Abstracts
The histological description of the urogenital papilla is an important tool to comprehension of the reproductive mechanisms in fish, as well as a pre-requisite to germ cell transplantation in adult fish, besides to be a good biological indicator to environmental changes. Was performed the histological description of the urogenital papilla and its component ducts in the tetra Astyanax altiparanae. The genital and urinay ducts pass separately throughout most part of its extension, joining in a single duct before opening. In males this opening is asymmetric and seems to have double origin, being completely surrounded by striated muscle fibers, while in females it is symmetric and the muscle fibers does not surround it totally. Spermatic duct and oviduct undergo changes throughout their extension, mainly in the morphology of the surrounding epithelium. In the spermatic duct, squamous epithelial cells change to columnar and cuboid with possible secretory activity, close to testes. In the oviduct, anteriorly epithelial cells are also squamous, however, close to ovary there are lamellae composed by a pseudostratified epithelium with columnar and cuboid cells. The urinary duct is highly similar for both sexes presenting globoid cells, which description is known in mammals, however, rare in fish.
Fish reproduction; Histological analysis; Oviduct; Sperm duct; Urinary duct
A descrição histológica da papila urogenital é uma importante ferramenta para a compreensão dos mecanismos reprodutivos em peixes, assim como um pré-requisito para a realização do transplante de células germinativas em peixes adultos, além de um bom indicador biológico de possíveis alterações ambientais. Foi realizada a investigação histológica da papila urogenital e seus ductos constituintes no lambari Astyanax altiparanae. Os ductos genital e urinário ocorrem separadamente ao longo de maior parte de sua extensão, entretanto, unem-se em um ducto simples antes de abrir para o meio externo. Nos machos esta abertura é assimétrica e parece ter dupla origem, sendo completamente envolvida por fibras musculares estriadas, enquanto nas fêmeas ela é simétrica e as fibras musculares não a envolve totalmente. O ducto espermático e oviducto sofrem alterações ao longo de sua extensão, principalmente na morfologia do epitélio que os envolve. No ducto espermático as células epiteliais passam de pavimentosas a colunares e cuboides, com possível atividade secretora, à medida que se aproxima dos testículos. No oviducto, anteriormente as células também são epiteliais pavimentosas, entretanto, próximo aos ovários, formam-se lamelas compostas por um epitélio pseudoestratificado composto por células cuboides e colunares. O ducto urinário é bastante similar em ambos os sexos apresentando células globosas, cuja descrição é conhecida em mamíferos, porém rara em peixes.
Introduction
The fish adaptability in distinct aquatic habitats reflects the numerous reproductive strategies adopted by these animals (Vazzoler, 1996Vazzoler, A. E. A. de M. 1996. Biologia da reprodução de peixes teleósteos: teoria e prática. Maringá; São Paulo, SBI, 169p.). As result fish show considerable anatomical, morphological and physiological differences in their reproductive systems, both in the gonads and in the genital ducts (Lopes et al., 2004Lopes, D. C. J. R., N., Bazzoli, M. F. G. Brito & T. A. Maria. 2004. Male reproductive system in the South American catfish Conorhynchus conirostris. Journal of Fish Biology, 64: 1419-1424.; Batlouni et al., 2006Batlouni, S. R., E. Romagosa & M. I. Borella. 2006. The reproductive cycle of male catfish Pseudoplatystoma fasciatum (Teleostei, Pimelodidae) revealed by changes of the germinal epithelium an approach addressed to aquaculture. Animal Reproduction Science, 96: 116-132.; Muñoz et al., 2011Muñoz, M. E., S. R. Batlouni, I. B. Franceschini Vicentini & C. A. Vicentini. 2011. Testicular structure and description of the seminal pathway in Leporinus macrocephalus (Anostomidae, Teleostei). Micron, 42: 892-897.). Therefore, examining the reproductive aspects of fish, including sexual dimorphism, fecundity, gonadal morphology and gametogenesis, is important for understanding how species have maximised their reproductive strategies in different environments (Grier & Taylor, 1998Grier, H. J. & R. G. Taylor. 1998. Testicular maturation and regression in the common snook. Journal of Fish Biology, 53: 521-542.; Brown-Peterson et al., 2002Brown-Peterson, N. J., H. J. Grier & R. M. Overstreet. 2002. Annual changes in germinal epithelium determine male reproductive classes of the cobia. Journal of Fish Biology, 60: 178-202.; Cassel et al., 2013Cassel, M., M. Mehanna, L. Mateus & A. Ferreira. 2013. Gametogenesis and reproductive cycle of Melanorivulus aff. punctatus (Boulenger, 1895) (Cyprinodontiformes, Rivulidae) in Chapada dos Guimarães, Mato Grosso, Brazil. Neotropical Ichthyology, 11: 179-192.; Siqueira-Silva et al., 2013Siqueira-Silva, D. H. de, C. A. Vicentini, A. Ninhaus-Silveira & R. Veríssimo-Silveira. 2013. Reproductive cycle of the Neotropical cichlid yellow peacock bass Cichla kelberi: A novel pattern of testicular development. Neotropical Ichthyology, 11: 587-596.; Wildner et al., 2013Wildner, D. D., H. Grier & I. Quagio-Grassiotto. 2013. Female germ cell renewal during the annual reproductive cycle in Ostariophysians fish. Theriogenology, 79: 709-724.).
However, apart from general knowledge about the fish reproductive system little is known about the morphology of their genital ducts, particularly the histological details of the urogenital papilla of freshwater fish. Most studies only describe its macroscopic morphology and anatomical position for use identifying the sex of individuals, in fish farming and for toxicological and ecological studies (Ferreira et al., 2010Ferreira, F., M. M. Santos, M. A. Reis-Henriques, N. M. Vieira & N. M. Monteiro. 2010. Sexing blennies using genital papilla morphology or ano-genital distance. Journal of Fish Biology, 77: 1432-1438.; Kruger et al., 2013Kruger, T., I. Barnhoorn, J. J. van Vuren & R. Bornman. 2013. The use of the urogenital papillae of male feral African sharptooth catfish (Clarias gariepinus) as indicator of exposure to estrogenic chemicals in two polluted dams in an urban nature reserve, Gauteng, South Africa. Ecotoxicology and Environmental Safety, 87: 98-107.). Moreover, most past histological studies were focused on marine fish species (Ross, 1984Ross, R. M. 1984. Anatomical changes associated with sex reversal in the fish Thalassoma duperrey (Teleostei: Labridae). Copeia, 1: 245-248.; Hastings & Petersen, 1986Hastings, P. A. & C. W. Petersen. 1986. A novel sexual pattern in serranid fishes: simultaneous hermaphrodites and secondary males in Serranus fasciatus. Environmental Biology of Fishes, 15: 59-68.; Kott et al., 1988Kott, E., C. B. Renaud & V. D. Vladykov. 1988. The urogenital papilla in the Holarctic lamprey (Petromyzontidae). Environmental Biology of Fishes, 23: 37-43.; Rasotto & Shapiro, 1998Rasotto, M. B. & D. Y. Shapiro. 1998. Morphology of gonoducts and male genital papilla, in the bluehead wrasse: implications and correlates on the control of gamete release. Journal of Fish Biology, 52: 716-725.; Suzuki & Shibata, 2004Suzuki, A. & N. Shibata. 2004. Developmental process of genital ducts in the medaka, Oryzias latipes. Zoological Science, 21: 397-406.; Kobayashi et al., 2012Kobayashi, Y., T. Usami, T. Sunobe, H. Manabe, Y. Nagahama & M. Nakamura. 2012. Histological observation of the urogenital papillae in the Bi-directional sex-changing Gobiid fish, Trimma okinawae. Zoological Science, 29: 121-126.).
Furthermore, histological descriptions of the urogenital papilla in freshwater fish could be valuable for biotechnological studies, such as germ cell transplantation in adult fish, whose researchers can use the studied specie as host such as Lacerda et al. (2010)Lacerda, S. M. S. N., S. R. Batlouni, G. M. J. Costa, T. M. Segatelli, B. R. Quirino, B. M. Queiroz, E. Kalapothakis & L. R. França. 2010. A new and fast technique to generate offspring after germ cells transplantation in adult fish: the Nile tilapia (Oreochromis niloticus) model. PloS One, 5: e10740 (9p)., that injected spermatogonial stem cell though Oreochomis niloticus (Linnaeus, 1758) urogenital papilla, after the description of this organ; it description can be also useful in systematic reviews, as an additional feature to be considered, as well as was already used by some authors (Kott et al., 1988; Hoshino et al., 2004Hoshino, K., K. Amaoka & T. A. Munroe. 2004. New records of sexual dimorphisms among the Pleuronectiformes exhibited by differences in urogenital papilla structure of Citharichthys platophrys (Paralichthyidae: Pleuronectiformes). Ichthyologycal Research, 51: 81-83.); and in toxicological studies, since this organ can be very sensitive to environmental changes (Rodrigues et al., 2006Rodrigues, P., M. A. Reis-Henriques, J. Campos & M. M. Santos. 2006. Urogenital papilla feminization in male Pomatoschistus minutus from two estuaries in northwestern Iberian Peninsula. Marine Environmental Research, 62: S258-S262.).
Thus, the Characidae species Astyanax altiparanae Garutti & Britski, 2000Garutti, V. & H. A. Britski. 2000. Descrição de uma espécie nova de Astyanax (Teleostei: Characidae) da bacia do alto rio Paraná e considerações sobre as demais espécies do gênero na bacia. Comunicações do Museu de Ciências da PUCRS. Série Zoologia, 13: 65-88., a typical fish from the upper rio Paraná Basin, Brazil (Garutti & Britski, 2000), seems to be a great candidate for this kind of morphological description, since it shows a great potential to be used as a host species in germ cell transplantation studies owing its reduced size, early maturation, prolificacy and management resistance (Orsi et al., 2004Orsi, M. L., E. D. Carvalho & F. Foresti. 2004. Biologia populacional de Astyanax altiparanae Garutti & Britski (Teleostei, Characidae) do médio rio Paranapanema, Paraná, Brasil. Revista Brasileira de Zoologia, 21: 207-218.).
Moreover, this species belongs to the Astyanax genus assigned as Incertae sedis, being a frequent target of systematic studies (Reis et al., 2003Reis, R. E., S. O. Kullander & C. J. Ferraris Jr. 2003. (Orgs.) Check list of the freshwater fishes of South and Central America. Porto Alegre: Edipucrs, 742p.; Orsi et al., 2004; Kantek et al., 2009Kantek, D. L. Z., M. R. Vicari, W. A. M. Peres, M. M. Cestari, R. F. Artoni, L. A. C. Bertollo & O. Moreira-Filho. 2009. Chromosomal location and distribution of As51 satellite DNA in five species of the genus Astyanax (Teleostei, Characidae, Incertae sedis). Journal of Fish Biology, 75: 408-421.; Martinez et al., 2012Martinez, E. R. M., A. L. Alves, S. M. Silveira, F. Foresti & C. Oliveira. 2012. Cytogenetic analysis in the incertae sedis species Astyanax altiparanae Garutti and Britzki, 2000 and Hyphessobrycon eques Steindachner, 1882 (Characiformes, Characidae) from the upper Paraná River basin. Comparative Cytogenetics, 6: 41-51.). Thus, one more described structure could be valuable in attempt to solve this phylogenetically question, and since A. altiparanae shows a great phenotypic plasticity, it could be useful as bio-indicator as some studies have already shown this organ can suffer changes under certain environmental conditions (Kirby et al., 2003Kirby, M. F., J. Bignell, E. Brown, J. A. Craft, I. Davies, R. A. Dyer, S. W. Feist, G. Jones, P. Matthiessen, C. Megginson, F. E. Robertson & C. Robinson. 2003. The presence of morphologically intermediate papilla syndrome in United Kingdom populations of sand goby (Pomatoschistus spp.): endocrine disruption? Environmental Toxicology and Chemistry, 22: 239-251.; Larsen et al., 2009Larsen, M. G., K. Bilberg & E. Baatrup. 2009. Reversibility of estrogenic sex changes in zebrafish (Danio rerio). Enviromental Toxicology and Chemistry, 28: 1783-1785.).
The present study aimed to provide a histological description of the urogenital papilla, the components ducts and the surrounding musculature in A. altiparanae.
Material and Methods
Animals. Ten sexually mature male and 10 sexually mature female of A. altiparanae of standard size ranging from 12 to 15 cm were used in this study. As mentioned before, the choice of this species was motivated by characteristics such as ease of handling, small size, sexual maturity at the fourth month of age, and ability to reproduction three to four times a year. These features are considered appropriate for the development of many different studies in laboratory conditions. Experimental procedures were carried out in strict accordance with the guide for care and use of laboratory animals of the Universidade Estadual Paulista (UNESP). The research ethics committee of UNESP approved the protocols (Permit Number: 006/2012/CEUA). All surgical procedures were performed under anesthesia with 0.5 g of benzocaine in 5 ml of absolute ethanol, and all efforts were made to minimize suffering. Fish were identified and a group of voucher individuals was deposited in the collection of fish of Laboratório de Ictiologia Departamento de Zoologia e Botânica IBILCE/UNESP (DZSJRP) under the register number DZSJRP 008999.
Sampling and analyses. The posterior part of the specimens' abdomen that contained the urogenital papilla and the distal portion of the gonads, gut and urinary duct were dissected. These samples were fixed in 4% paraformaldehyde with 2% glutaraldehyde solution in Sorensen phosphate buffer, pH 7.4, for a minimum period of 24 hours. Then, the samples were dehydrated in ethanolic solutions at increasing concentrations and were impregnated in glycol methacrylate (Technovit 7100/historesin; Heraeus Kulzer, Wehrheim, Germany). Finally, the samples were sectioned to a thickness of 3.0 μm using a microtome (LEICA RM 2145; Leica Instruments GmbH, Heidelberg Nussloch, Germany) equipped with a glass blade and stained with Haematoxylin and Eosin, Toluidine Blue and Metanil-Yellow/PAS/Harrys Haematoxylin. The photo-processing and analyses were completed with a Zeiss optical microscope equipped with an AXIOCAM-MRc5 camera (Carl Zeiss Microimaging GmbH, Göttingen, Germany).
Results
Microscopy similarities between males and females, such as the single and folded opening and the skin that was comprised by a squamous stratified epithelial tissue covering the urogenital papilla in both sexes were observed (Figs. 1a; 2b).
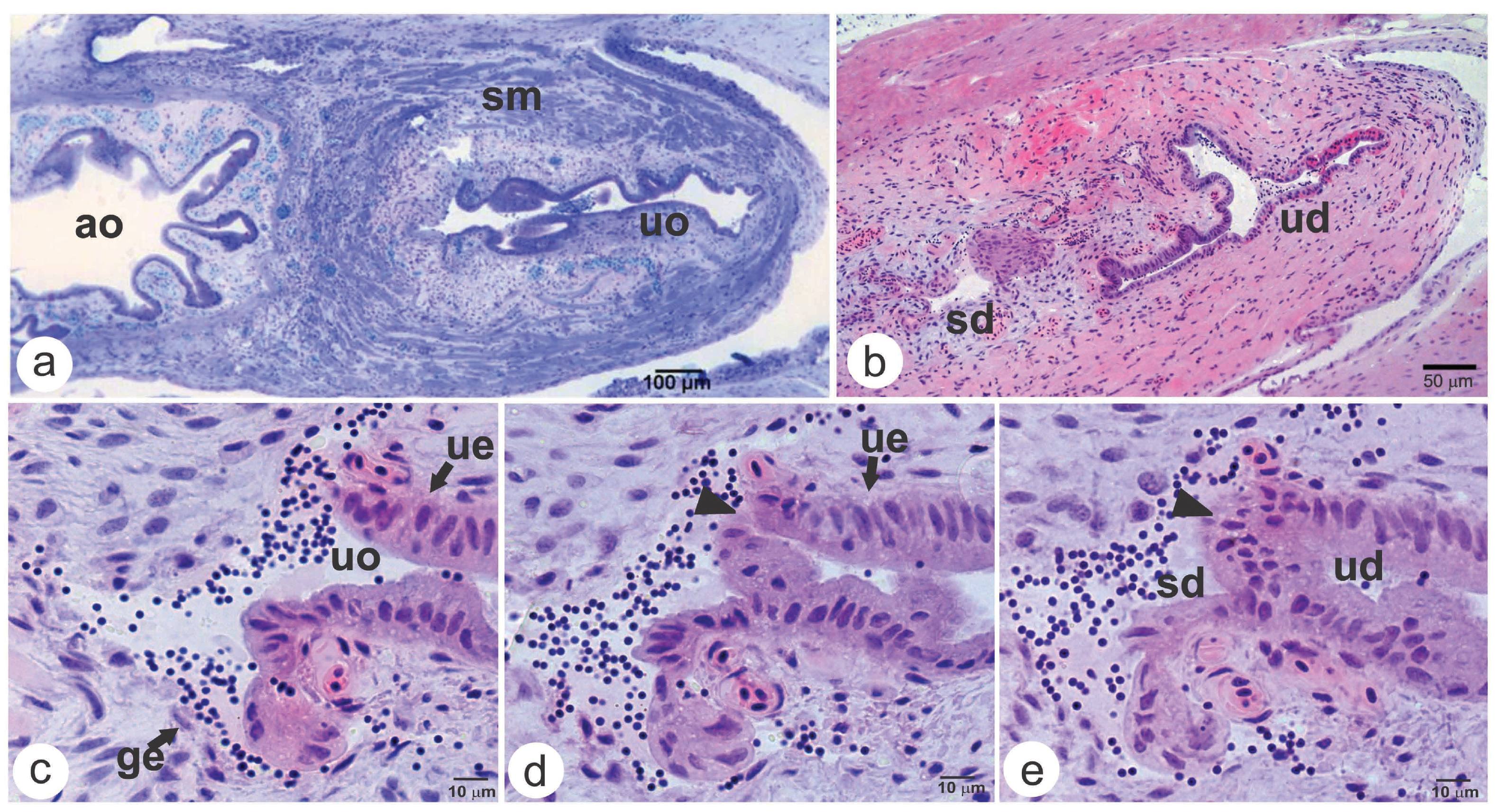
Histological characteristics of the urogenital opening in male Astyanax altiparanae. a) Urogenital opening (uo); b) Sperm duct (sd) and urinary duct (ud) split; c-e) Sequence showing the urogenital duct splitting in the sperm duct (sd) and urinary duct (ud). Abbreviations and symbols: ao = anal opening; ge = squamous genital epithelium formed from the genital tunic; sm = striated skeletal muscle surrounding the urogenital opening; ue = urogenital epithelium formed from the urinary duct; arrowhead = epithelial separation forming the sperm and urinary duct. Label: (a) = Toluidine Blue; (b-e) = Haematoxylin/Eosin.
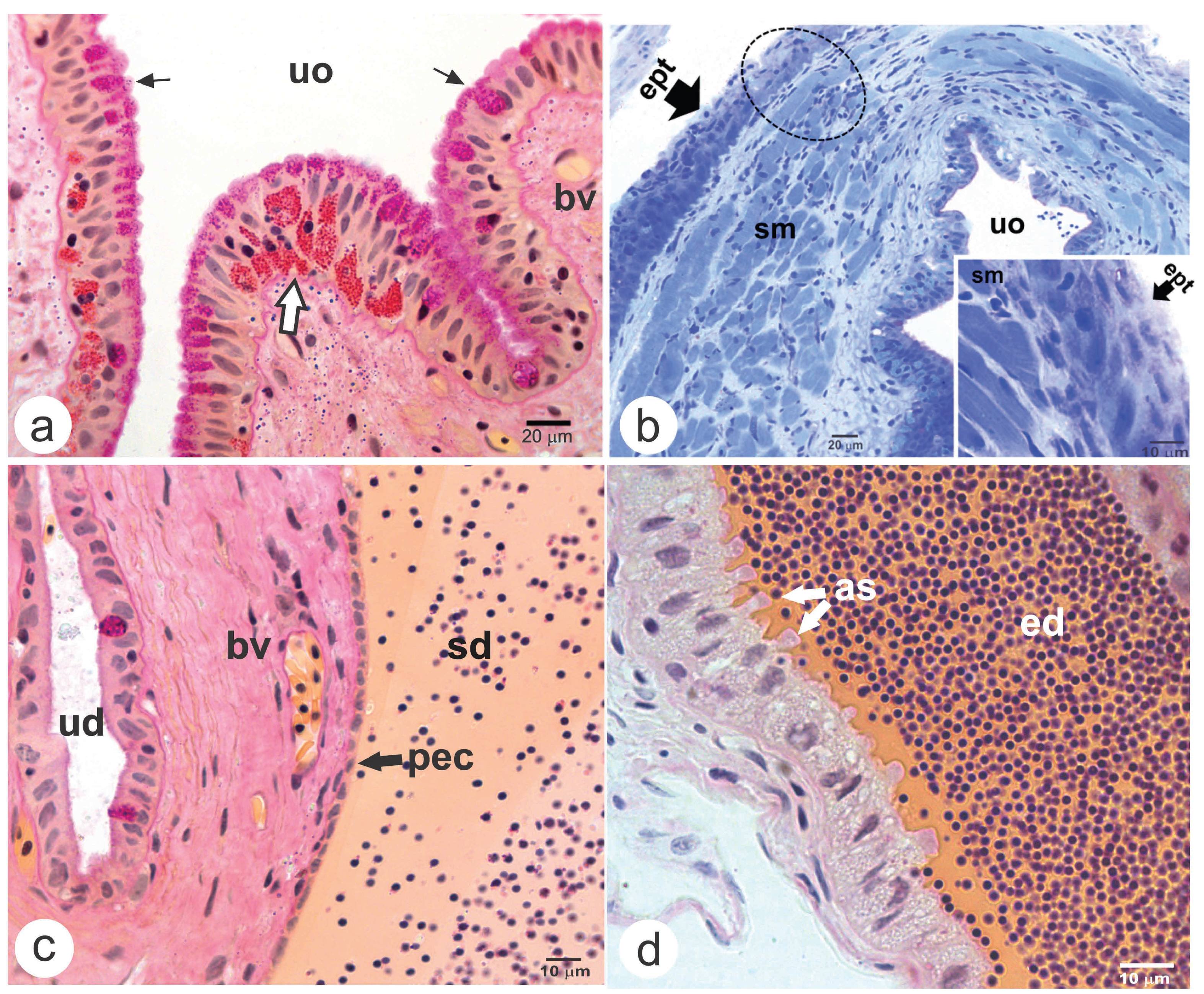
Histological characteristics of the genital duct in male Astyanax altiparanae. a) Urogenital opening (uo) highlighting the clusters of neutral polysaccharides (white arrow), the mucous-producing cells and the brush border (thin arrow); b) detail of the striated muscle (sm) that surround the urogenital opening and the stratified skin (ept) that involve the urogenital papilla; c) sperm duct (sd) highlighting the pavimentous epithelial cells (pec) and seminal fluid labelled in orange; d) efferent duct (ed) with secretory cells with possible apocrine activity (as). Abbreviations and symbols: bv = blood vessel; ept = squamous stratified epithelium; ud = urinary duct. Label: a, c-d = Metanil-Yellow/PAS/Harris Haematoxylin; b = Toluidine Blue.
However, there were significant differences in the morphology of this organ between males and females, mainly in the epithelium that borders the terminal portion of the urogenital opening (Figs. 1a; 3a; 4). In males, this epithelium was formed from the union of the epithelia stemming from both the genital and urinary ducts (Figs. 1a, c; 4b). The epithelium region formed from the genital duct was simple and composed by squamous cells (Fig. 1c; 4b), while the epithelium region formed from the urinary duct epithelium showed columnar and cuboid cells, many of which containing clusters of neutral polysaccharides in their cytoplasm beyond one brush border in their top (Fig. 2a; 4b). Mucus-producing cells were dispersed throughout the epithelium (Fig. 2a). In females, the epithelium bordering the urogenital duct opening has similar characteristics to the epithelium coming from the urinary duct described in males (Fig. 4c).
Histological characteristics of the urogenital papilla in female and urinary duct in Astyanax altiparanae. a) General view of the urogenital opening (uo) and its position in relation to the anal opening (ao). See the absence of muscle fibers (sm) between these two openings (sma); b) The beginning of the urogenital duct separation in genital (go) and urinary ducts (uro); c) The beginning of lamellae formation in the oviduct (od); d) Oviduct lamellae (L) Insert = highlight of the epithelial cells that composes the oviduct lamellae; e) Urinary duct highlighting the globoid cells (cg), mucus-producing cells (thin arrow) and smooth muscle fibers (asterisk) surrounding it; f) In detail, a binucleated globoid cell (bcg). Abbreviations and symbols: sm = striated skeletal muscle fibers; Label: a-c, e-f = Toluidine Blue; d = Metanil-Yellow/PAS/Harris Haematoxylin.
Scheme of the urogenital papilla in Astyanax altiparanae. a) Position of the urogenital papilla in relation to fish body. 1 = urogenital opening; 2 = split of the urogenital duct in genital and urinary duct; 3 = genital duct; 4 = urinary duct. Fish image adapted from: causoeacasodepescador.blogspot.com.br: b) Structure of the urogenital papilla and its components ducts in males; c) Structure of the urogenital papilla and its components ducts in females. Abbreviations and symbols: as = cells with possible apocrine activity; bv = blood vessels; ed = efferent ducts; L = oviduct lamellae; ode = epithelial cells of the oviduct lamellae; sd = spermatic duct; ud = urinary duct; large arrow = striated skeletal muscle fibers.
In males, the urogenital ducts were completely surrounded by a loose connective tissue containing numerous striated muscle fibers and blood vessels (Figs 1a; 2b). In females, this tissue did not involve totally the urogenital papilla, since there were no muscle fibers in the region between the anal and urogenital openings (Fig. 3a).
Posteriorly, the urogenital duct splits between the genital (positioned posterior to the rectum) and the urinary duct (caudally positioned in relation to the genital duct) (Figs. 1b-e; 3a-c; 4). In males, the genital duct (sperm duct) maintained a single, squamous epithelium until the efferent ducts, which also had a single epithelium but with cuboid and columnar secretory cells with possible apocrine activity (Figs. 2c-d). Seminal fluid was also present in this region (Figs. 2c-d; 4b). In females, a single, squamous epithelium was also observed at the beginning of the genital duct (oviduct) (Figs. 3b; 4c). However, close to the ovaries, the epithelium of the oviduct became pseudostratified with cuboid and columnar cells, forming lamellae from the tissue septa projected in the direction of the lumen (Figs. 3d; 4c). In the oviduct lumen no fluid was observed (Figs. 3a-d).
The urinary duct in both sexes had portions with both, single and stratified epithelia contained squamous, cuboid and columnar cells, many of which presented clusters of neutral polysaccharides in their cytoplasm beyond a brush border in their top (Figs. 3e-f). Furthermore, globoid cells, some of them binucleate, were observed among the epithelial cells (Fig. 3f). Smooth muscle fibers surrounded this epithelium (Fig. 3e).
Discussion
Unlike some fish species (e.g., Kirby et al., 2003; Rodrigues et al., 2006; Larsen et al., 2009; Dumont et al., 2011Dumont, P., J. D'Amours, S. Thibodeau, N. Dubuc, R. Verdon, S. Garceau, P. Pilodeau, Y. Mailhot & R. Fortin. 2011. Effects of the development of a newly created spawning ground in the Des Prairies River (Quebec, Canada) on the reproductive success of lake sturgeon (Acipenser fulvescens). Journal of Applied Ichthyology, 27: 394-404.; Kornis et al., 2012Kornis, M. S., N. Mercado-Silva & M. J. Vander Zanden. 2012. Twenty years of invasion: a review of round goby Neogobius melanostomus biology, spread and ecological implications. Journal of Fish Biology, 80: 235-285.), the sex of A. altiparanae cannot be identified by macroscopic examination of the urogenital papilla. However, histological analyses showed significant differences in this organ between the sexes, most likely correlated to reproductive events. These differences were only observed between the genital ducts and in the epithelium that encloses the common urogenital opening. In males, this epithelium has a double origin.
The structure of the urogenital opening is variable among teleost species and it can be different between two species from the same order, as in Cypriniformes whose the common carp Cyprinius carpio (Linnaeus, 1758), as in A. altiparanae, has a single opening, while the goldfish Carassius auratus (Linnaeus, 1758) has ducts opening separately on the papillary surface (Uematsu & Hibiya, 1983Uematsu, K. & T. Hibiya. 1983. Sphincter-like musculature surrounding the urino-genital duct of some teleosts. Japanese Journal of Ichthyology, 30: 72-76.). Furthermore, the structure can be different between the sexes of species from the same genus, as in the inseminating genus Astroblepus Humboldt, 1805 in which the females show separate openings while males have only one opening (Spadella et al., 2012Spadella, M. A., C. Oliveira, H. Ortega, I. Quagio-Grassiotto & J. R. Burns. 2012. Male and female reproductive morphology in the inseminating genus Astroblepus (Ostariophysi: Siluriformes: Astroblepidae). Zoologischer Anzeiger, 251: 38-48.).
Similarities in this structure can be found among evolutionarily distinct groups, such as the Neoteleostei bluehead wrasse Thalassoma bifasciatum (Bloch, 1791) (Labriniformes) and the Euteleostei chum salmon Onchorhynkus keta (Walbaum, 1792) (Salmoniforms), whose divergence time was more than 290 million years ago and both of them show the genital and urinary ducts opening independently on the papillary surface (Uematsu & Hibiya, 1983; Ross, 1984; Hastings & Petersen, 1986; Rasotto & Shapiro, 1998; Ortí & Li, 2009Ortí, G. & C. Li. 2009. Phylogeny, reproductive system, viviparity, spermatozoa. Phylogeny and classification. Pp. 1-24. In: Jamieson, B. G. M. (Ed.). Reproductive biology and phylogeny of fishes (agnathans and bony fishes). Enfield, NH, Science Publishers, v.1. (Reproductive biology and phylogeny series, v.8A-8B).; Kobayashi et al., 2012).
The arrangement of the muscle tissue fibers in A. altiparanae, may be involved in the control of gamete release during the reproductive act, working as a sphincter (Uematsu & Hibiya, 1983; Hastings & Petersen, 1986; Muñoz et al., 2002Muñoz, M., Y. Koya & M. Casadevall. 2002. Histochemical analysis of sperm storage in Helicolenus dactylopterus dactylopterus (Teleostei: Scorpaenidae). Journal of Experimental Zoology, 292: 156-164.). This mechanism may be a strategy to ensure a more efficient fertilization, since only the males have the muscle fibers running circularly around the entire urogenital papilla, making them able to control the timing of sperm release. Other sphincter-like musculature structure was described in T. bifasciatum. This ligament muscle, as the authors named it, was hypothesized to prevent water reflux into the genital and urinary ducts during the swimming movement (Rasotto & Shapiro, 1998).
Another important difference observed between A. altiparanae males and females is in the genital ducts and it can be assigned to the functional and physiological necessities of sexes. For example, the lamellae formation by the oviduct epithelium may be related to oocyte transportation to the urogenital opening (Kobayashi et al., 2012). This mechanism would be similar to the peristaltic movements of the uterine tube in mammals. By contrast, the sperm duct epithelium is related to seminal fluid production (Kobayashi et al., 2012; Spadella et al., 2012), and according to Lahnsteiner et al. (1998)Lahnsteiner, F., B. Berger, T. Weismann & R. A. Patzner. 1998. Determination of semen quality of the rainbow trout, Oncorhynchus mykiss, by sperm motility, seminal plasma parameters, and spermatozoal metabolism. Aquaculture, 163: 163-181., feeds the spermatozoa and keeps them motionless.
The epithelium that covers the urinary duct of the urogenital papilla, in tetra and in the marine species T. bifasciatum has a similar structure (Rasotto & Shapiro, 1998). Besides this, the urinary system of mammals also presents some similarities, since like in the tetra A. altiparanae it also has cells with voluminous cytoplasm, known as globoid cells, showing that this system has probably been preserved among the vertebrates (Junqueira & Carneiro, 2011Junqueira, L. C. U. & J. Carneiro, J. & , 2011. Histologia básica: Texto/Atlas. 11 ed., reimpr., Rio de Janeiro, Guanabara Koogan, 542p.).
Acknowledgments
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq for financial support, Francisco Langeani Neto by fish identification and its deposition in the collection of fishes of DZSJRP and the L.I.NEO group for collaboration during the development of this study.
References
- Batlouni, S. R., E. Romagosa & M. I. Borella. 2006. The reproductive cycle of male catfish Pseudoplatystoma fasciatum (Teleostei, Pimelodidae) revealed by changes of the germinal epithelium an approach addressed to aquaculture. Animal Reproduction Science, 96: 116-132.
- Brown-Peterson, N. J., H. J. Grier & R. M. Overstreet. 2002. Annual changes in germinal epithelium determine male reproductive classes of the cobia. Journal of Fish Biology, 60: 178-202.
- Cassel, M., M. Mehanna, L. Mateus & A. Ferreira. 2013. Gametogenesis and reproductive cycle of Melanorivulus aff. punctatus (Boulenger, 1895) (Cyprinodontiformes, Rivulidae) in Chapada dos Guimarães, Mato Grosso, Brazil. Neotropical Ichthyology, 11: 179-192.
- Dumont, P., J. D'Amours, S. Thibodeau, N. Dubuc, R. Verdon, S. Garceau, P. Pilodeau, Y. Mailhot & R. Fortin. 2011. Effects of the development of a newly created spawning ground in the Des Prairies River (Quebec, Canada) on the reproductive success of lake sturgeon (Acipenser fulvescens). Journal of Applied Ichthyology, 27: 394-404.
- Ferreira, F., M. M. Santos, M. A. Reis-Henriques, N. M. Vieira & N. M. Monteiro. 2010. Sexing blennies using genital papilla morphology or ano-genital distance. Journal of Fish Biology, 77: 1432-1438.
- Garutti, V. & H. A. Britski. 2000. Descrição de uma espécie nova de Astyanax (Teleostei: Characidae) da bacia do alto rio Paraná e considerações sobre as demais espécies do gênero na bacia. Comunicações do Museu de Ciências da PUCRS. Série Zoologia, 13: 65-88.
- Grier, H. J. & R. G. Taylor. 1998. Testicular maturation and regression in the common snook. Journal of Fish Biology, 53: 521-542.
- Hastings, P. A. & C. W. Petersen. 1986. A novel sexual pattern in serranid fishes: simultaneous hermaphrodites and secondary males in Serranus fasciatus. Environmental Biology of Fishes, 15: 59-68.
- Hoshino, K., K. Amaoka & T. A. Munroe. 2004. New records of sexual dimorphisms among the Pleuronectiformes exhibited by differences in urogenital papilla structure of Citharichthys platophrys (Paralichthyidae: Pleuronectiformes). Ichthyologycal Research, 51: 81-83.
- Junqueira, L. C. U. & J. Carneiro, J. & , 2011. Histologia básica: Texto/Atlas. 11 ed., reimpr., Rio de Janeiro, Guanabara Koogan, 542p.
- Kantek, D. L. Z., M. R. Vicari, W. A. M. Peres, M. M. Cestari, R. F. Artoni, L. A. C. Bertollo & O. Moreira-Filho. 2009. Chromosomal location and distribution of As51 satellite DNA in five species of the genus Astyanax (Teleostei, Characidae, Incertae sedis). Journal of Fish Biology, 75: 408-421.
- Kirby, M. F., J. Bignell, E. Brown, J. A. Craft, I. Davies, R. A. Dyer, S. W. Feist, G. Jones, P. Matthiessen, C. Megginson, F. E. Robertson & C. Robinson. 2003. The presence of morphologically intermediate papilla syndrome in United Kingdom populations of sand goby (Pomatoschistus spp.): endocrine disruption? Environmental Toxicology and Chemistry, 22: 239-251.
- Kobayashi, Y., T. Usami, T. Sunobe, H. Manabe, Y. Nagahama & M. Nakamura. 2012. Histological observation of the urogenital papillae in the Bi-directional sex-changing Gobiid fish, Trimma okinawae. Zoological Science, 29: 121-126.
- Kornis, M. S., N. Mercado-Silva & M. J. Vander Zanden. 2012. Twenty years of invasion: a review of round goby Neogobius melanostomus biology, spread and ecological implications. Journal of Fish Biology, 80: 235-285.
- Kott, E., C. B. Renaud & V. D. Vladykov. 1988. The urogenital papilla in the Holarctic lamprey (Petromyzontidae). Environmental Biology of Fishes, 23: 37-43.
- Kruger, T., I. Barnhoorn, J. J. van Vuren & R. Bornman. 2013. The use of the urogenital papillae of male feral African sharptooth catfish (Clarias gariepinus) as indicator of exposure to estrogenic chemicals in two polluted dams in an urban nature reserve, Gauteng, South Africa. Ecotoxicology and Environmental Safety, 87: 98-107.
- Lacerda, S. M. S. N., S. R. Batlouni, G. M. J. Costa, T. M. Segatelli, B. R. Quirino, B. M. Queiroz, E. Kalapothakis & L. R. França. 2010. A new and fast technique to generate offspring after germ cells transplantation in adult fish: the Nile tilapia (Oreochromis niloticus) model. PloS One, 5: e10740 (9p).
- Lahnsteiner, F., B. Berger, T. Weismann & R. A. Patzner. 1998. Determination of semen quality of the rainbow trout, Oncorhynchus mykiss, by sperm motility, seminal plasma parameters, and spermatozoal metabolism. Aquaculture, 163: 163-181.
- Larsen, M. G., K. Bilberg & E. Baatrup. 2009. Reversibility of estrogenic sex changes in zebrafish (Danio rerio). Enviromental Toxicology and Chemistry, 28: 1783-1785.
- Lopes, D. C. J. R., N., Bazzoli, M. F. G. Brito & T. A. Maria. 2004. Male reproductive system in the South American catfish Conorhynchus conirostris. Journal of Fish Biology, 64: 1419-1424.
- Martinez, E. R. M., A. L. Alves, S. M. Silveira, F. Foresti & C. Oliveira. 2012. Cytogenetic analysis in the incertae sedis species Astyanax altiparanae Garutti and Britzki, 2000 and Hyphessobrycon eques Steindachner, 1882 (Characiformes, Characidae) from the upper Paraná River basin. Comparative Cytogenetics, 6: 41-51.
- Muñoz, M., Y. Koya & M. Casadevall. 2002. Histochemical analysis of sperm storage in Helicolenus dactylopterus dactylopterus (Teleostei: Scorpaenidae). Journal of Experimental Zoology, 292: 156-164.
- Muñoz, M. E., S. R. Batlouni, I. B. Franceschini Vicentini & C. A. Vicentini. 2011. Testicular structure and description of the seminal pathway in Leporinus macrocephalus (Anostomidae, Teleostei). Micron, 42: 892-897.
- Orsi, M. L., E. D. Carvalho & F. Foresti. 2004. Biologia populacional de Astyanax altiparanae Garutti & Britski (Teleostei, Characidae) do médio rio Paranapanema, Paraná, Brasil. Revista Brasileira de Zoologia, 21: 207-218.
- Ortí, G. & C. Li. 2009. Phylogeny, reproductive system, viviparity, spermatozoa. Phylogeny and classification. Pp. 1-24. In: Jamieson, B. G. M. (Ed.). Reproductive biology and phylogeny of fishes (agnathans and bony fishes). Enfield, NH, Science Publishers, v.1. (Reproductive biology and phylogeny series, v.8A-8B).
- Rasotto, M. B. & D. Y. Shapiro. 1998. Morphology of gonoducts and male genital papilla, in the bluehead wrasse: implications and correlates on the control of gamete release. Journal of Fish Biology, 52: 716-725.
- Reis, R. E., S. O. Kullander & C. J. Ferraris Jr. 2003. (Orgs.) Check list of the freshwater fishes of South and Central America. Porto Alegre: Edipucrs, 742p.
- Rodrigues, P., M. A. Reis-Henriques, J. Campos & M. M. Santos. 2006. Urogenital papilla feminization in male Pomatoschistus minutus from two estuaries in northwestern Iberian Peninsula. Marine Environmental Research, 62: S258-S262.
- Ross, R. M. 1984. Anatomical changes associated with sex reversal in the fish Thalassoma duperrey (Teleostei: Labridae). Copeia, 1: 245-248.
- Siqueira-Silva, D. H. de, C. A. Vicentini, A. Ninhaus-Silveira & R. Veríssimo-Silveira. 2013. Reproductive cycle of the Neotropical cichlid yellow peacock bass Cichla kelberi: A novel pattern of testicular development. Neotropical Ichthyology, 11: 587-596.
- Spadella, M. A., C. Oliveira, H. Ortega, I. Quagio-Grassiotto & J. R. Burns. 2012. Male and female reproductive morphology in the inseminating genus Astroblepus (Ostariophysi: Siluriformes: Astroblepidae). Zoologischer Anzeiger, 251: 38-48.
- Suzuki, A. & N. Shibata. 2004. Developmental process of genital ducts in the medaka, Oryzias latipes. Zoological Science, 21: 397-406.
- Uematsu, K. & T. Hibiya. 1983. Sphincter-like musculature surrounding the urino-genital duct of some teleosts. Japanese Journal of Ichthyology, 30: 72-76.
- Vazzoler, A. E. A. de M. 1996. Biologia da reprodução de peixes teleósteos: teoria e prática. Maringá; São Paulo, SBI, 169p.
- Wildner, D. D., H. Grier & I. Quagio-Grassiotto. 2013. Female germ cell renewal during the annual reproductive cycle in Ostariophysians fish. Theriogenology, 79: 709-724.
Publication Dates
-
Publication in this collection
Apr-Jun 2015
History
-
Received
08 July 2014 -
Accepted
27 Feb 2015