. and ALTIG, R., 1988. Sequence of ontogenetic development and atrophy of the oral apparatus of six anuran tadpoles. Journal of Morphology, vol. 197, p. 63-69. http://dx.doi.org/10.1002/jmor.1051970106
- TOLEDO, LF., BRITO, FB., ARAÚJO, OGS., GIASSON, LOM. and HADDAD, CFB., 2006a. The occurrence of Batrachochytrium dendrobatidis in Brazil and the inclusion of 17 new cases of infection. South American Journal of Herpetology, vol. 1, p.185-191. http://dx.doi.org/10.2994/1808-9798(2006)1[185:TOOBDI]2.0.CO;2
- TOLEDO, LF., HADDAD, CFB., CARNAVAL, AOQC. and BRITTO, FB., 2006b. A Brazilian anuran (Hylodes magalhaesi: Leptodactylidae) infected by Batrachochytrium dendrobatidis: a conservation concern. Amphibian and Reptile Conservation, vol. 4, p. 17-21.
- VOYLES, J., RICHARDS-HRDLICKA, K., CASHINS, SD., ROSENBLUM, EB., HYATT, AD., BERGER, L. and SKERRATT, LF., 2010. Batrachochytrium dendrobatidis: requirement for further isolate collection and archiving. Diseases of Aquatic Organisms, vol. 92, no. 2-3, p. 109-1120. PMid:21268972. http://dx.doi.org/10.3354/dao02216
- VOYLES, J., CASHINS, SD., ROSENBLUM, EB. and PUSCHENDORF, R., 2009. Preserving pathogens for wildlife conservation: a case for action on amphibian declines. Oryx, vol. 43, p. 527-529. http://dx.doi.org/10.1017/S0030605309990469
Publication Dates
-
Publication in this collection
18 Apr 2013 -
Date of issue
Feb 2013
History
-
Received
28 Nov 2011 -
Accepted
16 Mar 2012