ABSTRACT
Comparisons of the external morphology and analysis of osteological features of the postcranial and appendicular skeletons of three southwestern Atlantic flatfish species of the genus Paralichthys (P. isosceles, P. orbignyanus and P. patagonicus) were carried out. Bones are described, and detailed morphological, morphometric and meristic characteristics of these flounders are given in order to provide information about the external and internal morphology of three species of Paralichthys occurring in the south-west Atlantic waters that add new information and will help regarding within the framework of a phylogenetic study of the group. Interspecific differences were found in the number of vertebrae and intermuscular bones, as well as in the morphology and morphometry of vertebrae, caudal skeletons, pectoral and pelvic girdle bones. Relationships between bones are discussed and bone characteristics compared with those found in other species of Paralichthys and in other pleuronectiform species. The position of Paralichthys isosceles within Paralichthys is discussed, along with other congeners such as P. triocellatus and P. oblongus.
Keywords:
Flatfishes; Identification key; Postcranial skeleton; Southwest-Atlantic; Taxonomy
RESUMEN
Se llevaron a cabo comparaciones de la morfología externa y el análisis de las características osteológicas de los esqueletos postcraneal y apendicular de tres especies de peces planos del Atlántico sudoccidental del género Paralichthys (P. isosceles, P. orbignyanus y P. patagonicus). Se describen los huesos, y se proporcionan características morfológicas, morfométricas y merísticas detalladas de estos lenguados con el fin de aportar información sobre la morfología externa e interna de tres especies de Paralichthys presentes en el Atlántico sudoccidental. Esta nueva información contribuirá al marco de un estudio filogenético del grupo. Se encontraron diferencias interespecíficas en el número de vértebras y huesos intermusculares, así como en la morfología y morfometría de las vértebras, los esqueletos caudales, los huesos de las cinturas pectoral y pélvica. Se discuten las relaciones entre los huesos y las características óseas en comparación con las encontradas en otras especies de Paralichthys y de otros Pleuronectiformes. Se discute la posición de Paralichthys isosceles dentro del género Paralichthys, junto con otros congéneres como P. oblongus y P. triocellatus.
Palabras-clave:
Atlántico suroccidental; Clave de identificacion; Esqueleto postcraneal; Peces planos; Taxonomía
Introduction
Paralichthys Girard, 1858 is a genus of the Paralichthyidae, a non-monophyletic family (Hensley, Ahlstrom, 1984Hensley DA, Ahlstrom EH. Pleuronectiformes: Relationships. In: Moser HG et al., editors. Ontogeny and Systematics of Fishes: Ahlstrom Symposium. California: American Society of Ichthyologists and Herpetologists; 1984. p.670-687. (Special publication of the American Society of Ichthyologists and Herpetologists; No. 1).; Chapleau, 1993Chapleau F. Pleuronectiform relationships: a cladistic reassessment. Bull Mar Sci. 1993; 52(1):516-40.; Munroe, 2015aMunroe TA. Systematic diversity of the Pleuronectiformes. In: Gibson RN, Nash RDM, Geffen AJ, van der Veer HW, editors. Flatfishes: Biology and Exploitation. 2nd edition. Hoboken (NJ): Wiley Blackwell; 2015a. p.13-51. (Fish and Aquatic Resources Series; 16).) of sinistral (usually) flatfishes. Members of Paralichthys are medium- to large-sized flatfishes (Norman, 1934Norman JR. A Systematic Monograph of the Flatfishes (Heterosomata). London: British Museum of Natural History; 1934. vol. 1, Psettodidae, Bothidae, Pleuronectidae.; Ginsburg, 1952Ginsburg I. Flounders of the genus Paralichthys and related genera in American waters. Fish Bull . 1952; 52(71):267-351.; Hensley, 1995Hensley DA. Guía FAO para identificación de especies para fines de la Pesca: Pacifico Centro-Oriental.. In: Fischer W, Krupp F, Schneider W, Sommer C, Carpenter KE, Niem V, editors). Rome: FAO.; 1995. vol 3, Vertebrados, pt. 2, Paralichthyidae: Lenguados.; Munroe, 2003Munroe TA. Family Paralichthyidae. In: Carpenter KE, editor. The living marine resources of the Western Central Atlantic. Rome: FAO ; 2003. p.1898-1921. vol 3, Bony fishes part 2 (Opistognathidae to Molidae), sea turtles and marine mammals. (FAO species identification guide for fishery purposes and American Society of Ichthyologists and Herpetologists Special Publication; No. 5).).
Except for the Japanese flounder, Paralichthys olivaceus (Temminck, Schlegel, 1846), which occurs off Japan, Korea and China (Wu, 1932Wu HW. Contribution à l’étude morphologique, biologique et systématique des poissons Hétérosomes (Pisces, Heterosomata) de la Chine. [PhD Thesis]. Paris: Faculté des Sciences de l’Université de Paris; 1932.; Amaoka, 1969Amaoka K. Studies on the sinistral flounders found in the waters around Japan: taxonomy, anatomy and phylogeny. J Shimonoseki Univ Fish. 1969; 18(2):65-340.; Li, Wang, 1995Li S, Wang H. Fauna Sinica, Osteichthyes, Pleuronectiformes. Beijing: Science Press; 1995.; Munroe, 2015bMunroe TA. Distributions and biogeography. In: Gibson RN, Nash RDM, Geffen AJ, van der Veer HW, editors. Flatfishes: Biology and Exploitation. 2nd edition. Hoboken (NJ): Wiley Blackwell ; 2015b. p.52-82. (Fish and Aquatic Resources Series; 16).), all other members of Paralichthys (ca. 25 nominal species) are distributed in temperate and tropical seas along the coasts and on the continental shelves of the American continent (Ginsburg, 1952Ginsburg I. Flounders of the genus Paralichthys and related genera in American waters. Fish Bull . 1952; 52(71):267-351.; Hensley, 1995Hensley DA. Guía FAO para identificación de especies para fines de la Pesca: Pacifico Centro-Oriental.. In: Fischer W, Krupp F, Schneider W, Sommer C, Carpenter KE, Niem V, editors). Rome: FAO.; 1995. vol 3, Vertebrados, pt. 2, Paralichthyidae: Lenguados.; Munroe, 2003Munroe TA. Family Paralichthyidae. In: Carpenter KE, editor. The living marine resources of the Western Central Atlantic. Rome: FAO ; 2003. p.1898-1921. vol 3, Bony fishes part 2 (Opistognathidae to Molidae), sea turtles and marine mammals. (FAO species identification guide for fishery purposes and American Society of Ichthyologists and Herpetologists Special Publication; No. 5).).
In the southwest Atlantic Ocean species of Paralichthys occur from northern Brazil just south of the Amazon river at ca. 1º N (Carvalho-Filho, 1999Carvalho-Filho A. Peixes: costa brasileira. 3th ed. São Paulo: Editora Melro; 1999.; Walsh et al., 2015Walsh SJ, Díaz de Astarloa JM, Poos JJ. Atlantic Flatfish Fisheries. In: Gibson RN, Nash RDM, Geffen AJ, van der Veer HW, editors. Flatfishes: Biology and exploitation. 2nd edition). Hoboken (NJ): Wiley Blackwell ; 2015. p.346-394. (Fish and Aquatic Resources Series; 16).) to central Patagonia, Argentina at ca. 47ºS (Díaz de Astarloa, Munroe, 1998Díaz de Astarloa JM, Munroe TA. Systematics, distribution and ecology of commercially important paralichthyid flounders ocurring in Argentinean-Uruguayan waters (Paralichthys, Paralichthyidae): an overview. J Sea Res. 1998; 39(1-2):1-9.). Throughout the southwest Atlantic region, species of Paralichthys occur in diverse habitats including coastal shallow-waters in areas containing muddy and silty substrata such as turbid estuaries, and also on sandy substrata in moderate depths on the continental shelf. In addition, some species of Paralichthys inhabit a variety of different substrata on the continental shelf, with some [P. isosceles Jordan, 1891; P. oblongus (Mitchill, 1815); P. triocellatus Miranda Ribeiro, 1903] also found on deep-water substrata located on the outer continental shelf.
Seven nominal species have long been reported from coastal seas of the Southwest Atlantic (Ginsburg, 1952Ginsburg I. Flounders of the genus Paralichthys and related genera in American waters. Fish Bull . 1952; 52(71):267-351.), including P. bicyclophorus Miranda Ribeiro, 1915, P. brasiliensis (Ranzani, 1842), P. isosceles, P. orbignyanus (Valenciennes, 1839), P. patagonicusJordan, 1889Jordan DS, Goss DK. A review of the flounders and soles (Pleuronectidae) of America and Europe. Rep U.S. Fish Comm. 1889; 14:225-42., P. triocellatus, and P. tropicus Ginsburg, 1933. Paralichthys bicyclophorus was later synonimized with P. patagonicus, in Jordan, Goss (1889) by Díaz de Astarloa (1996Díaz de Astarloa JM. Paralichthys patagonicus Jordan, in Jordan & Goss, 1889, a senior synonym of P. bicyclophorus Miranda Ribeiro, 1915 (Paralichthyidae: Pleuronectiformes). Copeia. 1996; (4):1035-37.).
Although the ecological and economic (Walsh et al., 2015Walsh SJ, Díaz de Astarloa JM, Poos JJ. Atlantic Flatfish Fisheries. In: Gibson RN, Nash RDM, Geffen AJ, van der Veer HW, editors. Flatfishes: Biology and exploitation. 2nd edition). Hoboken (NJ): Wiley Blackwell ; 2015. p.346-394. (Fish and Aquatic Resources Series; 16).) importance of Paralichthyid flounders have long been recognized (Mac Donagh, 1936Mac Donagh EJ. Sobre algunos peces marinos. Notas Mus. La Plata , Zool. 1936; 4:423-29.; Marini, López, 1963Marini TL, López RB. Recursos acuáticos vivos. Buenos Aires: Consejo Federal de Inversiones; 1963. (Evaluación de los recursos naturales de la Argentina, Primera Etapa; vol 1-2, pt. 7).; Bellisio et al., 1979Bellisio NB, López RB, Torno A. Peces marinos patagónicos. Publ Secret Int. Mar. Buenos Aires: Ed. Códex; 1979.; Cousseau, Fabré, 1990Fabré NN, Cousseau MB. Sobre la determinación de la edad y el crecimiento del lenguado Paralichthys isosceles aplicando retrocálculo. Rev Brazil Biol. 1990; 50(2):345-54.), species of South Atlantic Paralichthys, in general, have not been well studied. Most studies on these species have focused on a variety of subjects, but usually these have treated only some of the species of this genus from this area in each study. Studies have included those on traditional taxonomy (Cousseau, Díaz de Astarloa, 1991Cousseau MB, Díaz de Astarloa JM. Investigaciones sobre dos categorías específicas: Paralichthys bicyclophorus y Paralichthys patagonicus. Frente Marítimo. 1991; 8:51-59.; Díaz de Astarloa, 1995aDíaz de Astarloa JM. Determinación taxonómica de un grupo de lenguados del género Paralichthys colectado en aguas argentinas (Pleuronectiformes: Paralichthyidae). Rev Biol Mar Oceanogr. 1995a; 30(1):79-90., 1996Díaz de Astarloa JM. Paralichthys patagonicus Jordan, in Jordan & Goss, 1889, a senior synonym of P. bicyclophorus Miranda Ribeiro, 1915 (Paralichthyidae: Pleuronectiformes). Copeia. 1996; (4):1035-37.; Díaz de Astarloa, Munroe, 1998Díaz de Astarloa JM, Munroe TA. Systematics, distribution and ecology of commercially important paralichthyid flounders ocurring in Argentinean-Uruguayan waters (Paralichthys, Paralichthyidae): an overview. J Sea Res. 1998; 39(1-2):1-9.; Díaz de Astarloa et al., 2006aDíaz de Astarloa JM, Munroe TA, Desoutter M. Redescription and holotype clarification of Paralichthys orbignyanus (Valenciennes, 1839) (Pleuronectiformes, Paralichthyidae). Copeia . 2006a; (2):235-43.), partial aspects of the cranial osteology (Díaz de Astarloa, 2005Díaz de Astarloa JM. Comparative cranial osteology of three species of the flatfish genus Paralichthys (Pleuronectiformes, Paralichthyidae) from the southwestern Atlantic. Rev Chil Hist Nat. 2005; 78(3):343-91.), age and growth (Fabré, Cousseau, 1990Fabré NN, Cousseau MB. Sobre la determinación de la edad y el crecimiento del lenguado Paralichthys isosceles aplicando retrocálculo. Rev Brazil Biol. 1990; 50(2):345-54.; Lopez-Cazorla, 2005Lopez-Cazorla A. On the age and growth of flounder Paralichthys orbignyanus (Jenyns, 1842) in Bahía Blanca Estuary, Argentina. Hydrobiologia. 2005; 537(1-3):81-87.), distribution and abundance (Fabré, Díaz de Astarloa, 2001Fabré NN, Díaz de Astarloa JM. Distributional patterns and abundance of paralichthyid flounders in the south-west Atlantic (Pleuronectiformes: Paralichthyidae). Thalassas. 2001; 17(1):45-55.; Díaz de Astarloa, Fabré, 2002Díaz de Astarloa JM, Fabré NN. Abundance of three flatfishes (Pleuronectiformes: Paralichthyidae) off northern Argentina and Uruguay in relation to environmental factors. Arch Fish Mar Res. 2002; 50(2):121-40.), general aspects about their ecology (Díaz de Astarloa, Munroe, 1998Díaz de Astarloa JM, Munroe TA. Systematics, distribution and ecology of commercially important paralichthyid flounders ocurring in Argentinean-Uruguayan waters (Paralichthys, Paralichthyidae): an overview. J Sea Res. 1998; 39(1-2):1-9.), population dynamics (Fabré, 1992Fabré NN. Análisis de la distribución y dinámica poblacional de lenguados de la Provincia de Buenos Aires (Pisces, Bothidae). [PhD Thesis]. Mar del Plata, Buenos Aires: Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Mar del Plata; 1992.), food and feeding habits (García, 1987García ML. Pleuronectiformes de la Argentina, IV. Alimentación de Paralichthys isosceles (Bothidae, Paralichthyinae). Notas Mus. La Plata, Zool. 1987; 207:111-25.; Lopez-Cazorla, Forte, 2005Lopez-Cazorla A, Forte S. Food and feeding habits of flounder Paralichthys orbignyanus (Jenyns, 1842) in Bahía Blanca Estuary, Argentina. Hydrobiologia . 2005; 549(1):251-57.), abnormalities (Díaz de Astarloa, 1995bDíaz de Astarloa JM. Ambicoloration in two flounders, Paralichthys patagonicus and Xystreuris rasile. J Fish Biol. 1995b; 47(1):168-70., 1997Díaz de Astarloa JM. A case of reversal in Paralichthys orbignyanus a shallow-water flounder from the south-western Atlantic. J Fish Biol . 1997; 50(4):900-02., 1998aDíaz de Astarloa JM. Vertebral anomalies in the patagonian flounder Paralichthys patagonicus (Pleuronectiformes: Paralichthyidae) from Río de la Plata estuary. Physis. 1998a; 56(130-131):7-11., 1998bDíaz de Astarloa JM. An ambicolorate flounder, Paralichthys isosceles, collected off Península Valdés, Argentina. Cybium . 1998b; 22(2):187-91.; Díaz de Astarloa et al., 2006bDíaz de Astarloa JM, Rico R, Acha M. First report of a totally ambicoloured Patagonian flounder Paralichthys patagonicus (Paralichthyidae) with dorsal fin anomalies. Cybium . 2006b; 30(1):73-76.) and reproduction (Macchi, Díaz de Astarloa, 1996Macchi GJ, Díaz de Astarloa JM. Ciclo reproductivo y fecundidad del lenguado, Paralichthys patagonicus. Rev Inv Des Pesq. 1996; (10):73-83.; Radonic et al., 2007Radonic M, Müller MI, López AV, Bambill GA, Spinedi M, Boccanfuso JJ. Improvement in flounder Paralichthys orbignyanus controlled spawning in Argentina. Cienc Mar. 2007; 33(2):187-96.; Militelli, 2011Militelli MI. Paralichthys patagonicus spawning areas and reproductive potential in the Bonaerense Coastal Zone, Argentina (34º-42ºS). Lat Am Aquat Res. 2011; 39(1):131-37.) of some species of the genus. Despite of these contributions, gaps still remain in the available knowledge about biological, anatomical (especially osteology) and ecological information available for several species of Paralichthys from this region.
In some demersal fish communities, especially those inhabiting soft-bottom habitats in the southwestern Atlantic Ocean (Walsh et al., 2015Walsh SJ, Díaz de Astarloa JM, Poos JJ. Atlantic Flatfish Fisheries. In: Gibson RN, Nash RDM, Geffen AJ, van der Veer HW, editors. Flatfishes: Biology and exploitation. 2nd edition). Hoboken (NJ): Wiley Blackwell ; 2015. p.346-394. (Fish and Aquatic Resources Series; 16).), paralichthyids can be abundant and may account for a significant portion of the fish biomass. For example, the Patagonian flounder (P. patagonicus) is widely fished on the continental shelf from Rio de Janeiro State to at least as far south as northern Patagonia (Díaz de Astarloa, Munroe, 1998Díaz de Astarloa JM, Munroe TA. Systematics, distribution and ecology of commercially important paralichthyid flounders ocurring in Argentinean-Uruguayan waters (Paralichthys, Paralichthyidae): an overview. J Sea Res. 1998; 39(1-2):1-9.). Although landing statistics of flatfishes for this region are aggregated, Patagonian flounder is the most abundant flatfish species in these landings, representing 61% of the total of flatfish species captured in Argentina (Rico, 2010Rico MR. Pesquería de lenguados en el ecosistema costero bonaerense al norte de 39° S. Frente Marítimo . 2010; 21:129-35.). In southern Brazil, it is the main species of flatfish landed in the bottom trawl fisheries on the continental shelf and in coastal shallow-waters (Carneiro, 1995Carneiro MH. Reprodução e alimentação dos linguados Paralichthys patagonicus e Paralichthys orbignyanus (Pleuronectiformes: Bothidae), no Rio Grande do Sul, Brasil. [Msc. Dissertation]. Rio Grande, RS: Universidade Federal do Rio Grande; 1995.), representing 96.3% of the total flatfish landings (Haimovici, 1998Haimovici M. Present state and perspectives for the southern Brazil shelf demersal fisheries. Fish Manag Ecol. 1998; 5:277-89.). Other species of paralichthyids taken commercially include the Mud flounder (P. orbignyanus), a shallow-water flatfish occurring from Rio de Janeiro southward to San Matías Gulf, northern Patagonia, in Argentina, as well as the Isosceles flounder, P. isosceles. The Mud flounder represents 2.3% of the total flatfish landings in Rio Grande do Sul. At Mar del Plata harbor (Argentina), where almost 70% of the fishing fleet is located, the Mud and Patagonian flounders combined rank eighth in the total amount of kilograms of fish biomass sold. Paralichthys isosceles is marketed as small or moderate-sized flounder in Mar del Plata harbor, representing between 2.4% and 2.6% of the total amount of fish sold there (Fabré, Díaz de Astarloa, 2001Fabré NN, Díaz de Astarloa JM. Distributional patterns and abundance of paralichthyid flounders in the south-west Atlantic (Pleuronectiformes: Paralichthyidae). Thalassas. 2001; 17(1):45-55.). It is only occasionally caught in Brazilian waters (Haimovici, Mendonça, 1996Haimovici M, Mendonça JT. Descartes da fauna acompanhante na pesca de arrasto de tangones dirigida a linguados e camarões na plataforma continental do sul do Brasil. Atlântida. 1996; 18:161-77.). In Argentine waters (Díaz de Astarloa, 2002Díaz de Astarloa JM. A review of the flatfish fisheries of the south Atlantic Ocean. Rev Biol Mar. Oceanogr. 2002; 37(2):113-25.), three species of Paralichthys (P. isosceles, P. orbignyanus, and P. patagonicus) are the most important flatfishes in commercial fisheries conducted in those waters (Fig. 1a, b, c). Here, these fishes constitute the most abundant flounders in the commercial landings of Mar del Plata harbor (Cousseau, Fabré, 1990Cousseau MB, Fabré NN. Lenguados. In: Cousseau, MB, editor. Muestreo bioestadístico de desembarque del Puerto de Mar del Plata Período 1980-1985. Mar del Plata: Contribución INIDEP; 1990. No. 5.; Fabré, 1992Fabré NN. Análisis de la distribución y dinámica poblacional de lenguados de la Provincia de Buenos Aires (Pisces, Bothidae). [PhD Thesis]. Mar del Plata, Buenos Aires: Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Mar del Plata; 1992.; Rico, 2010Rico MR. Pesquería de lenguados en el ecosistema costero bonaerense al norte de 39° S. Frente Marítimo . 2010; 21:129-35.) and they also play an important role in artisanal fisheries conducted in shallow waters of the Rio de la Plata estuary on the Uruguayan side and on the Atlantic coasts of Argentina and Uruguay, as well (Silva Junior et al., 2007da Silva Junior LC, De Andrade AC, De Andrade-Tubino MF, Vianna M. Reversal and ambicoloration in two flounder species (Paralichthyidae, Pleuronectiformes). Panam J Aquat Sci, 2007; 2(1):23-26.).
Photographs of three flatfishes of Paralichthys from the Southwestern Atlantic: a. Paralichthys isosceles, b. Paralichthys orbignyanus, c. Paralichthys patagonicus. Scale bar indicates 5 cm.
Ginsburg´s (1952Ginsburg I. Flounders of the genus Paralichthys and related genera in American waters. Fish Bull . 1952; 52(71):267-351.) study remains the most comprehensive systematic treatment of species of Paralichthys. Other taxonomic and systematic information is scattered among several works (Gutherz, 1967Gutherz EJ. Field guide to the flatfishes of the family Bothidae in the western North Atlantic. Washington (DC): US Fish and wildlife service; Bureau of commercial fisheries; 1967. (Circular 263).; Amaoka, 1969Amaoka K. Studies on the sinistral flounders found in the waters around Japan: taxonomy, anatomy and phylogeny. J Shimonoseki Univ Fish. 1969; 18(2):65-340.; Hensley, 1995Hensley DA. Guía FAO para identificación de especies para fines de la Pesca: Pacifico Centro-Oriental.. In: Fischer W, Krupp F, Schneider W, Sommer C, Carpenter KE, Niem V, editors). Rome: FAO.; 1995. vol 3, Vertebrados, pt. 2, Paralichthyidae: Lenguados.; Díaz de Astarloa, Munroe, 1998Díaz de Astarloa JM, Munroe TA. Systematics, distribution and ecology of commercially important paralichthyid flounders ocurring in Argentinean-Uruguayan waters (Paralichthys, Paralichthyidae): an overview. J Sea Res. 1998; 39(1-2):1-9.; Figueiredo, Menezes, 2000Figueiredo JL, Menezes NA. Manual de Peixes Marihnos do sudeste do Brasil. São Paulo: Museu de Zoologia da Universidade de São Paulo; 2000. vol 6, Telostei (5).; Munroe, 2003Munroe TA. Family Paralichthyidae. In: Carpenter KE, editor. The living marine resources of the Western Central Atlantic. Rome: FAO ; 2003. p.1898-1921. vol 3, Bony fishes part 2 (Opistognathidae to Molidae), sea turtles and marine mammals. (FAO species identification guide for fishery purposes and American Society of Ichthyologists and Herpetologists Special Publication; No. 5).). However, Paralichthys is still not adequately defined based on shared derived morphological characters and even the species composition of this genus as presently known remains controversial. For example, some nominal species now included in Paralichthys were previously considered as members of the related paralichthyid Hippoglossina Steindachner, 1876 or Pseudorhombus Bleeker, 1862. Such is the case for the nominal species P. isosceles, which was placed in Pseudorhombus isosceles by Ginsburg (1952Ginsburg I. Flounders of the genus Paralichthys and related genera in American waters. Fish Bull . 1952; 52(71):267-351.) but without further comment or investigation as to why Norman (1934Norman JR. A Systematic Monograph of the Flatfishes (Heterosomata). London: British Museum of Natural History; 1934. vol. 1, Psettodidae, Bothidae, Pleuronectidae.) recognized it as a member of Paralichthys. More contemporary authors (Figueiredo, Menezes, 2000Figueiredo JL, Menezes NA. Manual de Peixes Marihnos do sudeste do Brasil. São Paulo: Museu de Zoologia da Universidade de São Paulo; 2000. vol 6, Telostei (5).) also recognize this species as a member of Paralichthys. Placement of these nominal taxa into other paralichthyid genera has not always been based upon objective criteria supported by morphological features, but rather, placements or re-assignments have been based on authoritative opinions without substantiation supported by the possession of shared, derived characters among these taxa. Furthermore, none of the previous studies on Paralichthys has examined systematic relationships among members of this genus and our knowledge of the evolutionary relationships among these taxa remains at a basic level.
This paper provides new information about the external and internal morphology of the three most commercially important species of Paralichthys occurring in the Southwest Atlantic Ocean. This new information complements information provided in earlier studies by Cousseau, Díaz de Astarloa (1991Cousseau MB, Díaz de Astarloa JM. Investigaciones sobre dos categorías específicas: Paralichthys bicyclophorus y Paralichthys patagonicus. Frente Marítimo. 1991; 8:51-59.), Díaz de Astarloa (1995aDíaz de Astarloa JM. Determinación taxonómica de un grupo de lenguados del género Paralichthys colectado en aguas argentinas (Pleuronectiformes: Paralichthyidae). Rev Biol Mar Oceanogr. 1995a; 30(1):79-90., 1996Díaz de Astarloa JM. Paralichthys patagonicus Jordan, in Jordan & Goss, 1889, a senior synonym of P. bicyclophorus Miranda Ribeiro, 1915 (Paralichthyidae: Pleuronectiformes). Copeia. 1996; (4):1035-37.), Díaz de Astarloa, Munroe (1998Díaz de Astarloa JM, Munroe TA. Systematics, distribution and ecology of commercially important paralichthyid flounders ocurring in Argentinean-Uruguayan waters (Paralichthys, Paralichthyidae): an overview. J Sea Res. 1998; 39(1-2):1-9.), Figueiredo, Menezes (2000Figueiredo JL, Menezes NA. Manual de Peixes Marihnos do sudeste do Brasil. São Paulo: Museu de Zoologia da Universidade de São Paulo; 2000. vol 6, Telostei (5).), Díaz de Astarloa (2005Díaz de Astarloa JM. Comparative cranial osteology of three species of the flatfish genus Paralichthys (Pleuronectiformes, Paralichthyidae) from the southwestern Atlantic. Rev Chil Hist Nat. 2005; 78(3):343-91.), and Díaz de Astarloa et al. (2006aDíaz de Astarloa JM, Munroe TA, Desoutter M. Redescription and holotype clarification of Paralichthys orbignyanus (Valenciennes, 1839) (Pleuronectiformes, Paralichthyidae). Copeia . 2006a; (2):235-43.). In addition, this new information will be helpful regarding studies in comparative anatomy, flatfish bone identification in archaeozoology or paleontology, and construction of a framework for phylogenetic study of the group. Also, comparisons of this new information with that of congeners will help in the assignment of species under study to the correct genus. These assignments will facilitate further investigation regarding the phylogenetic analyses of relationships among nominal species of Paralichthys. Finally, to assist other investigators interested in identifying and studying these fishes, we provide updated identification keys to the species occurring in each ocean based on new information derived from this study.
Material and Methods
A total of 553 specimens of Paralichthys were examined in the present study. The taxonomic analysis for the specimens occurring in the Southwest Atlantic waters was based on examination of both external (morphometrics and meristics) and internal (osteological) characters of 23 specimens of Paralichthys isosceles (130-370 mm SL), 35 specimens of P. patagonicus (250-480 mm SL) and 23 specimens of P. orbignyanus (390-1030 mm SL). The maximum length ranges correspond to the largest size of each species. All specimens were collected on the south-west Atlantic continental shelf between 34°30’S and 44°54’S, and between 40 and 100 m depth.
Thirteen morphometric characters on the ocular side were measured to the nearest 0.1 mm using dial calipers or a metal ruler. Measurements are expressed either as percentages of standard length (SL) or percentages of head length (HL) and are defined as follows (abbreviations in parentheses). Standard length - measured as a straight line from the anteriormost end of the lower lip to the caudal-fin base (posterior end of hypural plate). Head and snout lengths (SNL) - measured from anteriormost end of lower lip to posterior end of opercular flap and to anterior fleshy margin of lower eye, respectively. Pectoral-fin length (PL) - measured as length of longest finray. Upper jaw length (UJL) - measured from anterior margin of upper lip to posterior end of maxilla. Eye diameter (ED) - measured as greatest fleshy diameter of the lower eye. Interorbital width (IW) - measured as least fleshy width between the orbits. Predorsal (PDL), prepelvic (PVL) , preanal (PAL) and prepectoral (PPL) lengths - all measured from anteriormost end of lower lip to bases of first finrays in the dorsal, ventral, anal and pectoral fins, respectively. Caudal-peduncle depth (CPD) - measured as least depth across caudal the peduncle.
Meristic data were also taken from the ocular side of each specimen. Numbers of perforated lateral-line scales were counted starting from the scale above the pectoral-fin base and ending with that located at the base of the caudal-fin rays. Counts of oblique rows of scales along the horizontal region of the lateral line were made from the point just posterior to the lateral-line arch above the pectoral-fin rays to bases of the caudal-fin rays. Vertebral numbers were obtained either from dissection of the fish, or from cleared and stained specimens, or were taken from radiographs of type and non-type specimens curated in institutions. The urostyle was included as one vertebra. Numbers of infraorbital bones, epineurals and ribs, premaxilla and dentary teeth were also counted. Abbreviations for meristic characters are: DO, dorsal-fin rays; AN, anal-fin rays; PE, pectoral-fin rays, CA, caudal-fin rays; GR, gill rakers on first arch; LL, lateral-line scales; VE, total vertebrae.
Regression analysis was performed to compare body proportions among the three species of Paralichthys. Standard length and morphometric relationships were calculated separately between sexes for each species, and between species. The null hypothesis of no difference between slopes of the linear regressions was tested with the Student´s t-test (Zar, 1984Zar JH. Biostatistical Analysis. Englewood Cliffs, Prentice Hall; 1984.). Differences among species in meristic features were tested by a Kruskal-Wallis analysis of variance (ANOVA) (Hoaglin et al., 1991Hoaglin DC, Mosteller F, Tukey J, editors. Fundamentals of exploratory analysis of variance. New York: John Wiley & Sons, Inc.; 1991. (Wiley Series in Probability and Statistics).). Contrast comparisons between flatfish species were made using a Wilcoxon rank test with those characters that showed significant differences in the ANOVA. In all statistical tests, the probability of a type I error was set equal to 0.05.
Specimens examined included both fresh and alcohol-preserved fish that were dissected, and also cleared and stained specimens. Observations of anatomical features were made macroscopically and under a stereomicroscope. Drawings were made with the aid of a camera lucida attachment. Methods for preparing disarticulated skeletons followed Ossian (1970Ossian CR. Preparation of disarticulated skeletons using enzyme-based laundry “pre-soakers”. Copeia . 1970; (1):199-200.) and Mayden, Wiley (1984Mayden RL, Wiley EO. A method of preparing disarticulated skeletons of small fishes. Copeia . 1984; (1):230-32.). Methods of clearing and staining for bone and cartilage followed Dingerkus, Uhler (1977Dingerkus G, Uhler LH. Enzyme clearing of alcian blue stained whole small vertebrates for demonstration of cartilage. Stain Technol. 1977; 52(4):229-32.), Potthoff (1984Potthoff T. Clearing and staining techniques. In: Moser HG et al., editors. Ontogeny and Systematics of Fishes: Ahlstrom Symposium. California: American Society of Ichthyologists and Herpetologists; 1984. p.35-37. (Special publication of the American Society of Ichthyologists and Herpetologists; No. 1).) and Kawamura, Hosoya (1991Kawamura K, Hosoya K. A modified double staining technique for making a transparent fish-skeletal specimen. Bull Natl Res Inst Aquacult (Jpn). 1991; 20:11-18.). Terminology for osteology mainly followed that of Cervigón (1985Cervigón F. Las especies de los géneros Achirus y Trinectes (Pisces: Soleidae) de las costas de Venezuela (Osteología, Musculatura y ligamentos fasciales, y Sistemática). Caracas: Fundación Científica Los Roques, Monografía; 1985.) and Hoshino, Amaoka (1998Hoshino K, Amaoka K. Osteology of the flounder, Tephrinectes sinensis (Lacèpede) (Teleostei: Pleuronectiformes), with comments on its relationships. Ichthyol Res . 1998; 45(1):69-77.), except for intermuscular bones where Patterson, Johnson (1995Patterson C, Johnson GD. The intermuscular bones and ligaments of teleostean fishes. Washington, (DC): Smithsonian Institution Press; 1995. (Smithsonian Contributions to Zoology; No. 559).) were followed, and terminology of Hoshino (2001Hoshino K. Homologies of the caudal fin rays of Pleuronectiformes (Teleostei). Ichthyol Res. 2001; 48(3):231-46.) was used for elements of the caudal skeleton. Other osteological terms followed those in Rojo (1988Rojo AL. Diccionario enciclopédico de anatomía de peces. Madri: Ministerio de Agricultura, Pesca y Alimentación; 1988. (Monografias del Instituto Español de oceanografía; No. 3).).
Abbreviations used for names of bones are as follows, or are provided under individual illustrations. ba - Basipterygium, ep - Epineurals, ri - Ribs, cl - Cleithrum, Cp. 1-2 - Preural centra, is - Interhaemal spine, hs - Haemal spine, ns - Neural spine, ep1-2 - Epurals 1 and 2, sca - Scapula, H. 1-5 - Hypurals, lp - Lower postcleithrum, up - Upper postcleithrum, phy - Parhypural, PoZ - Postzygapophyses, PrZ - Prezygapophyses, dp - Distal pterygiophore, pp - Proximal pterygiophore, pra - Pectoral radials, pr - Pectoral rays, sc - Supracleithrum, uh Urohyal, cv - Caudal vertebra, pv - Precaudal vertebra.
Museum acronyms follow those listed in Sabaj Pérez (2016Sabaj Pérez MH, editor. Standard symbolic codes for institutional resource collections in herpetology and ichthyology: an online reference. Washington (DC), version 6.5. Available from: http://www.asih.org/resources/standard-symbolic-codes-institutional-resource-collections-herpetology-ichthyology
http://www.asih.org/resources/standard-s...
).
Results
Morphology. Scale morphology. All species of Paralichthys examined herein have either ctenoid or cycloid scales on their ocular sides, but only P. isosceles has ctenoid scales on both the ocular side as well as on the blind side of the body. In addition, the species have accessory scales on their ocular sides with the exception of P. isosceles, P. triocellatus and P. oblongus, which lack accessory scales.
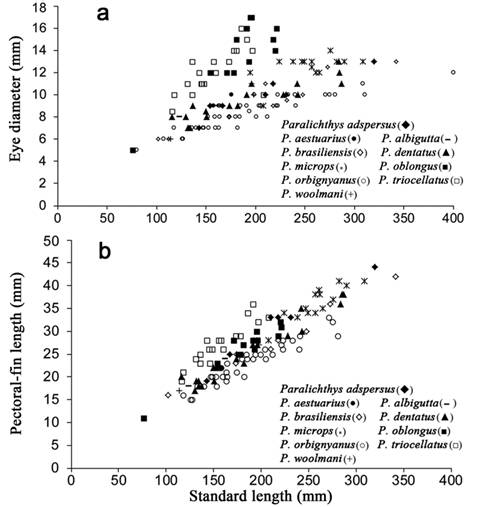
Morphometric comparisons. Comparisons of morphometric features examined in the three southwest Atlantic species of Paralichthys revealed that some features were not significantly different between species, whereas other morphometric features were significantly different between these species. Regression analysis revealed no differences (P>0.05) in relationships of head length to standard length for the three southwest Atlantic species examined (Fig. 2a). In contrast, significant differences were found between these species in several other morphometric features. For example, in P. orbignyanus, the IOW (Fig. 2b) is 2.25 times wider [mean 12.5% (range 9.1-16.6%) HL] than that of P. isosceles [5.5% (4-7%) of HL] (P<0.0001), and 2.0 times as wide as that of P. patagonicus [mean 6.0% (3.5-9.0%) of HL] (P<0.001). Paralichthys isosceles tends to have a longer ocular-side pectoral fin [mean 16.0% (range 13.8-19.5%) of SL] than that of both P. patagonicus [12.8% (10.2-16.4%) of SL] (P<0.0001) and P. orbignyanus [11.0% (9.4-12.9%) of SL) (P<0.0001) (Fig. 2c).
Relationships of head length to standard length: a. interorbital width to head length, b. pectoral length to standard length, c. eye diameter to head length, d. caudal peduncle depth to standard length, e. among the following species: Paralichthys isosceles, P. orbignyanus and P. patagonicus.
Of the three species, P. orbignyanus has the shortest eye diameter [mean 12.0% (range 8.0-15.6%) of HL], while P. isosceles has the longest eye diameter [26.0% (23.0-29.0%) of HL] (P<0.001). Eye diameter values of P. patagonicus [mean 20.0% (16.0-25.0%) of HL] were intermediate between those of P. isosceles and P. orbignyanus (P<0.01) (Fig. 2d). Furthermore, P. orbignyanus tends to have a deeper caudal peduncle [mean 11.6% (range 10.0-14.0%) of SL] than either that of P. patagonicus [9.9% (7.9-12.3%) of SL] (P<0.0001) or P. isosceles [10.6% (9.0-12.2%) of SL] (P<0.0001) (Fig. 2e).
Meristic comparisons. Comparisons of meristic features of the three species revealed that P. isosceles has relatively higher counts of dorsal-and anal-fin rays [mode 84 (range 79-91) and mode 67 (63-73), respectively] than those of P. orbignyanus [mode 76 (68-82) and mode 55 (51-61), respectively] and P. patagonicus [mode 80 (71-91) and mode 63 (57-70), respectively] (Tabs. 1, 2). Paralichthys isosceles also has higher counts for total vertebrae (precaudal plus caudal) [mode 39 (38-39)] than those of P. orbignyanus [mode 35 (35-36)] and P. patagonicus [mode 38 (36-39) (Tab. 3). All three species have 10 precaudal vertebrae. Modes of counts for pectoral-fin-rays overlapped in the three species of Paralichthys, however the range of counts for each species was different (Tab. 4).
Frequency comparison for counts of total vertebrae in species of Paralichthys. * include types.
Frequency comparison for counts of pectoral-fin rays in Paralichthys species. *include types.
The number of gill-rakers is easily countable and constitutes an important taxonomic character in differentiating these three species. Paralichthys orbignyanus has higher counts for gill-rakers (16-24) compared with those of P. patagonicus [11-15 (rarely 16, 1 of 213 specimens)], and especially when compared with those for P. isosceles [8-11 (rarely 13, 1 of 58 specimens] (Tab. 5).
Paralichthys patagonicus has the highest numbers of both lateral-line scales and oblique rows of scales [mode 103 (range 93-112) and mode 71 (64-82), respectively] compared with those of P. orbignyanus with [mode 94 (90-107) and mode 67 (61-75), respectively], and especially when compared with those of P. isosceles, which is distinctive among all three species (Tab. 6) in having the fewest numbers of lateral-line scales [mode 75 (range 71-81)] and oblique rows of scales [mode 50 (47-56), respectively].
Frequency comparison for counts of lateral-line scales in species of Paralichthys. * include types.
Osteology. Postcranial axial skeleton. This section is divided into three parts: vertebral column, ribs and epineurals, and caudal skeleton.
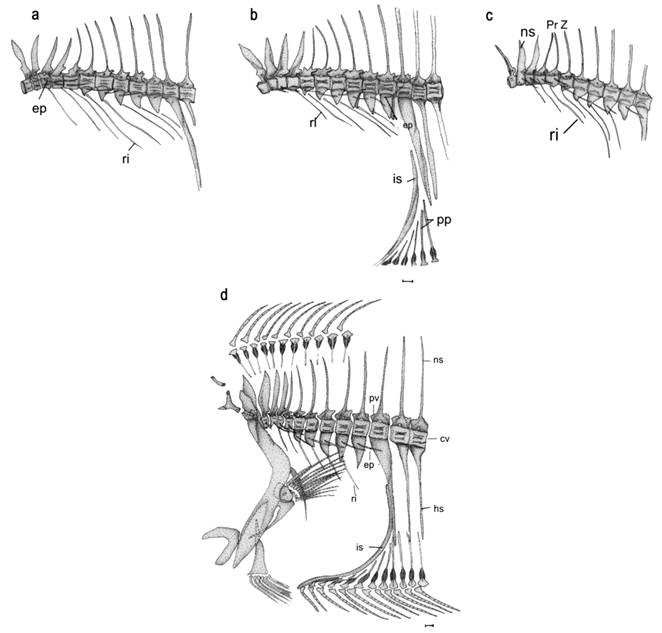
Vertebral column. The vertebral column in all three species comprises 10 precaudal vertebrae. These species differ in the ranges of caudal vertebrae: 28-29 in P. isosceles, 25-26 in P. orbignyanus and 27-29 (rarely 26, 1 of 177 specimens examined) caudal vertebrae in P. patagonicus.
In all three Paralichthys of the SW Atlantic examined here the first precaudal vertebra is small with its corresponding neural spine fused to the centrum, also the neural spines of the first to the fourth vertebrae are stout and compressed. Starting from the third or fourth vertebra, the neural spines narrow posteriorly toward the hypural complex and they become more directed posteriorly, with this deflection becoming most conspicuous on the caudal vertebrae. Haemal spines are only present on the caudal vertebrae. On each successive anterior caudal vertebra, the neural spine is more elongate than that of the respective corresponding neural spine of the previous precaudal centrum. The haemal spine of the first caudal vertebra (Fig. 3b) is a very strong plate, supporting the first anal-fin pterygiophore (Amaoka´ s interhemal spine or Woolcott´ s abdominal rod).
Skeleton of the precaudal vertebrae region of Paralichthys isosceles (a); P. orbignyanus (b and d); and P. patagonicus (c). See abbreviations in text. Scale bar indicates 1 mm.
All vertebral centra are amphicoelous, concave both anteriorly and posteriorly. In the three species of Paralichthys in our study, there are one or two small elliptical concavities present between the dorsal and ventral concave portions only on the last precaudal and the first caudal vertebrae. Beginning with the sixth or seventh and continuing on all subsequent precaudal vertebrae, paired parapophyses are present. On the sixth or seventh to the posteriormost precaudal vertebrae, the distal ends of the paired parapophyses unite, forming a closed haemal arch, which is referred to as haemapophyses by Amaoka (1969Amaoka K. Studies on the sinistral flounders found in the waters around Japan: taxonomy, anatomy and phylogeny. J Shimonoseki Univ Fish. 1969; 18(2):65-340.). These haemopophyses have a single non-bifurcated tip. Haemapophyses appear on the 7th to 10th precaudal vertebrae in P. patagonicus, on the 7th or 8th to 10th vertebrae in P. isosceles, and on the 6th or 7th to 10th vertebrae in P. orbignyanus. The preneural and postzygapophyses are well developed on all vertebrae of Paralichthys. On the precaudal vertebrae and on the first caudal vertebrae, the zygapophyses are tightly interlocked with each other, but in the posteriormost caudal vertebrae they are only slightly articulated, or are entirely free from each other. The prehaemal - and postzygapophyses are present only in P. orbignyanus, begining from the second caudal vertebra (Fig. 4). No haemal zygapophyses were found in the other two South Atlantic species of Paralichthys examined.
Ribs and epineurals. In the three southwest Atlantic species examined, the first, or the first two, ribs are attached directly to the anterolateral side of the centrum, while the other ribs attach to the distal ends of the haemapophyses (Fig. 3). Ribs are directed downward following the joint line of the myosepta with the wall of the coeloma. In contrast, epineurals [epipleural (dorsal) ribs sensu Cervigón, 1980Cervigón F. Ictiología Marina. Caracas: Editora Arte; 1980. vol. 1.] extend outward from the vertebral centra following the horizontal myoseptum (Cervigón, 1980Cervigón F. Ictiología Marina. Caracas: Editora Arte; 1980. vol. 1.).
Fourth caudal vertebrae of Paralichthys: a. P. orbignyanus, b. P. patagonicus. Arrow indicates haemal zygapophyses. Scale bar indicates 9 mm.
Of the three South Atlantic of interest to our study species, P. orbignyanus has the fewest ribs (6 pairs), P. patagonicus has the highest number of ribs (7-8 pairs), and P. isosceles has 7 pairs of ribs.
Epineurals occur on the 2nd to 10th precaudal vertebrae in the three species of Southwest Atlantic Paralichthys examined (Fig. 3). The first epineural bone is attached to the base of the neural prezygapophyses. The second epineural articulates slightly ventral to the prezygapophyses, and the third epineural is directly connected to the anterodorsal side of the vertebral centrum, where it shares the joint with the second epineural rib. The remaining epineurals are attached to the central portion of the parapophyses. Paralichthys patagonicus has the fewest number of epineurals (9 pairs), followed by that of P. isosceles [range (9-10); mean 9.6], whereas P. orbignyanus has the highest number [range (10-11); mean 10.3] of epineurals among the three species studied.
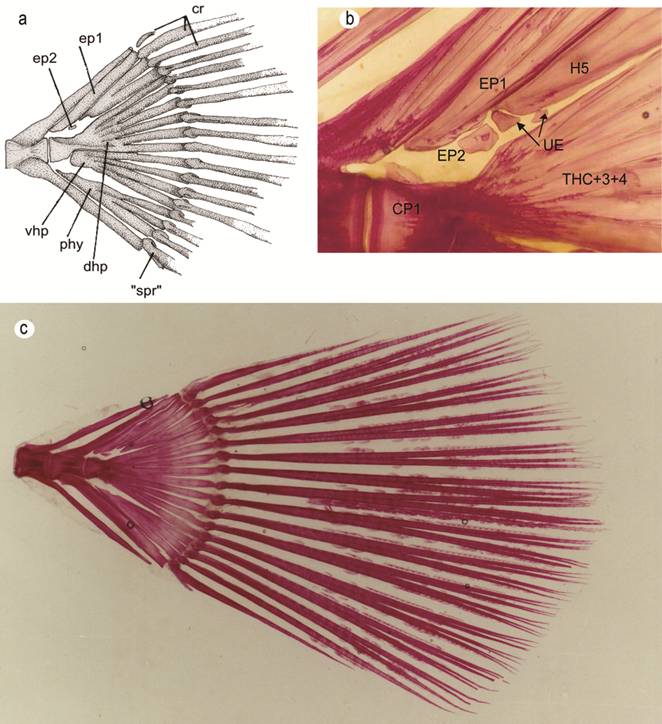
Caudal skeleton. All three species of Paralichthys examined in this paper have the hypural complex pattern 6 (Hensley, Ahlstrom, 1984Hensley DA, Ahlstrom EH. Pleuronectiformes: Relationships. In: Moser HG et al., editors. Ontogeny and Systematics of Fishes: Ahlstrom Symposium. California: American Society of Ichthyologists and Herpetologists; 1984. p.670-687. (Special publication of the American Society of Ichthyologists and Herpetologists; No. 1).) which is characterized by two preural centra (pc 1-2); a detached autogenous parhypural (phy); hypurals 1 and 2 fused together forming a broad ventral hypural plate (vhp) articulated with the posteroventral surface of the first preural centrum (cp1); hypurals 3 and 4 fused together and fused to the tip of first preural centrum forming a broad dorsal hypural plate (dhp); an autogenous fifth hypural (H5) with its proximal end closest to the second epural; and with this caudal complex supporting a total number of 18 caudal-fin rays, of which 13 central-fin rays are branched (Fig. 5a). In the present study, we found two autogenous epurals in all cleared and stained specimens of P. isosceles and P. patagonicus, and in only 5 of 14 specimens of P. orbignyanus. In one specimen of P. patagonicus, we also noticed two unidentified bony elements, situated adjacent to the two epurals (Fig. 5b). Some scissures are present on distal margins of the parhypural and hypural elements.
Caudal skeleton of a dissected specimen of P. isosceles (a). Cleared and stained specimens of Paralichthys patagonicus (b); and P. isosceles (c). Abrreviattions are: CP1: preural centrum 1; EP1-2: epurals 1 and 2; H1+2: ventral hypural plate; Phy: pahypural; Rca: caudal rays; “Sp.r”: “splinter ray”; H5: hypural 5; THC+3+4: dorsal hypural plate; UE: unidentified elements.
Of the 18 caudal-fin rays, two segmented fin rays occur in the upper and lower distal parts of the fin, 13 branched fin rays are present in the middle section of the fin, and one unsegmented fin ray, also called “procurrent ray” (sensu Hoshino, 2001Hoshino K. Homologies of the caudal fin rays of Pleuronectiformes (Teleostei). Ichthyol Res. 2001; 48(3):231-46.), is present at the upper and lower distal extremities of the fin. The uppermost fin ray is very small, covered by skin and musculature, and supported by the neural spine of preural centrum 2. The ventralmost fin ray is also very small and fused to its neighboring segmented ray (Fig. 5c).
Dorsal and anal fins. Each fin ray is supported basally by a bipartite structure consisting of a small distal pterygiophore and an elongate proximal pterygiophore. The two small lentiform halves of the distal pterygiophore are located between the two halves of lepidotrichia. The upper extremity of the proximal pterygiophore is cup-shaped and bears two lamellar projections laterally. The proximal pterygiophores interdigitate by sutures with the neural or hemal spines (Fig. 3d). The anteriormost eight proximal pterygiophores of the dorsal fin articulate with the dorsal part of the supraoccipital. The anteriormost of these pterygiophores supports the first two dorsal-fin rays. Generally, two pterygiophores interdigitate between neural spines, but sometimes three pterygiophores insert in an interneural space (Fig. 3d). The first anal-fin pterygiophore is modified into a large, curved rod that together with the first haemal spine closes the abdominal cavity posteriorly. This pterygiophore supports three fin rays in P. isosceles. The following 11 proximal pterygiophores rest on the posterior face of the abdominal rod in P. patagonicus. In P. orbignyanus, 8 or 9 proximal pterygiophores rest on the abdominal rod (Figs 3b,d).
Appendicular skeleton. Pectoral girdle. The pectoral girdle consists of the girdle itself (cleithrum, coracoid and scapula), four radials and a chain of bones that connect the girdle to the cranium (supracleithrum and two postcleithra). Some authors also include the posttemporal in this series (Balart, 1985Balart EF. Development of median and paired fin skeleton of Paralichthys olivaceus (Pleuronectiformes: Paralichthyidae). Jpn J Ichthyol. 1985; 31(4):398-410.; Collette, Gillis, 1992Collette BB, Gillis GB. Morphology, systematics and biology of the double-lined mackerels (Grammatorcynus, Scombridae). Fish Bull. 1992; 90(1):13-53.).
Cleithrum- The cleithrum is a half moon-shaped bone with both dorsal and lower limbs sharply pointed. The inferior limb bears a medial groove on its lateral surface and a well-developed ridge on the inner face that delimits a deep furrow where the sternohioideus muscle is inserted. The upper limb of the cleithrum bears a shallow lateral depression into which fits the overlapping supracleithrum. At the posterior angle of the cleithrum where the upper and lower limbs join together, a lateral process expands from this bone to overlap and support, partly by suture, the scapula and the body of the coracoid. This lateral process also contacts the anterior tip of the first postcleithrum. The elongate anterior projections of the basipterygia are included between the lower limbs of the two cleithra (Fig. 6). In P. patagonicus and P. orbignyanus, the lateral process of the trailing edge of the cleithrum is more expanded, and has a more irregular border compared with that of P. isosceles (Figs. 6b, c, d).
Inner view of left pectoral girdle of Paralichthys patagonicus (a). Left cleithra of Paralichthys patagonicus (b), P. orbignyanus (c), and P. isosceles (d). Left and right basipterygia in outer and inner views of Paralichthys patagonicus (e), P. orbignyanus (f), and P. isosceles (g). See abbreviations in text. Scale bar indicates 1 mm.
Scapula-The scapula is a flattened bony plate with a slender caudal process. Anteriorly, the lateral surface fits into a depression on the medial face of the cleithrum. Ventrally, it is connected to the coracoid by means of a cartilaginous strip. Two of the four radials are attached posteriorly to the scapula. The other two arise from the posterior border of the scapula and do not have a facetal articulation (Fig. 6a). No differences were found among the scapulae in the three South Atlantic species of Paralichthys, examined in this study.
Coracoid-The coracoids are elongate bones, compressed at their dorsal end. They are partially overlapped by the expanded inner lamina of the cleithra. Dorsally, they are separated slightly from the scapula. Right and left coracoids are asymmetric in the three South Atlantic species of Paralichthys, with the right bone being smaller than that of the left (ocular) side. No consistent differences were noticed among the coracoids in the specimens of Paralichthys examined.
Supracleithrum-This leaflike bone has its upper tip overlapped by the posttemporal to which it is joined by means of connective tissue. The lower part of the supracleithrum overlaps the anterior portion of the cleithrum (Fig. 6a). The maximum width of the supracleithrum varies from 15.0 to 23.0% of the total length of the bone in the three species. It is widest in P. isosceles (range 21.0-23.0%; mean 22.0%) and narrowest in P. orbignyanus (15.0-19.0%; 16.7%), with intermediate values found in P. patagonicus (16.0-18.0%; 17.2%).
Postcleithrum-Both upper and lower postcleithra are elongate, thin bones located posterior to the cleithrum where they are partially overlapped by the pectoral-fin rays (Fig. 6a). The upper (first) postcleithrum joins the inner face of the cleithrum. The bone is curved and elongate with its lower end pointed in P. isosceles. Both upper and lower ends are pointed, with lamellar expansions in P. patagonicus and P. orbignyanus.
Pelvic girdle. The pelvic girdle consists of paired basipterygia each associated with six pelvic-fin rays (the first and second pelvic-fin rays being simple, and the remainder on each side branched). Each basipterygium is composed of an anterior process shaped like an elongate shaft obliquely directed into a space that is hidden by the lower limbs of the cleithra; a wide basal plate that posteroventrally supports the pelvic-fin rays; a strong posterior process; and a spiny process directed forward and located on the inner face of the basipterygium (Fig. 6). Both basipterygia meet in an extended sutural joint. The posterior process of the basipterygium is sharp in P. isosceles, wider and blunt in P. patagonicus, and slightly curved upwards in P. orbignyanus.
Based on these findings, and for a comparison with all currently known species of Paralichthys occurring on both sides of America and the single species occurring in western Pacific Ocean, keys to the Atlantic and Pacific species of Paralichthys were created to assist in the identification of these species.
Key to species of Paralichthys of the Atlantic Ocean
1a. Ctenoid scales on one or both sides of the body .................... 2
1b. Cycloid scales on both sides of the body .................... 4
2a. Lateral-line scales 93 to 112. Gill rakers on lower limb of first gill arch 9 to 12. Two conspicuous black spots on the ocular side, one below the curve of the lateral line, the other on the lateral line just anterior to the caudal peduncle .................... Paralichthys patagonicus
2b. Lateral-line scales 55 to 81. Gill rakers on lower limb of first gill arch 7 to 10. Three conspicuous ocellated spots forming a triangle on the ocular side, two ocelli located on a horizontal line with one above and the other below the lateral line and both located near the dorsal and ventral body margins, the third ocellus situated on the lateral line anterior to the caudal peduncle .................... 3
3a. Ctenoid scales on both sides of body. Lateral-line scales 77 to 81. Distal margin of ocular-side pelvic fin with a small, oblong black spot .................... Paralichthys isosceles
3b. Ctenoid scales on ocular side of body, cycloid scales on blind side of body. Lateral-line scales 55 to 72. No spot on distal margin of pelvic fin of ocular side .................... Paralichthys triocellatus
4a. Three to five prominent ocelli on ocular side of body .................... 5
4b. No prominent ocelli on ocular side of body .................... 7
5a. Gill rakers on lower limb of first gill arch 15 or more. Dorsal-fin rays 84 to 95. Anal-fin rays 65 to 74. Lateral-line scales 95 to 110. Five prominent ocellated dark spots on posterior half of ocular side of body .................... Paralichthys dentatus
5b. Gill rakers on lower limb of first gill arch 8 to 12. Dorsal-fin rays 73 to 84. Anal-fin rays 58 to 65. Laleral-line scales 83 to 98. Three or four ocellated spots on ocular side of body .................... 6
6a. Eyes relatively large and close set, nearly meeting, separated only by a narrow, bony ridge. Four large ocelli each with a dark center encircled by a ring of lighter colour on ocular side of body, ocelli arranged in a trapezoid with 2 in midbody (one above the other on opposite sides of the lateral line) and 2 on the body (one above the other on opposite sides of the lateral line) at a point slightly anterior to caudal peduncle .................... Paralichthys oblongus
6b. Eyes separated by a flat space without a bony ridge. Three prominent ocellated dark spots on body arranged in a triangle with 2 (one above the other) in the midbody and 1 on the lateral line in the posterior part of the body .................... Paralichthys albigutta
7a. Gill rakers on lower limb of first gill arch 13 to 18. Interorbital width wide, 9.1 to 16.6 % of head length (HL). Eye diameter short, 8.0 to 15.6 % of HL. Pectoral-fin length short, 30.4 to 47.7% of HL .................... Paralichthys orbignyanus
7b. Gill rakers on lower limb of first gill arch 7 to 12. Interorbital width narrow, 5.6 to 14.5 % of head length (HL). Eye diameter long, 13.6 to 23.2 % of HL. Pectoral-fin length long, 41.7 to 61.3% of HL .................... 8
8a. Dorsal-fin rays 67 to 75. Anal-fin rays 51 to 56. Caudal-peduncle depth 12.5 to 14.3 % of standard length (SL) .................... Paralichthys brasiliensis
8b. Dorsal-fin rays 74 to 91. Anal-fin rays 58 to 72. Caudal-peduncle depth 9.2 to 11.7 % of standard length (SL) .................... 9
9a. Body depth greater than 47% (47.6 to 52.5%) of SL. Blind side of larger specimens dusky. Lateral-line scales 110 to 119 .................... Paralichthys squamilentus
9b. Body depth 47% or less of standard length, (usually 42.2 to 45.5 %). Blind side immaculate or dusky. Lateral-line scales 81 to 99 .................... 10
10a. Dorsal-fin rays 74 to 78. Anal-fin rays 58 to 59. Gill rakers on lower limb of first arch 11 to 12 .................... Paralichthys tropicus
10b. Dorsal-fin rays 76 to 91. Anal-fin rays 62 to 72. Gill rakers on lower limb of first arch 9 to 11 .................... Paralichthys lethostigma
Key to species of Paralichthys of the Pacific Ocean
1a- Cycloid scales on both sides of body .................... 2
1b- Ctenoid scales on ocular side of body .................... 3
2a. Gill rakers on lower limb of first gill arch 10 to 14. Anal-fin rays 55 to 58 .................... Paralichthys woolmani
2b. Gill rakers on lower limb of first gill arch 18 to 21. Anal-fin rays 59 to 64 .................... Paralichthys aestuarius
3a. Gill rakers on lower limb of first gill arch 9 to 10 .................... Paralichthys fernandezianus
3b. Gill rakers on lower limb of first gill arch 14 to 23 .................... 4
4a. Three distinct ocelli on ocular side of body; two (one above and one below) the beginning of the straight part of the lateral line. A third ocellus on the straight part of the lateral line just anterior to the caudal peduncle .................... Paralichthys adspersus
4b. No disctinct ocelli on body, but some dark blotches may be present .................... 5
5a. Dorsal-fin rays 59 to 64. Anal-fin rays 47 to 51. Gill rakers on lower limb of first gill arch 14 to 16. Several dark blotches on ocular side of body .................... Paralichthys delfini
5b. Dorsal-fin rays 67 to 85. Anal-fin rays 51 to 66. Gill rakers on lower limb of first gill arch 14 to 23. Ocular side of body mottled or spotted with paler and darker spots or blotches .................... 6
6a. Lateral-line scales 80 to 90. Dorsal-fin rays 71 to 80. Anal-fin rays 58 to 66. Gill rakers on lower limb of first arch 18 to 23 .................... Paralichthys microps
6b. Lateral-line scales 101 to 126. Dorsal-fin rays 67 to 84. Anal-fin rays 51 to 65. Gill rakers on lower limb of first arch 14 to 22 .................... 7
7a. Gill rakers on lower limb of first arch 14 to 16. Dorsal-fin rays 77 to 81. Lateral-line scales 119 to 126 .................... Paralichthys olivaceus
7b. Gill rakers on lower limb of first arch 18 to 22. Dorsal-fin rays 67 to 76. Lateral-line scales 101 to 110 .................... Paralichthys californicus
Material examined.Paralichthys isosceles: Brazil: Bahia: USNM 43335, 1, 211 mm SL, Paralectotype of Paralichthys isosceles Jordan, 1891. USNM 43371, 1, 203 mm SL, Lectotype of Paralichthys isosceles Jordan, 1891. USNM 43368, 1, 204 mm SL. Argentina: Chubut: INIDEP 145, 8, 170-290 mm SL. INIDEP 146, 1, 276 mm SL. MACN 2520, 1, 106 mm SL. MACN 6312, 3, 87-198 mm SL. MACN 6468, 1, 185 mm SL. Rio Grande do Sul: MZUSP 72370, 2, 149-171 mm SL. MZUSP 72379, 2, 117-120 mm SL. Santa Catarina: MZUSP 72332, 1, 177 mm SL. MZUSP 72377, 1, 110 mm SL. Paralichthys orbignyanus: Argentina: Buenos Aires: MNHN 1999-0295, 1, 345 mm SL, holotype of Paralichthys orbignyanus Valenciennes, 1839. USNM 77389, 1, 140 mm SL. USNM 77389, 1, 140 mm SL. Mar del Plata: MACN 793, 1, 185 mm SL. Quequén: MACN 2802, 4, 30-293 mm SL. MACN 2804, 3, 43-53 mm SL. MACN 6703, 1, 221 mm SL. Mar Chiquita coastal lagoon: INIDEP 441, 2, 107-160 mm SL. Rio Negro: NMW 42781, 2, 153-160 mm SL. San Antonio Bay: NMW 42786, 1, 296 mm SL. San Antonio Oeste: MACN 7547, 5, 80-281 mm SL. San Blas Bay: MACN 4210, 2, 182-194 mm SL. San Clemente del Tuyú: MACN 6296, 1, 153 mm SL. MACN 6170, 2, 271-275 mm SL. Brazil: Rio de Janeiro: NMW 42777, 3, 205-265 mm SL. Rio de Janeiro: NMW 42780, 2, 118-154 mm SL.Rio de Janeiro: MNHN 1975-0390, 1, 292 mm SL. Rio Grande: MCZ 11509, 1, 240 mm SL. Rio Grande do Sul: MNRJ 14360, 2, 173-178 mm SL. Rio Grande do Sul: MCZ 11125, 2, 191-400 mm SL. Rio Grande do Sul: MZUSP 14360, 2, 173-178 mm SL.Rio Grande do Sul: MCZ 11406, 1, 233 mm SL. Santos: MCZ 11406, 8, 133-204 mm SL. Santos: MCZ 11410, 8, 133-233 mm SL. Rio de Janeiro: MNRJ 5618, 1, 174 mm SL. Brazil: Rio de Janeiro: ZMB 5808, 1, 195 mm SL. Rio de Janeiro: BMNH 1993.3.31.24, 1, 207 mm SL. Rio de Janeiro: MNRJ 8789, 1, 203 mm SL. Rio de Janeiro: MZUSP 27883, 1, 136 mm SL. Uruguay: INIDEP 445, 1, 400 mm SL. Montevideo: MACN 1925, 1 ,182 mm SL. MACN 6780, 1, 281 mm SL. USNM 77388, 10, 126-174 mm SL. USNM 77388, 6, 144-213 mm SL. USNM 86733, 1, 212 mm SL. USNM 86733, 1, 212 mm SL. Rio de Janeiro: USNM 83404, 1, 161 mm SL, holotype of Xystreurys riberoi Fowler and Bean, 1923. Rio de Janeiro: USNM 83399, 1, 123 mm SL, paratype of Xystreurys riberoi Fowler and Bean, 1923. Paralichthys patagonicus: Argentina: San Antonio Oeste: MCZ 11399, 1, 132 mm SL, lectotype of Paralichthys patagonicus Jordan, 1889. MCZ 135300, 2, 135-156 mm SL, paralectotypes of Paralichthys patagonicus Jordan, 1889. Quequén: MACN 2804, 2, 53-63 mm SL. Mar del Plata: INIDEP 71, 5, 224-268 mm SL. Patagonia: FAKU AP 315, 1, 426 mm SL. Patagonia: FAKU AP 825, 1, 348 mm SL. Patagonia: FAKU 43051, 1, 368 mm SL. Rawson: MACN 6467, 1, 127 mm SL. San Antonio Este: INIDEP 153, 1, 104 mm SL. San José Gulf: INIDEP 104, 2, 108-120 mm SL. San Matías Gulf: MACN 3490, 1, 260 mm SL. San Matías Gulf: INIDEP 158, 1, 107 mm SL. Brazil: Rio de Janeiro: MNRJ 3022, 2, 354-445 mm SL, types of Paralichthys bicyclophorus Miranda Ribeiro, 1915. Brazil: MNHN 1975-0287, 1, 160mm SL. MNHN 1975-0288, 1, 164 mm SL. MNHN 1975-0289, 1, 69 mm SL. MNHN 1975-0290, 1, 262 mm SL. MNHN 1989-0481, 1, 176 mm SL. MNHN 1989-0482, 1, 153 mm SL. MNRJ 9138, 1, 180 mm SL. Rio Grande do Sul: MZUSP 72357, 1, 126 mm SL. MZUSP 72436, 1, 141 mm SL. Rio de Janeiro: MNRJ 2093, 1, 278 mm SL. MZUSP 72444, 1, 210 mm SL. Santos: MZUSP 72429, 2, 158-185 mm SL. Santa Catarina: MZUSP 52483, 1, 122 mm SL. MZUSP 72432, 1, 115 mm SL. MZUSP 72440, 1, 123 mm SL. Uruguay: USNM 87778, 1, 337 mm SL.
Discussion
Comparisons with congeneric species.Paralichthys orbignyanus differs from P. dentatus, P. triocellatus and P. lethostigma in having fewer dorsal-and anal-fin rays, and vertebrae. Also, this species has fewer vertebrae compared with those of P. oblongus, P. olivaceus, P. squamilentus and P. albigutta (see Tabs 1-3). Although P. isosceles, P. orbignyanus and P. patagonicus have 10 precaudal vertebrae, other species of Paralichthys, such as P. olivaceus (Amaoka, 1969Amaoka K. Studies on the sinistral flounders found in the waters around Japan: taxonomy, anatomy and phylogeny. J Shimonoseki Univ Fish. 1969; 18(2):65-340., USNM 71996) and P. dentatus (Woolcott et al., 1968Woolcott WS, Beirne C, Hall WM Jr. Descriptive and comparative osteology of the young of three species of flounders, genus Paralichthys. Chesapeake Sci. 1968; 9(2):109-20., USNM 187286) have 11 precaudal vertebrae, whereas P. oblongus (USNM 286112) has 12 precaudal vertebrae. Paralichthys orbignyanus differs from P. brasiliensis, P. triocellatus, P. albigutta and P. tropicus in having a greater number of total gill-rakers and more lateral-line scales (Tabs 5, 6). Paralichthys orbignyanus also has a smaller eye and a shorter pectoral fin compared with those of its congeners (Fig. 7a, b). Additionally, P. orbignyanus has a wider interorbital width compared to those of other species of Paralichthys (Fig. 8).
Relationships of eye diameter to standard length (a); and pectoral-fin length to standard length (b); in Paralichthys adspersus, P. aestuarius, P. albigutta, P. brasiliensis, P. dentatus, P. microps, P. oblongus, P. orbignyanus, P. triocellatus and P. woolmani.
Relationships of interorbital width to head length in Paralichthys californicus, P. dentatus, P. lethostigma, P. oblongus, P. orbignyanus, P. squamilentus, P. triocellatus, P. tropicus and P. woolmani.
Among species of Paralichthys, P. isosceles shares two features in common with both P. triocellatus and P. oblongus: one is the absence of accessory scales and the other is the fewest gill-rakers. Paralichthys isosceles differs from P. triocellatus in having more lateral-line scales (mean 75.9 vs.65.2) and pectoral-fin rays (11.2 vs. 10.3). Paralichthys isosceles and P. triocellatus are very similar in external appearance in that both species have three conspicuous ocelli, and most of their meristic features, except those mentioned above, overlap (Bittencourt, 1982Bittencourt MM. Estudo comparativo de aspectos da distribuiçao, morfologia e biologia de Paralichthys isosceles (Jordan, 1890) e Paralichthys triocellatus (Ribeiro, 1904) (Pleuronectiformes: Bothidae) da regiao da plataforma continental comprendida entre Cabo Frio e Torres (23°S-29°21’S). [Msc Dissertation]. São Paulo, SP: Instituto Oceanográfico da Universidade de São Paulo; 1982.). However, these species are easily differentiated by the type of scales present on their blind sides (ctenoid in P. isosceles and cycloid in P. triocellatus), and also by the presence of a black spot on the ocular-side pelvic fin of P. isosceles which is absent in P. triocellatus (Figueiredo, Menezes, 2000Figueiredo JL, Menezes NA. Manual de Peixes Marihnos do sudeste do Brasil. São Paulo: Museu de Zoologia da Universidade de São Paulo; 2000. vol 6, Telostei (5).; Pers. obs.). Paralichthys isosceles differs from P. oblongus in having a greater number of dorsal-and anal-fin rays (mean 84.3 vs. 77.4; and mean 67.0 vs. 62.4, respectively), and it has fewer pectoral-fin rays, vertebrae and lateral-line scales (mean 11.2 vs. 11.7, 38.6 vs. 42.0 and 75.9 vs. 91.2, respectively). Also, P. isosceles has 10 precaudal vertebrae, while P. oblongus has either 11 or 12. Paralichthys isosceles differs from the other species of Paralichthys, except P. oblongus and P. triocellatus, in having a greater eye diameter and a longer ocular-side pectoral fin (Fig. 9a, b).
Relationships of eye diameter to standard length (a); and prepectoral-fin length to standard length (b); in Paralichthys adspersus, P. aestuarius, P. albigutta, P. brasiliensis, P. californicus, P. dentatus, P. isosceles, P. lethostigma, P. microps, P. olivaceus, P. squamilentus, P. tropicus and P. woolmani, P. californicus.
Paralichthys patagonicus differs from P. brasiliensis in having greater numbers of lateral-line scales, vertebrae and dorsal and anal-fin rays (mean 102.8 vs. 84.2; mean 38.0 vs. 35.0; mean 81.0 vs. 71.0 and mean 63.0 vs. 53.8, respectively). Paralichthys patagonicus has lower gill-raker counts than those of P. adspersus, P. aestuarius, P. dentatus, P. californicus, P. microps and P. olivaceus (mean 13.1 vs. 23.8; 27.5; 20.2; 29.2; 29.2 and 20.2, respectively). Also, it has fewer vertebrae than that found in P. dentatus and P. oblongus (mean 38.0 vs. 41.7 and 42.0, respectively). Paralichthys patagonicus also has a narrower interorbital width than that of P. adspersus, P. albigutta, P. brasiliensis, P. dentatus, P. microps and P. olivaceus (Fig. 10a), but this measurement is wider than that found in P. oblongus and P. triocellatus (Fig. 10b).
Relationships of interorbital width to standard length in Paralichthys adspersus, P. albigutta, P. brasiliensis, P. microps, P. olivaceus and P. patagonicus (a). Relationships of interorbital width to standard length (b) in Paralichthys patagonicus, P. oblongus and P. triocellatus.
Osteology. In the three southwest Atlantic species examined in the present paper, the first neural spine is fused to the centrum. In other Pleuronectiformes, the first neural spine is autogenous (Cervigón, 1985Cervigón F. Las especies de los géneros Achirus y Trinectes (Pisces: Soleidae) de las costas de Venezuela (Osteología, Musculatura y ligamentos fasciales, y Sistemática). Caracas: Fundación Científica Los Roques, Monografía; 1985.) or is lacking altogether (Amaoka, 1969Amaoka K. Studies on the sinistral flounders found in the waters around Japan: taxonomy, anatomy and phylogeny. J Shimonoseki Univ Fish. 1969; 18(2):65-340.). Although Amaoka (1969Amaoka K. Studies on the sinistral flounders found in the waters around Japan: taxonomy, anatomy and phylogeny. J Shimonoseki Univ Fish. 1969; 18(2):65-340.) reported that plate-like neural spines occurred on the first to the third vertebrae in Pseudorhombus, versus on the first to the fourth vertebrae in Paralichthys, we found that broad plate-like neural spines occurred only on the first three vertebrae in P. isosceles, P. triocellatus and P. oblongus. Conversely, all other species of Paralichthys, including P. patagonicus and P. orbignyanus, have broad plate-like neural spines on their first four vertebrae (Fig. 3).
Haemapophyses appear on the 7th to 10th precaudal vertebrae in P. patagonicus, on the 7th or 8th to 10th vertebrae in P. isosceles, and on the 6th or 7th to 10th vertebrae in P. orbignyanus. In comparison, Woolcott et al. (1968Woolcott WS, Beirne C, Hall WM Jr. Descriptive and comparative osteology of the young of three species of flounders, genus Paralichthys. Chesapeake Sci. 1968; 9(2):109-20.) reported that in P. albigutta, P. lethostigma and P. dentatus the haemal arch was completely formed beginning with the 7th precaudal vertebra. Ribs are attached directly to the anterolateral side of the centrum, while the other ribs attach to the distal ends of the haemapophyses. Ribs are present in all members of Paralichthyidae, but they purportedly are not present in species of the family Bothidae (Amaoka, 1969Amaoka K. Studies on the sinistral flounders found in the waters around Japan: taxonomy, anatomy and phylogeny. J Shimonoseki Univ Fish. 1969; 18(2):65-340.). However, Hensley, Ahlstrom (1984Hensley DA, Ahlstrom EH. Pleuronectiformes: Relationships. In: Moser HG et al., editors. Ontogeny and Systematics of Fishes: Ahlstrom Symposium. California: American Society of Ichthyologists and Herpetologists; 1984. p.670-687. (Special publication of the American Society of Ichthyologists and Herpetologists; No. 1).) concluded that those elements termed “abdominal hypomerals” by Amaoka (1969Amaoka K. Studies on the sinistral flounders found in the waters around Japan: taxonomy, anatomy and phylogeny. J Shimonoseki Univ Fish. 1969; 18(2):65-340.) are actually pleural ribs (Patterson, Johnson, 1995Patterson C, Johnson GD. The intermuscular bones and ligaments of teleostean fishes. Washington, (DC): Smithsonian Institution Press; 1995. (Smithsonian Contributions to Zoology; No. 559).). Chanet et al. (2004Chanet B, Chapleau F, Desoutter M. Os et ligaments intermusculaires chez les poisson plats (Teleostei: Pleuronectiformes): interprétations phylogénétiques. Cybium. 2004; 28 (S1):9-14.) presented a table of the number of the anteriormost ribs occurring in members of different flatfish Families, including those of the Paralichthyidae and Bothidae. Amaoka (1969Amaoka K. Studies on the sinistral flounders found in the waters around Japan: taxonomy, anatomy and phylogeny. J Shimonoseki Univ Fish. 1969; 18(2):65-340.) noted the presence of ribs on the 3rd to 10th precaudal vertebrae in Paralichthys, however, we found differences and more variation from this arrangement among species of Paralichthys we examined. For example, we found ribs occurring only on the 3rd to 9th precaudal vertebrae in P. isosceles, and to the 10th vertebra (one specimen) in P. patagonicus, and on the 4th to 9th vertebrae (one case beginning on the 3rd vertebra) in P. orbignyanus.
The caudal fin and caudal skeleton of species of Paralichthys have been illustrated and discussed by several authors (Woolcott et al., 1968Woolcott WS, Beirne C, Hall WM Jr. Descriptive and comparative osteology of the young of three species of flounders, genus Paralichthys. Chesapeake Sci. 1968; 9(2):109-20.; Amaoka, 1969Amaoka K. Studies on the sinistral flounders found in the waters around Japan: taxonomy, anatomy and phylogeny. J Shimonoseki Univ Fish. 1969; 18(2):65-340.; Hensley, Ahlstrom, 1984Hensley DA, Ahlstrom EH. Pleuronectiformes: Relationships. In: Moser HG et al., editors. Ontogeny and Systematics of Fishes: Ahlstrom Symposium. California: American Society of Ichthyologists and Herpetologists; 1984. p.670-687. (Special publication of the American Society of Ichthyologists and Herpetologists; No. 1).; Balart, 1985Balart EF. Development of median and paired fin skeleton of Paralichthys olivaceus (Pleuronectiformes: Paralichthyidae). Jpn J Ichthyol. 1985; 31(4):398-410.; Díaz de Astarloa, 1991Díaz de Astarloa JM. Estudios osteológicos del sincráneo y complejo caudal en dos formas nominales de Paralichthys: Paralichthys patagonicus y Paralichthys bicyclophorus. Frente Marítimo . 1991; 9:15-27.; Hoshino, 2001Hoshino K. Homologies of the caudal fin rays of Pleuronectiformes (Teleostei). Ichthyol Res. 2001; 48(3):231-46.). The hypural complex of the Pleuronectiformes corresponds to the stegural acentral V-b2 type of Monod (1968Monod T. Le complexe urophore des poissons Téléostéens. Bull Inst Fondam Afr Noire, Ser A. 1968; 81:1-705.). However, and according to several patterns of fusions among hypurals 1-4, Hensley, Ahlstrom (1984) situate Paralichthys in hypural pattern 6. Hoshino (2001Hoshino K. Homologies of the caudal fin rays of Pleuronectiformes (Teleostei). Ichthyol Res. 2001; 48(3):231-46.), in contrast, regards the small spur on the ventralmost surface near the base of the first ventral caudal-fin ray, called the “splinter ray” by Hensley, Ahlstrom (1984), as an unsegmented ray fused to a segmented ray in the ventral part of the caudal fin (Fig. 3).
Balart (1985Balart EF. Development of median and paired fin skeleton of Paralichthys olivaceus (Pleuronectiformes: Paralichthyidae). Jpn J Ichthyol. 1985; 31(4):398-410.) mentioned the presence of two epurals of contrasting size in P. olivaceus, with the anteriormost epural elongate whilst the posterior epural is very reduced in size compared to that of its counterpart. Woolcott et al. (1968Woolcott WS, Beirne C, Hall WM Jr. Descriptive and comparative osteology of the young of three species of flounders, genus Paralichthys. Chesapeake Sci. 1968; 9(2):109-20.) also described two autogenous epurals in species of Paralichthys they examined, but they erroneously pointed out hypurals 3, 4 and 5, and overlooked in their illustration the correct two epurals (illustrated in their Figs. 3, 5). Díaz de Astarloa (1991Díaz de Astarloa JM. Estudios osteológicos del sincráneo y complejo caudal en dos formas nominales de Paralichthys: Paralichthys patagonicus y Paralichthys bicyclophorus. Frente Marítimo . 1991; 9:15-27.) found only one autogenous epural in two nominal species of Paralichthys he examined.
Remarks. On the generic assignment of Paralichthys isosceles, P. triocellatus and P. oblongus. Paralichthys isosceles was originally described as a member of Paralichthys. Later, this species was transferred to Pseudorhombus by Ginsburg (1952Ginsburg I. Flounders of the genus Paralichthys and related genera in American waters. Fish Bull . 1952; 52(71):267-351.) based primarily on the absence of accessory scales. Accessory scales are present in all other species currently assigned to Paralichthys, except P. triocellatus and P. oblongus (assigned to Hippoglossina by Ginsburg) which also lack accesory scales. Ginsburg stated that P. triocellatus is a species of doubtful relationship, and according to its diagnostic characteristics should be close to “Pseudorhombus” isosceles. Ginsburg (1952Ginsburg I. Flounders of the genus Paralichthys and related genera in American waters. Fish Bull . 1952; 52(71):267-351.) did not examine the Holotype of P. triocellatus. Here, we examined the type of Miranda Ribeiro´s P. triocellatus and conclude that it is similar to, but can be clearly distinguished from, P. isosceles in having cycloid scales on the blind side and by other diagnostic features that were discussed above. Although P. isosceles and P. triocellatus may be closely related as hypothesized by Ginsburg (1952Ginsburg I. Flounders of the genus Paralichthys and related genera in American waters. Fish Bull . 1952; 52(71):267-351.), they clearly do not belong to Pseudorhombus since these two species have 18 caudal-fin rays including a splinter ray on the ventralmost caudal-fin ray (vs. 17 caudal-fin rays and no splinter ray in Pseudorhombus). This splinter ray is likely a remnant of a ray lost through fusion with an adjacent ray (Hensley, Ahlstrom, 1984Hensley DA, Ahlstrom EH. Pleuronectiformes: Relationships. In: Moser HG et al., editors. Ontogeny and Systematics of Fishes: Ahlstrom Symposium. California: American Society of Ichthyologists and Herpetologists; 1984. p.670-687. (Special publication of the American Society of Ichthyologists and Herpetologists; No. 1).) and this structure was observed in all species examined in the present paper that we consider belonging to Paralichthys.
Regarding P. oblongus, the generic placement of this species is problematic because it shares some features in common with those of members of both Paralichthys and Hippoglossina. During its history, this species has been placed in several different genera, and together with Hippoglossina tetrophthalma, it was even placed in a separate subgenus within Hippoglossina (see synonymy in Ginsburg, 1952Ginsburg I. Flounders of the genus Paralichthys and related genera in American waters. Fish Bull . 1952; 52(71):267-351.). Some disagreement occurs within contemporary works (Munroe, 2003Munroe TA. Family Paralichthyidae. In: Carpenter KE, editor. The living marine resources of the Western Central Atlantic. Rome: FAO ; 2003. p.1898-1921. vol 3, Bony fishes part 2 (Opistognathidae to Molidae), sea turtles and marine mammals. (FAO species identification guide for fishery purposes and American Society of Ichthyologists and Herpetologists Special Publication; No. 5).) regarding the correct generic assignment for this species. Paralichthys oblongus differs from members of Hippoglossina in placement of the origin of the dorsal fin, which in these species is well behind the posterior nostril of blind side and over the posterior part of the pupil. In P. oblongus, the dorsal fin commences just behind of the blind-side posterior nostril and the origin of the dorsal fin is located above the anterior margin of the upper eye. Additionally, members of Hippoglossina have 17 caudal-fin rays and they lack a splinter ray in the caudal fin (vs. 18 caudal-fin rays and splinter ray present in P. oblongus).
Most contemporary works assign Paralichthys isosceles, P. triocellatus and P. oblongus to Paralichthys. However, several external and internal morphological features of these species differ from those found in other species currently assigned to Paralichthys. According to Amaoka (1969Amaoka K. Studies on the sinistral flounders found in the waters around Japan: taxonomy, anatomy and phylogeny. J Shimonoseki Univ Fish. 1969; 18(2):65-340.), broad plate-like neural spines occur on the first four vertebrae in Paralichthys, whereas in P. isosceles, P. oblongus and P. triocellatus, these broad-shaped neural spines are found only on the first to the third vertebrae, as is also observed in members of Pseudorhombus. This feature, along with the lack of accessory scales in these species, reinforces the hypothesis that these three nominal species currently assigned to Paralichthys should be reassigned to another genus or to other genera, and likely not Pseudorhombus or Hippoglossina, especially as currently defined. However, before a more definitive answer can be provided about the generic placement of these species, more investigation is needed to provide strong support for hypotheses regarding the relationships of these species.
Comparative material examined. Paralichthys adspersus: Perú: Chinchas islands: NMW 42743, 1, 246 mm SL, holotype of Paralichthys adspersus Steindachner, 1867.Chile: MNHN 2014-0125, 1, 200 mm SL. MNHN 2014-0126, 1, 197 mm SL. Antofagasta: MNHN 1907-0343, 1, 320 mm SL. MNHN 1907-0344, 1, 218 mm SL. MNHN 1907-0345, 1, 231 mm SL. MNHN 1907-0346, 1, 210 mm SL. Coquimbo: ZMB 16282, 1, 255 mm SL. Iquique: ZMB 9505, 1, 157 mm SL. ZMB 16281, 1, 315 mm SL. Lota: BMNH 1930.9.2.1, 1, 79 mm SL. BMNH 1935.8.29.23, 1, 84 mm SL. Talcahuano: NMW 9540, 1, 275 mm SL. NMW 9541, 1, 228 mm SL. NMW 9542, 1, 227 mm SL. México: Mazatlán: NMW 42740, 1, 292 mm SL. Perú: Callao: NMW 42788, 1, 215 mm SL. Boca del Rio: MNHN 2001-0510, 1, 143 mm SL. La Lagunilla: USNM 128153, 2, 154- 167 mm SL. Paralichthys aestuarius: Mexico: Sonora: USNM 48128, 2, 158-176 mm SL, types of Paralichthys aestuarius Gilbert and Scofield, 1898. BMNH 1897.1.12.61, 1, 157 mm SL, paratype of Paralichthys aestuarius Gilbert and Scofield, 1898. Paralichthys albigutta: Central America: NMW 42784, 1, 285 mm SL. U.S.A: Florida: NMW 87251, 1, 130 mm SL. BMNH 1933.2.18.1, 1, 118 mm SL. USNM 4887, 1, 123 mm SL, syntype of Paralichthys albigutta Jordan and Gilbert, 1882. USNM 30818, 1, 162 mm SL, syntype of Paralichthys albigutta Jordan and Gilbert, 1882. Texas: BMNH 1948.8.6.1224-1229, 4, 139-170 mm SL. Paralichthys brasiliensis: Brazil: MZUB 955, 1, 325 mm TL, holotype of Paralichthys brasiliensis Ranzani, 1842. NMW 43928, 1, 214 mm SL. ZMB 8216, 1, 310 mm SL Bahia: MNHN 1999-0437, 1, 273 mm SL. MNHN 1999-1930, 2, 233-369 mm SL. MNRJ 1932, 1, 232 mm SL. MCZ 4669, 1, 173 mm SL. MCZ 11404, 1, 218 mm SL. Cajutuba: NMW 42775, 2, 370-371 mm SL. Espírito Santo: MCZ 11407, 2, 80-85 mm SL. MCZ 11408, 1, 342 mm SL. Ilha de São Luis: MZUSP 72303, 2, 112-122 mm SL. Recife:.USNM 104260, 1, 85 mm SL. Rio Grande do Sul: BMNH 1848-12-21-63, 1, 333 mm SL. BMNH 1885.2.3.73-74, 1, 270 mm SL. Rio de Janeiro: FAKU 37967, 1, 210 mm SL. NMW 85572, 2, 295-313 mm SL. MZUSP 72304, 1, 102 mm SL. MCZ 11409, 1, 248 mm SL. Pará: MCZ 11403, 1, 174 mm SL. Paralichthys californicus: U.S.A: California: BMNH 1881.3.14.26, 1, 176 mm SL, BMNH 1923.2.26.561-565, 5, 90-241 mm SL. MNHN A 3297, 2, 115-139 mm SL. NMW 43926, 2, 95-116 mm SL. NMW 43929, 1, 358 mm SL. USNM 64039, 2, 130-151 mm SL. ZMB 7777, 1, 181 mm SL. ZMB 11521, 2, 124-181 mm SL. San Francisco: NMW 12483, 1, 245 mm SL. Colombia: Magdalena: MNHN 1896-0151, 1, 226 mm SL. Pacific Ocean, Albatros Expedition: NMW 86113, 1, 393 mm SL. Locality unkown: MNHN A-8770, 1, 848 mm SL, dry specimen. Paralichthys dentatus: Antilles: Martinique: MNHN 1999-0426, 3, 116-207 mm SL. Cuba: Antilles: MNHN 1999-0459, 1, 132 mm SL. USA: Connecticut: ZMB 10224, 1, 335 mm SL. Delaware: USNM 187286, 1, 134 mm SL. Massachussetts: NMW 93988, 1, (mounted skeleton). New York: MNHN A 9964, 3, 243-287 mm SL. MNHN 1999-0458, 1, 121 mm SL. MNHN 1999-0463, 1, 228 mm SL. MNHN 1999-0461, 4, 116-284 mm SL. MNHN 1999-0469, 1, 134 mm SL. North Carolina: NMW 43925, 1, 185 mm SL. South Carolina: BMNH 1933.8.10.6-9, 4, 91-114 mm SL. MNHN A 5186, 1, 150 mm SL. MNHN 1999-0464, 1, 196 mm SL. MNHN 1999-0999, 1, 174 mm SL. USNM 127184, 2, 151-182 mm SL.Virginia: BMNH 1930.9.4.4-5, 1, 104 mm SL. MNHN 1999-0462, 1, 191 mm SL. Locality unknown: MNHN 1999-0648, 3, 217-227 mm SL. Paralichthys fernandezianus: Chile: Juan Fernández: ZMB 16279, 1, 426 mm SL, holotype of Paralichthys fernandezianus Steindachner, 1903. ZMB 16280, 1, 206 mm SL, holotype of Paralichthys hilgendorfii Steindachner, 1903. USNM 88831, 1, 374 mm SL, holotype of Paralichthys schmitti Ginsburg, 1933. Paralichthys lethostigma: USA: New York: MNHN 1999-0458, 2, 98-151 mm SL. MNHN 2006-1795, 1, 116 mm SL. North Carolina: NMW 43925, 1, 165 mm SL. South Carolina: MNHN 1999-0999, 1, 242 mm SL. Texas: BMNH 1948.8.6.1473-1477, 5, 157-216 mm SL. Paralichthys microps: Chile: BMNH 1880.7.28.6, 1, 86 mm SL, holotype of Paralichthys microp Günther, 1881. BMNH 1935.4.23.63-66, 3, 188-204 mm SL. ZMB 2376, 1, 171 mm SL. Bío-Bío: BMNH 1936.8.26.1211-1220, 3, 124-150 mm SL. Chilean Patagonia: FAKU 117415, 1, 261 mm SL. FAKU 117417, 1, 247 mm SL. FAKU 117418, 1, 262 mm SL. FAKU 117696, 1, 208 mm SL. FAKU 117702, 1, 257 mm SL. FAKU 124797, 1, 276 mm SL. FAKU 124804, 1, 265 mm SL. FAKU 125016, 1, 238 mm SL. Port Montt: ZMB 15697, 3, 180-237 mm SL. Locality unkown: FAKU CP 189, 1, 257 mm SL. Locality unkown: FAKU CP 622, 1, 283 mm SL. Locality unkown: FAKU CP 624, 1, 290 mm SL. Locality unkown: FAKU CP 628, 1, 224 mm SL. Locality unkown: FAKU 110474, 1, 309 mm SL. Locality unkown: FAKU 111265, 1, 195 mm SL. Locality unkown: FAKU 111267, 1, 249 mm SL. Paralichthys oblongus: USA: USNM 302461, 3, 196-221 mm SL. Connecticut: ZMB 10317, 1, 290 mm SL. Florida: USNM 286117, 4, 154-195 mm SL. USNM 286122, 4, 171-220 mm SL. Massachusetts: MNHN A 2428, 1, 193 mm SL. MNHN 1896-0150, 1, 76 mm SL. NMW 43931, 1, 290 mm SL. Paralichthys olivaceus: China: MNHN 1999-0471, 1, 251 mm SL. Foutcheou: MNHN 1941-0205, 2, 43-48 mm SL. Ningpo: ZMB 11236, 1, 138 mm SL. Port Arthur: BMNH 1923.2.26.557-559, 3, 138-141 mm SL. San-Tung: BMNH 1931.9.18.13, 1, 205 mm SL. Shangai:MNHN 1891-0653, 1, 605 mm SL. Japan: USNM 71996, 3, 158-186 mm SL. Kobe: NMW 43927, 1, 311 mm SL. Japan: Rikuzen: BMNH 1923.2.26.560, 1, 201 mm SL. Yokohama: ZMB 5278, 1, 148 mm SL. Locality unkown: FAKU 34344, 1, 436 mm SL. Paralichthys squamilentus: USA: Florida: USNM 30862, 98 mm SL, syntype of Paralichthys squamilentus Jordan and Gilbert, 1882. Arkansas Port: BMNH 1948.8.6.1223, 1, 154 mm SL. USA: Florida: BMNH 1883.12.14.99-100, 1, 99 mm SL. USA: Florida: BMNH 1933.10.12.124, 1, 295 mm SL. USA: Florida: MNHN 1937-0274, 1, 315 mm SL. Paralichthys triocellatus: Brazil: Rio de Janeiro:MNRJ 3154, 2, 197-208 mm SL, syntype of Paralichthys triocellatus Miranda Ribeiro, 1903. Brazil: MNHN 1975-0292, 3, 145-191 mm SL. MNHN 1975-0293, 1, 148 mm SL.MNHN 1975-0294, 2, 146-173 mm SL. MNHN 2001-1242, 2, 116-118 mm SL. Estado de São Paulo: MZUSP 72448, 1, 136 mm SL. Rio Grande do Sul: MZUSP 72457, 3, 131-186 mm SL. MZUSP 72451, 1, 155 mm SL. MZUSP 72456, 1, 136 mm SL. Rio de Janeiro: MNRJ 9723, 1, 181 mm SL. Uruguay: MZUSP 72452, 1, 143 mm SL. Paralichthys tropicus: Trinidad And Tobago: USNM 34919, 1, 259 mm SL, holotype of Paralichthys tropicus Ginsburg, 1933. Venezuela: Sucre: MCZ 41042, 1, 258 mm SL. Paralichthys woolmani: Ecuador: Galápagos Is.: USNM 47575,1, 196 mm SL, holotype of Paralichthys woolmani Jordan and Williams, 1897. Panamá: USNM 82698, 1, 198 mm SL. Panamá:USNM 81634, 1, 114 mm SL.
Acknowledgements
Many people helped us to make this paper possible. Special thanks to F. Cervigón (deceased), M. B. Cousseau, A. Gosztonyi, A. Miquelarena, R. Perrotta (deceased) and M. Desoutter for the valuable commnents and suggestions on early drafts of this manuscript.
The senior author is much indebted to the following collection managers for their kind assistance and support in their respective institutions: A. Palandacic (Naturhistorisches Museum in Vienna, Austria), P. Bartsch (Museum für Naturkunde Leibniz Institute for Evolution and Biodiversity Science, Berlin), J. Maclaine, Patrick Campbell and Oliver Crimmen (Natural History Museum of United Kingdom), Patrice Pruvost and Romain Causse (Museum national d´histoire naturelle, Paris), Gustavo Chiaramonte (Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires Argentina), S. Jewett (National Museum of Natural History, Washington, D.C.), K. Hartel (Museum of Comparative Zoology, Harvard University), I. Nakamura (Faculty of Agriculture [formerly Department of Fisheries], Kyoto University, Maizuru, Japan), the late G. Nunan (Museu Nacional, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil), N. Menezes and J.L. de Figueiredo (Museu de Zoologia da Universidade de São Paulo, São Paulo, Brazil), M.B. Cousseau (Instituto Nacional de Investigación y Desarrollo Pesquero fish collection, Mar del Plata, Argentina). S. Grosjean and M. Silvain (MNHN) kindly assisted in preparing the photographs and taking the X-rays. The senior author is also grateful to Pr. Guy Duhamel, Director of the Département Milieux et Peuplements Aquatiques, Muséum national d’Histoire naturelle, Paris for providing laboratory space and equipment. The technical assistance of C. Ferrara, Z. Gabsi and L. Duque-Vélez in supplying the material examined is greatly appreciated. We sincerely thank the anonymous reviewers for making important and critical suggestions to improve the manuscript. We are also much indebted to Diane Pitassy (National Museum of Natural History, Washington, D.C.) for help with type specimens and catalogue information regarding type specimens curated at the USNM.
B.B. Collette supervised and provided assistance and support during JMDA’s visit to the National Museum of Natural History, Smithsonian Institution by means of a research visitor grant. Also, JMDA extends his appreciation to Muséum National d’ Histoire Naturelle, Paris (MNHN) for financial assistance that provided opportunities to conduct research in its respective fish collections and libraries. M. Herrera and C. Milloc assisted in the drawings and M. Tobío in taking the photographs.
References
- Amaoka K. Studies on the sinistral flounders found in the waters around Japan: taxonomy, anatomy and phylogeny. J Shimonoseki Univ Fish. 1969; 18(2):65-340.
- Balart EF. Development of median and paired fin skeleton of Paralichthys olivaceus (Pleuronectiformes: Paralichthyidae). Jpn J Ichthyol. 1985; 31(4):398-410.
- Bellisio NB, López RB, Torno A. Peces marinos patagónicos. Publ Secret Int. Mar. Buenos Aires: Ed. Códex; 1979.
- Bittencourt MM. Estudo comparativo de aspectos da distribuiçao, morfologia e biologia de Paralichthys isosceles (Jordan, 1890) e Paralichthys triocellatus (Ribeiro, 1904) (Pleuronectiformes: Bothidae) da regiao da plataforma continental comprendida entre Cabo Frio e Torres (23°S-29°21’S). [Msc Dissertation]. São Paulo, SP: Instituto Oceanográfico da Universidade de São Paulo; 1982.
- Carneiro MH. Reprodução e alimentação dos linguados Paralichthys patagonicus e Paralichthys orbignyanus (Pleuronectiformes: Bothidae), no Rio Grande do Sul, Brasil. [Msc. Dissertation]. Rio Grande, RS: Universidade Federal do Rio Grande; 1995.
- Carvalho-Filho A. Peixes: costa brasileira. 3th ed. São Paulo: Editora Melro; 1999.
- Cervigón F. Ictiología Marina. Caracas: Editora Arte; 1980. vol. 1.
- Cervigón F. Las especies de los géneros Achirus y Trinectes (Pisces: Soleidae) de las costas de Venezuela (Osteología, Musculatura y ligamentos fasciales, y Sistemática). Caracas: Fundación Científica Los Roques, Monografía; 1985.
- Collette BB, Gillis GB. Morphology, systematics and biology of the double-lined mackerels (Grammatorcynus, Scombridae). Fish Bull. 1992; 90(1):13-53.
- Cousseau MB, Díaz de Astarloa JM. Investigaciones sobre dos categorías específicas: Paralichthys bicyclophorus y Paralichthys patagonicus Frente Marítimo. 1991; 8:51-59.
- Cousseau MB, Fabré NN. Lenguados. In: Cousseau, MB, editor. Muestreo bioestadístico de desembarque del Puerto de Mar del Plata Período 1980-1985. Mar del Plata: Contribución INIDEP; 1990. No. 5.
- Chanet B, Chapleau F, Desoutter M. Os et ligaments intermusculaires chez les poisson plats (Teleostei: Pleuronectiformes): interprétations phylogénétiques. Cybium. 2004; 28 (S1):9-14.
- Chapleau F. Pleuronectiform relationships: a cladistic reassessment. Bull Mar Sci. 1993; 52(1):516-40.
- Díaz de Astarloa JM. Estudios osteológicos del sincráneo y complejo caudal en dos formas nominales de Paralichthys: Paralichthys patagonicus y Paralichthys bicyclophorus Frente Marítimo . 1991; 9:15-27.
- Díaz de Astarloa JM. Determinación taxonómica de un grupo de lenguados del género Paralichthys colectado en aguas argentinas (Pleuronectiformes: Paralichthyidae). Rev Biol Mar Oceanogr. 1995a; 30(1):79-90.
- Díaz de Astarloa JM. Ambicoloration in two flounders, Paralichthys patagonicus and Xystreuris rasile J Fish Biol. 1995b; 47(1):168-70.
- Díaz de Astarloa JM. Paralichthys patagonicus Jordan, in Jordan & Goss, 1889, a senior synonym of P. bicyclophorus Miranda Ribeiro, 1915 (Paralichthyidae: Pleuronectiformes). Copeia. 1996; (4):1035-37.
- Díaz de Astarloa JM. A case of reversal in Paralichthys orbignyanus a shallow-water flounder from the south-western Atlantic. J Fish Biol . 1997; 50(4):900-02.
- Díaz de Astarloa JM. Vertebral anomalies in the patagonian flounder Paralichthys patagonicus (Pleuronectiformes: Paralichthyidae) from Río de la Plata estuary. Physis. 1998a; 56(130-131):7-11.
- Díaz de Astarloa JM. An ambicolorate flounder, Paralichthys isosceles, collected off Península Valdés, Argentina. Cybium . 1998b; 22(2):187-91.
- Díaz de Astarloa JM. A review of the flatfish fisheries of the south Atlantic Ocean. Rev Biol Mar. Oceanogr. 2002; 37(2):113-25.
- Díaz de Astarloa JM. Comparative cranial osteology of three species of the flatfish genus Paralichthys (Pleuronectiformes, Paralichthyidae) from the southwestern Atlantic. Rev Chil Hist Nat. 2005; 78(3):343-91.
- Díaz de Astarloa JM, Fabré NN. Abundance of three flatfishes (Pleuronectiformes: Paralichthyidae) off northern Argentina and Uruguay in relation to environmental factors. Arch Fish Mar Res. 2002; 50(2):121-40.
- Díaz de Astarloa JM, Munroe TA. Systematics, distribution and ecology of commercially important paralichthyid flounders ocurring in Argentinean-Uruguayan waters (Paralichthys, Paralichthyidae): an overview. J Sea Res. 1998; 39(1-2):1-9.
- Díaz de Astarloa JM, Munroe TA, Desoutter M. Redescription and holotype clarification of Paralichthys orbignyanus (Valenciennes, 1839) (Pleuronectiformes, Paralichthyidae). Copeia . 2006a; (2):235-43.
- Díaz de Astarloa JM, Rico R, Acha M. First report of a totally ambicoloured Patagonian flounder Paralichthys patagonicus (Paralichthyidae) with dorsal fin anomalies. Cybium . 2006b; 30(1):73-76.
- Dingerkus G, Uhler LH. Enzyme clearing of alcian blue stained whole small vertebrates for demonstration of cartilage. Stain Technol. 1977; 52(4):229-32.
- Fabré NN. Análisis de la distribución y dinámica poblacional de lenguados de la Provincia de Buenos Aires (Pisces, Bothidae). [PhD Thesis]. Mar del Plata, Buenos Aires: Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Mar del Plata; 1992.
- Fabré NN, Cousseau MB. Sobre la determinación de la edad y el crecimiento del lenguado Paralichthys isosceles aplicando retrocálculo. Rev Brazil Biol. 1990; 50(2):345-54.
- Fabré NN, Díaz de Astarloa JM. Distributional patterns and abundance of paralichthyid flounders in the south-west Atlantic (Pleuronectiformes: Paralichthyidae). Thalassas. 2001; 17(1):45-55.
- Figueiredo JL, Menezes NA. Manual de Peixes Marihnos do sudeste do Brasil. São Paulo: Museu de Zoologia da Universidade de São Paulo; 2000. vol 6, Telostei (5).
- García ML. Pleuronectiformes de la Argentina, IV. Alimentación de Paralichthys isosceles (Bothidae, Paralichthyinae). Notas Mus. La Plata, Zool. 1987; 207:111-25.
- Ginsburg I. Flounders of the genus Paralichthys and related genera in American waters. Fish Bull . 1952; 52(71):267-351.
- Gutherz EJ. Field guide to the flatfishes of the family Bothidae in the western North Atlantic. Washington (DC): US Fish and wildlife service; Bureau of commercial fisheries; 1967. (Circular 263).
- Haimovici M. Present state and perspectives for the southern Brazil shelf demersal fisheries. Fish Manag Ecol. 1998; 5:277-89.
- Haimovici M, Mendonça JT. Descartes da fauna acompanhante na pesca de arrasto de tangones dirigida a linguados e camarões na plataforma continental do sul do Brasil. Atlântida. 1996; 18:161-77.
- Hensley DA. Guía FAO para identificación de especies para fines de la Pesca: Pacifico Centro-Oriental.. In: Fischer W, Krupp F, Schneider W, Sommer C, Carpenter KE, Niem V, editors). Rome: FAO.; 1995. vol 3, Vertebrados, pt. 2, Paralichthyidae: Lenguados.
- Hensley DA, Ahlstrom EH. Pleuronectiformes: Relationships. In: Moser HG et al, editors. Ontogeny and Systematics of Fishes: Ahlstrom Symposium. California: American Society of Ichthyologists and Herpetologists; 1984. p.670-687. (Special publication of the American Society of Ichthyologists and Herpetologists; No. 1).
- Hoaglin DC, Mosteller F, Tukey J, editors. Fundamentals of exploratory analysis of variance. New York: John Wiley & Sons, Inc.; 1991. (Wiley Series in Probability and Statistics).
- Hoshino K. Homologies of the caudal fin rays of Pleuronectiformes (Teleostei). Ichthyol Res. 2001; 48(3):231-46.
- Hoshino K, Amaoka K. Osteology of the flounder, Tephrinectes sinensis (Lacèpede) (Teleostei: Pleuronectiformes), with comments on its relationships. Ichthyol Res . 1998; 45(1):69-77.
- Jordan DS, Goss DK. A review of the flounders and soles (Pleuronectidae) of America and Europe. Rep U.S. Fish Comm. 1889; 14:225-42.
- Kawamura K, Hosoya K. A modified double staining technique for making a transparent fish-skeletal specimen. Bull Natl Res Inst Aquacult (Jpn). 1991; 20:11-18.
- Li S, Wang H. Fauna Sinica, Osteichthyes, Pleuronectiformes. Beijing: Science Press; 1995.
- Lopez-Cazorla A. On the age and growth of flounder Paralichthys orbignyanus (Jenyns, 1842) in Bahía Blanca Estuary, Argentina. Hydrobiologia. 2005; 537(1-3):81-87.
- Lopez-Cazorla A, Forte S. Food and feeding habits of flounder Paralichthys orbignyanus (Jenyns, 1842) in Bahía Blanca Estuary, Argentina. Hydrobiologia . 2005; 549(1):251-57.
- Mac Donagh EJ. Sobre algunos peces marinos. Notas Mus. La Plata , Zool. 1936; 4:423-29.
- Macchi GJ, Díaz de Astarloa JM. Ciclo reproductivo y fecundidad del lenguado, Paralichthys patagonicus Rev Inv Des Pesq. 1996; (10):73-83.
- Marini TL, López RB. Recursos acuáticos vivos. Buenos Aires: Consejo Federal de Inversiones; 1963. (Evaluación de los recursos naturales de la Argentina, Primera Etapa; vol 1-2, pt. 7).
- Mayden RL, Wiley EO. A method of preparing disarticulated skeletons of small fishes. Copeia . 1984; (1):230-32.
- Militelli MI. Paralichthys patagonicus spawning areas and reproductive potential in the Bonaerense Coastal Zone, Argentina (34º-42ºS). Lat Am Aquat Res. 2011; 39(1):131-37.
- Monod T. Le complexe urophore des poissons Téléostéens. Bull Inst Fondam Afr Noire, Ser A. 1968; 81:1-705.
- Munroe TA. Family Paralichthyidae. In: Carpenter KE, editor. The living marine resources of the Western Central Atlantic. Rome: FAO ; 2003. p.1898-1921. vol 3, Bony fishes part 2 (Opistognathidae to Molidae), sea turtles and marine mammals. (FAO species identification guide for fishery purposes and American Society of Ichthyologists and Herpetologists Special Publication; No. 5).
- Munroe TA. Systematic diversity of the Pleuronectiformes. In: Gibson RN, Nash RDM, Geffen AJ, van der Veer HW, editors. Flatfishes: Biology and Exploitation. 2nd edition. Hoboken (NJ): Wiley Blackwell; 2015a. p.13-51. (Fish and Aquatic Resources Series; 16).
- Munroe TA. Distributions and biogeography. In: Gibson RN, Nash RDM, Geffen AJ, van der Veer HW, editors. Flatfishes: Biology and Exploitation. 2nd edition. Hoboken (NJ): Wiley Blackwell ; 2015b. p.52-82. (Fish and Aquatic Resources Series; 16).
- Norman JR. A Systematic Monograph of the Flatfishes (Heterosomata). London: British Museum of Natural History; 1934. vol. 1, Psettodidae, Bothidae, Pleuronectidae.
- Ossian CR. Preparation of disarticulated skeletons using enzyme-based laundry “pre-soakers”. Copeia . 1970; (1):199-200.
- Patterson C, Johnson GD. The intermuscular bones and ligaments of teleostean fishes. Washington, (DC): Smithsonian Institution Press; 1995. (Smithsonian Contributions to Zoology; No. 559).
- Potthoff T. Clearing and staining techniques. In: Moser HG et al, editors. Ontogeny and Systematics of Fishes: Ahlstrom Symposium. California: American Society of Ichthyologists and Herpetologists; 1984. p.35-37. (Special publication of the American Society of Ichthyologists and Herpetologists; No. 1).
- Radonic M, Müller MI, López AV, Bambill GA, Spinedi M, Boccanfuso JJ. Improvement in flounder Paralichthys orbignyanus controlled spawning in Argentina. Cienc Mar. 2007; 33(2):187-96.
- Rico MR. Pesquería de lenguados en el ecosistema costero bonaerense al norte de 39° S. Frente Marítimo . 2010; 21:129-35.
- Rojo AL. Diccionario enciclopédico de anatomía de peces. Madri: Ministerio de Agricultura, Pesca y Alimentación; 1988. (Monografias del Instituto Español de oceanografía; No. 3).
- Sabaj Pérez MH, editor. Standard symbolic codes for institutional resource collections in herpetology and ichthyology: an online reference. Washington (DC), version 6.5. Available from: http://www.asih.org/resources/standard-symbolic-codes-institutional-resource-collections-herpetology-ichthyology
» http://www.asih.org/resources/standard-symbolic-codes-institutional-resource-collections-herpetology-ichthyology - da Silva Junior LC, De Andrade AC, De Andrade-Tubino MF, Vianna M. Reversal and ambicoloration in two flounder species (Paralichthyidae, Pleuronectiformes). Panam J Aquat Sci, 2007; 2(1):23-26.
- Walsh SJ, Díaz de Astarloa JM, Poos JJ. Atlantic Flatfish Fisheries. In: Gibson RN, Nash RDM, Geffen AJ, van der Veer HW, editors. Flatfishes: Biology and exploitation. 2nd edition). Hoboken (NJ): Wiley Blackwell ; 2015. p.346-394. (Fish and Aquatic Resources Series; 16).
- Woolcott WS, Beirne C, Hall WM Jr. Descriptive and comparative osteology of the young of three species of flounders, genus Paralichthys Chesapeake Sci. 1968; 9(2):109-20.
- Wu HW. Contribution à l’étude morphologique, biologique et systématique des poissons Hétérosomes (Pisces, Heterosomata) de la Chine. [PhD Thesis]. Paris: Faculté des Sciences de l’Université de Paris; 1932.
- Zar JH. Biostatistical Analysis. Englewood Cliffs, Prentice Hall; 1984.
Data availability
Data citations
Sabaj Pérez MH, editor. Standard symbolic codes for institutional resource collections in herpetology and ichthyology: an online reference. Washington (DC), version 6.5. Available from: http://www.asih.org/resources/standard-symbolic-codes-institutional-resource-collections-herpetology-ichthyology
Publication Dates
-
Publication in this collection
16 July 2018 -
Date of issue
2018
History
-
Received
22 Dec 2017 -
Accepted
16 Apr 2018