Abstract
The South American giant fishes of the genus Arapaima, commonly known as pirarucu, are one of the most iconic among Osteoglossiformes. Previously cytogenetic studies have identified their karyotype characteristics; however, characterization of cytotaxonomic differentiation across their distribution range remains unknown. In this study, we compared chromosomal characteristics using conventional and molecular cytogenetic protocols in pirarucu populations from the Amazon and Tocantins-Araguaia river basins to verify if there is differentiation among representatives of this genus. Our data revealed that individuals from all populations present the same diploid chromosome number 2n=56 and karyotype composed of 14 pairs of meta- to submetacentric and 14 pairs of subtelo- to acrocentric chromosomes. The minor and major rDNA sites are in separate chromosomal pairs, in which major rDNA sites corresponds to large heterochromatic blocks. Comparative genomic hybridizations (CGH) showed that the genome of these populations shared a great portion of repetitive elements, due to a lack of substantial specific signals. Our comparative cytogenetic data analysis of pirarucu suggested that, although significant genetic differences occur among populations, their general karyotype patterns remain conserved.
Keywords:
CGH; Chromosome banding; Fish cytotaxonomy; Osteoglossomorpha; rDNA FISH
Resumo
Os peixes gigantes da América do Sul do gêneroArapaima, comumente conhecidos como pirarucus, são um dos mais icônicos de Osteoglossiformes. Estudos citogenéticos prévios identificaram suas características cariotípicas, entretanto a caracterização da diferenciação citotaxonômica através de suas distribuições geográficas ainda são desconhecidas. Nesse estudo, nós comparamos características cromossômicas utilizando técnicas de citogenética clássica e molecular em populações das bacias dos rios Amazonas e Tocantins-Araguaia, a fim de verificar se há alguma diferenciação entre representantes desse gênero. Nossos dados revelaram que indivíduos de todas as populações apresentam número diploide de 2n=56 cromossomos e que seus cariótipos são compostos de 14 pares de cromossomos meta- e submetacêntricos e 14 pares de subtelo- e acrocêntricos. Os sítios maiores e menores de rDNA estão localizados em pares cromossômicos separados, onde os sítios maiores de rDNA correspondem a grandes blocos heterocromáticos. Hibridizações genômicas comparativas (CGH) mostraram que o genoma dos espécimes dessas populações é amplamente compartilhado, devido à falta de sinais substanciais específicos. Nossos dados de citogenética comparativa do pirarucu sugerem que embora diferenças genéticas significativas ocorram entre populações, os padrões cariotípicos gerais se mantêm conservados.
Palabras-chave:
Bandamento cromossômico; CGH; Citotaxonomia; Osteoglossomorpha; DNAr FISH
INTRODUCTION
Distributed throughout South America, Africa, Australia, and South Asia realms, the Osteoglossiformes represents one of the ancient teleost lineages containing several taxa important for fisheries and aquaculture (Castello et al., 2009Castello L, Viana JP, Watkins G, Pinedo-Vasquez M, Luzadis VA. Lessons from integrating fishers of Arapaima in small-scale fisheries management at the Mamirauá Reserve, Amazon. Environ Manage. 2009; 43:197-209. https://doi.org/10.1007/s00267-008-9220-5
https://doi.org/10.1007/s00267-008-9220-...
; Medipally et al., 2016Medipally SR, Yusoff FM, Sharifhuddin N, Shariff M. Sustainable aquaculture of Asian arowana - a review. J Environ Biol. 2016; 37:829-38.; Maldonado et al., 2017Maldonado AG, Lopes PFM, Fernández CAR, Lasso-Alcala CA, Summalia UR. Transboundary fisheries management in the Amazon: Assessing current policies for the management of the ornamental silver arawana (Osteoglossum bicirrhosum). Mar Policy. 2017; 76:192-99. https://doi.org/10.1016/j.marpol.2016.11.021
https://doi.org/10.1016/j.marpol.2016.11...
).
Fishes of the genus Arapaima Müller, 1843, commonly named as pirarucu, are among the largest freshwater fish species, reaching up to two and a half meters in length and 200 kg in weight (Nelson et al., 2016Nelson JS, Grande TC, Wilson MVH. Fishes of the World. Hoboken: John Wiley & Sons; 2016.). Their natural distribution extends throughout the Brazilian basins of Tocantins-Araguaia, Amazon, and Essequibo (Guyana) rivers, usually in lakes and showing a preference for calm waters, known as “várzeas” (Lowe-McConnell, 1987Lowe-McConnell RH. Ecological studies in Tropical Fish communities. Cambridge: Cambridge University Press; 1987.; Queiroz, 2000Queiroz HL. Natural history and conservation of Pirarucu, Arapaima gigas, at the Amazonian Várzea: Red giants in muddy waters. [PhD Thesis]. St Andrews: University of St. Andrews; 2000. Available from: http://hdl.handle.net/10023/2818
http://hdl.handle.net/10023/2818...
; Castello, 2008Castello L. Lateral migration of Arapaima gigas in floodplains of the Amazon. Ecol Freshw Fish. 2008; 17(1):38-46. https://doi.org/10.1111/j.1600-0633.2007.00255.x
https://doi.org/10.1111/j.1600-0633.2007...
; Reis et al., 2016Reis RE, Albert JS, Di Dario F, Mincarone MM, Petry P, Rocha LA. Fish biodiversity and conservation in South America. J Fish Biol. 2016; 89(1):12-47. https://doi.org/10.1111/jfb.13016
https://doi.org/10.1111/jfb.13016...
).
Despite their high economic and conservation importance, as well as the voluminous literature about them, few information is available focusing on karyotype structure and mapping of chromosomal markers in pirarucu (Marques et al., 2006Marques DK, Venere PC, Galetti PM. Chromosomal characterization of the bonytongueArapaima gigas (Osteoglossiformes: Arapaimidae). Neotrop Ichthyol. 2006; 4(2):215-18. https://doi.org/10.1590/S1679-62252006000200007
https://doi.org/10.1590/S1679-6225200600...
; Rosa et al., 2009Rosa R, Rubert M, Caetano-Filho M, Giuliano-Caetano L. Conserved cytogenetic features in the Amazonian Arapaima, Arapaima gigas(Schinz 1822) from Jamari River, Rondônia-Brazil. Open Biol. 2009; 2:91-94. http://doi.org/10.2174/1874196700902010091
http://doi.org/10.2174/18741967009020100...
; Oliveira et al., 2019Oliveira EA, Bertollo LAC, Ráb P, Ezaz T, Yano CF, Hatanaka T, Jegede OI, Tanomtong A, Liehr T, Sember A, Maruyama SR, Feldberg E, Viana PF, Cioffi MB. Cytogenetics, genomics and biodiversity of the South American and African Arapaimidae fish family (Teleostei, Osteoglossiformes). PLoS ONE. 2019; 14(3):e0214225. https://doi.org/10.1371/journal.pone.0214225
https://doi.org/10.1371/journal.pone.021...
). The first karyotype description of pirarucu, under the name Arapaima gigas(Schinz, 1822) (from Araguaia River, Araguaiana - MT), reported a diploid chromosome number 2n=56, karyotype containing 14 m-sm + 14 st-a pair of chromosomes and only one chromosome pair bearing Nuclear Organizer Regions (NORs) (Marques et al., 2006Marques DK, Venere PC, Galetti PM. Chromosomal characterization of the bonytongueArapaima gigas (Osteoglossiformes: Arapaimidae). Neotrop Ichthyol. 2006; 4(2):215-18. https://doi.org/10.1590/S1679-62252006000200007
https://doi.org/10.1590/S1679-6225200600...
). These results were also observed in a recent reassessment (Oliveira et al., 2019Oliveira EA, Bertollo LAC, Ráb P, Ezaz T, Yano CF, Hatanaka T, Jegede OI, Tanomtong A, Liehr T, Sember A, Maruyama SR, Feldberg E, Viana PF, Cioffi MB. Cytogenetics, genomics and biodiversity of the South American and African Arapaimidae fish family (Teleostei, Osteoglossiformes). PLoS ONE. 2019; 14(3):e0214225. https://doi.org/10.1371/journal.pone.0214225
https://doi.org/10.1371/journal.pone.021...
), and it was observed that the karyotype of pirarucu remarkably differs from its sister taxon, the African Heterotis niloticus(Cuvier, 1829), the latter possessing 2n=40 with different content and distribution patterns of repetitive DNAs.
The genus Arapaima was considered monotypic since Günther (1867)Günther A. Contribution to the anatomy of Hatteria (Rhynchocephalus, Owen). Philos Trans R Soc Lond B Biol Sci. 1867; 157:595-629. https://doi.org/10.1098/rstl.1867.0019
https://doi.org/10.1098/rstl.1867.0019...
, who synonymized earlier described species with A. gigas. However, a recent taxonomic revision of species diversity in the genus indicated the validity of the species, namely A. arapaima (Valenciennes, 1847), A. mapae (Valenciennes, 1847), A. agassizii (Valenciennes, 1847) and the proposition of the new species A. leptosoma Stewart, 2013Stewart DJ. A New Species of Arapaima (Osteoglossomorpha: Osteoglossidae) from the Solimões River, Amazonas State, Brazil. Copeia. 2013a; 2013(3):470-76. https://doi.org/10.1643/CI-12-017
https://doi.org/10.1643/CI-12-017...
. But, recent publications analyzing the genetic diversity present in Arapaima considered it as a monotypic genus (Farias et al., 2019Farias IP, Willis S, Leão A, Verba JT, Crossa M, Foresti F, Porto-Foresti F, Sampaio I, Hrbek T. The largest fish in the world’s biggest river: Genetic connectivity and conservation of Arapaima gigasin the Amazon and Araguaia-Tocantins drainages. PLoS ONE. 2019; 14(8):e0220882. https://doi.org/10.1371/journal.pone.0220882
https://doi.org/10.1371/journal.pone.022...
; Torati et al., 2019Torati LS, Taggart JB, Varela ES, Araripe J, Wehner S, Migaud H. Genetic diversity and structure in Arapaima gigaspopulations from Amazon and Araguaia-Tocantins river basins. BMC Genet. 2019; 20(13):1-13. https://doi.org/10.1186/s12863-018-0711-y
https://doi.org/10.1186/s12863-018-0711-...
). In this study, although all analyzed specimens have been classified as A. gigasbased on morphological characteristics during voucher deposit, we still accept the potential existence of other cryptic species as proposed by Stewart (2013aStewart DJ. A New Species of Arapaima (Osteoglossomorpha: Osteoglossidae) from the Solimões River, Amazonas State, Brazil. Copeia. 2013a; 2013(3):470-76. https://doi.org/10.1643/CI-12-017
https://doi.org/10.1643/CI-12-017...
, bStewart DJ. Re-description of Arapaima agassizii (Valenciennes), a rare fish from Brazil (Osteoglossomorpha: Osteoglossidae). Copeia. 2013b; 2013(1):38-51. https://doi.org/10.1643/CI-12-013
https://doi.org/10.1643/CI-12-013...
).
Cytogenetic techniques have been frequently used as helpful tools on fish species with complex taxonomy, as well as to characterize fish biodiversity (Artoni et al., 2006Artoni RF, Shibatta OA, Gross MC, Schneider CH, Almeida MC, Vicari MR, Bertollo LAC. Astyanax aff. fasciatus Cuvier, 1819 (Teleostei; Characidae): evidences of a species complex in the upper rioTibagi basin (Paraná, Brazil). Neotrop Ichthyol. 2006; 4(2):197-202. https://doi.org/10.1590/S1679-62252006000200005
https://doi.org/10.1590/S1679-6225200600...
; Bertollo, 2007Bertollo LAC. Chromosome evolution in the Neotropical Erythrinidae fish family: an overview. In: Pisano E, Ozouf-Costaz C, Foresti F, Kapoor BG, editors. Fish cytogenetics. Boca Raton: CRC Press; 2007. p.195-211.; Pansonato-Alves et al., 2013Pansonato-Alves JC, Serrano EA, Utsunomia R, Scacchetti PC, Oliveira C, Foresti F. Mapping five repetitive DNA classes in sympatric species of Hypostomus (Teleostei: Siluriformes: Loricariidae): analysis of chromosomal variability. Rev Fish Biol Fish. 2013; 23:477-89. https://doi.org/10.1007/s11160-013-9303-0
https://doi.org/10.1007/s11160-013-9303-...
; Cioffi et al., 2018Cioffi MB, Moreira-Filho O, Ráb P, Sember A, Molina WF, Bertollo LAC. Conventional cytogenetic approaches - useful and indispensable tools in discovering fish biodiversity. Curr Genet Med Rep. 2018; 6:176-86. https://doi.org/10.1007/s40142-018-0148-7
https://doi.org/10.1007/s40142-018-0148-...
; Pazza et al., 2018Pazza R, Dergam JA, Kavalco KF. Trends in karyotype evolution in Astyanax (Teleostei, Characiformes, Characidae): insights from molecular data. Front Genet. 2018; 9(131):1-11. https://doi.org/10.3389/fgene.2018.00131
https://doi.org/10.3389/fgene.2018.00131...
). Furthermore, it is now possible to gain insights into evolutionary relationships using comparative genomic hybridization (CGH), a useful approach for fish genomes characterization (Symonová et al., 2015Symonová R, Sember A, Majtánová Z, Ráb P. Characterization of fish genomes by GISH and CGH. In: Ozouf-Costaz C, Pisano E, Foresti F, Toledo LFA, editors. Fish cytogenetic techniques: ray-fin fishes and chondrichthyans. Boca Raton: CRC Press; 2015. p.118-31.), as demonstrated for Lebiasinidae (Sassi et al., 2020Sassi FMC, Hatanaka T, Moraes RLR, Toma GA, Oliveira EA, Liehr T, Ráb P, Bertollo LAC, Viana PF, Feldberg E, Nirchio M, Marinho MMF, Souza JFS, Cioffi MB. An insight into the chromosomal evolution of Lebiasinidae (Teleostei, Characiformes). Genes. 2020; 11(365):1-14. https://doi.org/10.3390/genes11040365
https://doi.org/10.3390/genes11040365...
), Notopteridae (Barby et al., 2019Barby FF, Bertollo LAC, Oliveira EA, Yano CF, Hatanaka T, Ráb P, Sember A, Ezaz T, Artoni RF, Liehr T, Al-Rikabi ABH, Trifonov V, Oliveira EHC, Molina WF, Jegede OI, Tanomtong A, Cioffi MB. Emerging patterns of genome organization in Notopteridae species (Teleostei, Osteoglossiformes) as revealed by Zoo-FISH and Comparative Genomic Hybridization (CGH). Sci Rep. 2019; 9(1112):1-21. https://doi.org/10.1038/s41598-019-38617-4
https://doi.org/10.1038/s41598-019-38617...
) and Osteoglossidae (Cioffi et al., 2019Cioffi MB, Ráb P, Ezaz T, Bertollo LAC, Lavoué S, Oliveira EA, Sember A, Molina WF, Souza FHS, Majtánová Z, Liehr T, Al-Rikabi ABH, Yano CF, Viana P, Feldberg E, Unmack P, Hatanaka T, Tanomtong A, Perez MF. Deciphering the evolutionary history of arowana fishes (Teleostei, Osteoglossiformes, Osteoglossidae): insight from comparative cytogenomics. Int J Mol Sci. 2019; 20(4296):1-19. https://doi.org/10.3390/ijms20174296
https://doi.org/10.3390/ijms20174296...
) fish families.
In this study, we analysed individuals of Arapaima gigas from regions covering part of its geographical distribution from the Amazon and Tocantins-Araguaia rivers basins. We applied conventional and molecular cytogenetic procedures, including chromosomal distributions of repetitive DNAs and CGH among populations from both river systems to assess potential divergences in their genomes. The main objective of our study was to investigate whether the cytogenetic data support different evolutionary patterns among populations of pirarucu fishes or if they represent a case of taxonomic diversity not associated with cytotaxonomic differentiation despite their wide geographical distribution.
MATERIAL AND METHODS
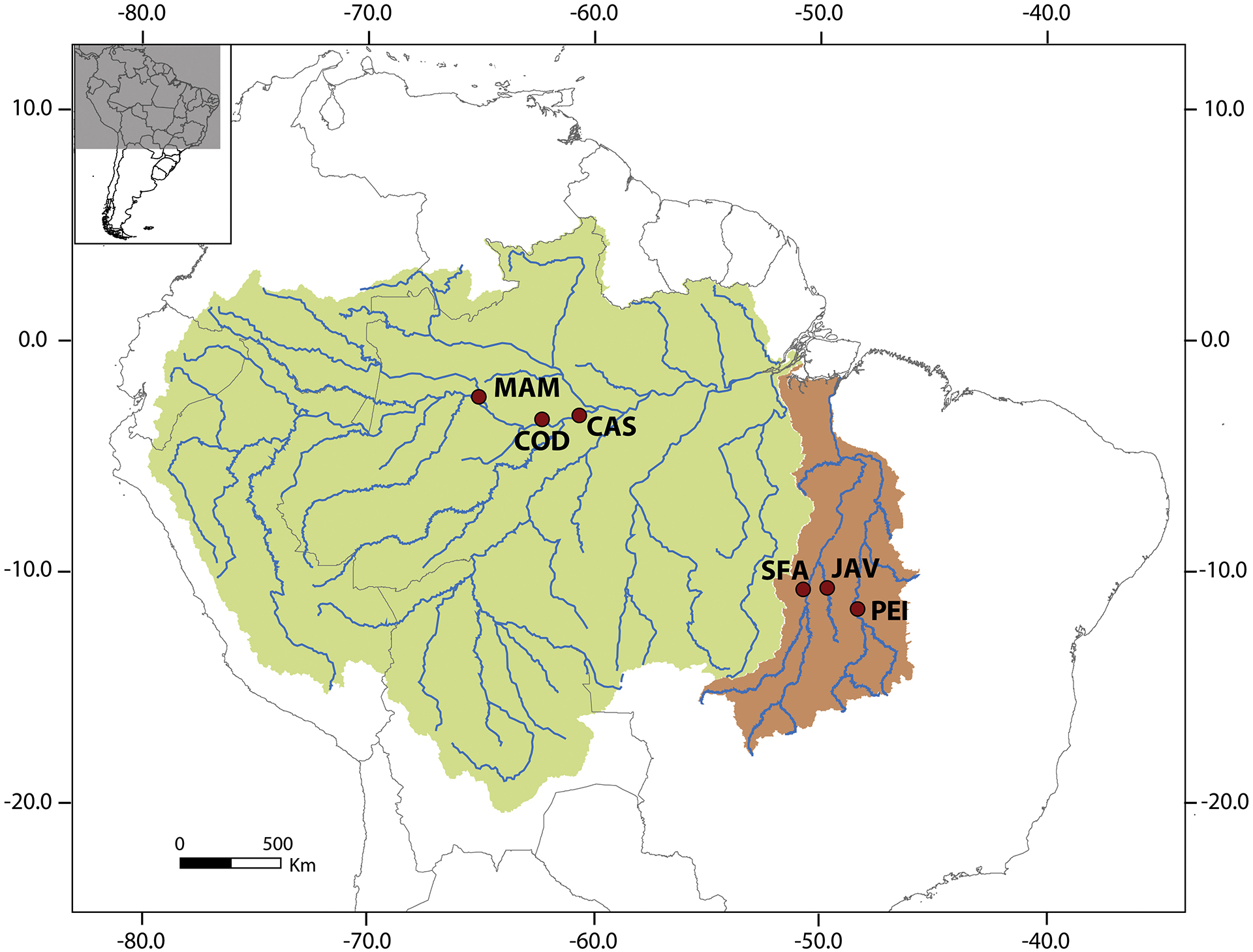
Material, conventional and molecular cytogenetic protocols. Samples of pirarucu from six localities (three from the Amazon river basin and three from the Tocantins-Araguaia river basin) were analyzed (Tab. 1, Fig. 1). Voucher specimens were deposited in the Museu de Zoologia da Universidade de São Paulo (MZUSP) (Tab. 1).
Metaphase chromosomes were obtained using the conventional air-drying method (Bertollo et al., 2015Bertollo LAC, Cioffi MB, Moreira-Filho O. Direct chromosome preparation from freshwater teleost fishes. In: Ozouf-Costaz C, Pisano E, Foresti F, Toledo LFA, editors. Fish cytogenetic techniques: Ray-Fin Fishes and Chondrichthyans. Boca Raton: CRC Press; 2015. p.21-26.), with some adaptations given by Oliveira et al. (2019)Oliveira EA, Bertollo LAC, Ráb P, Ezaz T, Yano CF, Hatanaka T, Jegede OI, Tanomtong A, Liehr T, Sember A, Maruyama SR, Feldberg E, Viana PF, Cioffi MB. Cytogenetics, genomics and biodiversity of the South American and African Arapaimidae fish family (Teleostei, Osteoglossiformes). PLoS ONE. 2019; 14(3):e0214225. https://doi.org/10.1371/journal.pone.0214225
https://doi.org/10.1371/journal.pone.021...
. Conventional staining was done using 5% Giemsa solution in phosphate buffer, pH 6.8, for 10 min. C-positive heterochromatin was detected following Sumner (1972)Sumner AT. A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res. 1972; 75(1):304-06. https://doi.org/10.1016/0014-4827(72)90558-7
https://doi.org/10.1016/0014-4827(72)905...
. A total of 10 repetitive DNA sequences, namely three multigene families (U2 snDNA, 5S and 18S rDNAs) and seven microsatellite repeat motifs (A)30, (CA)15, (GA)15, (CAC)10, (CGG)10, (GAA)10 and (GAG)10 were isolated and mapped by fluorescence in situ hybridization (FISH) procedures, following Yano et al. (2017)Yano CF, Bertollo LAC, Rebordinos L, Merlo MA, Liehr T, Portela-Bens S, Cioffi MB. Evolutionary dynamics of rDNAs and U2 small nuclear DNAs in Triportheus(Characiformes, Triportheidae): high variability and particular syntenic organization. Zebrafish. 2017; 14(2):146-54. https://doi.org/10.1089/zeb.2016.1351
https://doi.org/10.1089/zeb.2016.1351...
. The microsatellite probes were directly labeled with Cy3 during synthesis according to Kubat et al. (2008)Kubat Z, Hobza R, Vyskot B, Kejnovsky E. Microsatellite accumulation on the Y chromosome in Silene latifolia. Genome. 2008; 51(5):350-56. https://doi.org/10.1139/G08-024
https://doi.org/10.1139/G08-024...
. Both rDNA sequences (18S and 5S rDNAs) were produced following Cioffi et al. (2009)Cioffi MB, Martins C, Bertollo LAC. Comparative chromosome mapping of repetitive sequences. Implications for genomic evolution in the fish, Hoplias malabaricus. BMC Genet. 2009; 10(34):1-11. https://doi.org/10.1186/1471-2156-10-34
https://doi.org/10.1186/1471-2156-10-34...
and Pendás et al. (1994)Pendás AM, Moran P, Freije JP, Garcia-Vázquez E. Chromosomal mapping and nucleotide sequence of two tandem repeats of Atlantic salmon 5S rDNA. Cytogenet Genome Res. 1994; 67(1):31-36. https://doi.org/10.1159/000133792
https://doi.org/10.1159/000133792...
, respectively. The U2 snDNA probe was produced according to Silva et al. (2015)Silva DMZA, Utsunomia R, Pansonato-Alves JC, Oliveira C, Foresti F. Chromosomal mapping of repetitive DNA sequences in five species of Astyanax (Characiformes, Characidae) reveals independent location of U1 and U2 snRNA sites and association of U1 snRNA and 5S rDNA. Cytogenet Genome Res. 2015; 146:144-52. https://doi.org/10.1159/000438813
https://doi.org/10.1159/000438813...
. All these probes were directly labeled with Spectrum Orange-dUTP by nick translation, according to manufacturer’s recommendations (Roche, Mannheim, Germany), with the exception of 5S rDNA, which was directly labeled with Spectrum Green-dUTP, also by nick translation (Roche, Mannheim, Germany).
Localities, numbers, sex and voucher codes of Arapaima individuals analyzed in this study. Voucher specimens were deposited in the Museu de Zoologia da Universidade de São Paulo (MZUSP).
Map of northern part of South America showing the sampling sites of Arapaima individuals analyzed in this study from Araguaia-Tocantins (brown) and Amazon (green) River basins, coded in the Tab. 1.
Comparative Genomic Hybridization (CGH). CGH experiments were performed to compare the genomic composition of individuals from populations located in different hydrographic basins. The gDNA of individuals from the JAV population (Tocantins-Araguaia basin) was compared with the gDNA of individuals from the MAM population (Amazon basin), by using probes of labeled gDNA isolated from individuals of each basin against metaphase chromosomes from samples of each basin. For this purpose, the gDNA of individuals from the MAM population was labeled with digoxigenin-11-dUTP using DIG-nick-translation Mix (Roche, Mannheim, Germany), while the gDNA of individuals from JAV population was labeled with biotin-16-dUTP using BIO-nick-translation Mix (Roche). For blocking the repetitive sequences, we used C0t-1 DNA prepared according to Zwick et al. (1997)Zwick MS, Hanson RE, Islam-Faridi MN, Stelly DM, Wing RA, Price HJ, McKnight TD. A rapid procedure for the isolation of C0t-1 DNA from plants. Genome. 1997; 40(1):138-42. https://doi.org/10.1139/g97-020
https://doi.org/10.1139/g97-020...
, in all experiments. The final probe cocktail for each slide was composed by 500ng of gDNA of individuals from the MAM population + 500ng of gDNA of individuals from the JAV population + 15μg of derived C0t-1 DNA of each specimen. The probes were ethanol-precipitated, and the dry pellets were suspended in hybridization buffer containing 50% formamide, 2×SSC, 10% SDS, 10% dextran sulfate and Denhardt´s buffer, pH 7.0.
Microscopic Analysis and Image Processing. At least 30 metaphase spreads per individual were analyzed to confirm the 2n, karyotype structure, and results of FISH experiments. Images were captured using an Olympus BX50 microscope (Olympus Corporation, Ishikawa, Japan) with Cool SNAP, and processed using Image-Pro Plus 4.1 software (Media Cybernetics, Silver Spring, MD, USA). Chromosomes were classified as metacentric (m), submetacentric (sm), subtelocentric (st), or acrocentric (a) and according to Levan et al. (1964)Levan A, Fredga K, Sandberg AA. Nomenclature for centromeric position on chromosomes. Hereditas. 1964; 52(2):201-20. https://doi.org/10.1111/j.1601-5223.1964.tb01953.x
https://doi.org/10.1111/j.1601-5223.1964...
.
RESULTS
Karyotypes, chromosome banding, and rDNA FISH. Both males and females from all populations had conserved diploid chromosome number 2n=56, with karyotypes composed of 28 m-sm + 28 st-a chromosomes and a number of chromosomal arms (NF value) of 84 (Fig. 2). C-positive heterochromatic bands were distributed in the pericentromeric regions of all chromosomes, except for some additional conspicuous telomeric blocks in some chromosome pairs (Fig. 2). The 5S and 18S rDNA sites were interstitially located on the long (q) arms of the pair No. 1 and on the short (p) arms of the pair No. 2, respectively (Fig. 2).
Karyotypes of Arapaima gigasmale (A-C) and female (D-F) from Javaé (JAV) population (Araguaia-Tocantins River basin), sequentially arranged from Giemsa-stained (A and D), C-banded (B and E), and double-FISH (C and F) with 5S rDNA (green) and 18S rDNA (red) labeled chromosomes. Bar=5 µm.
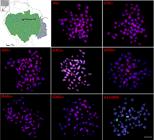
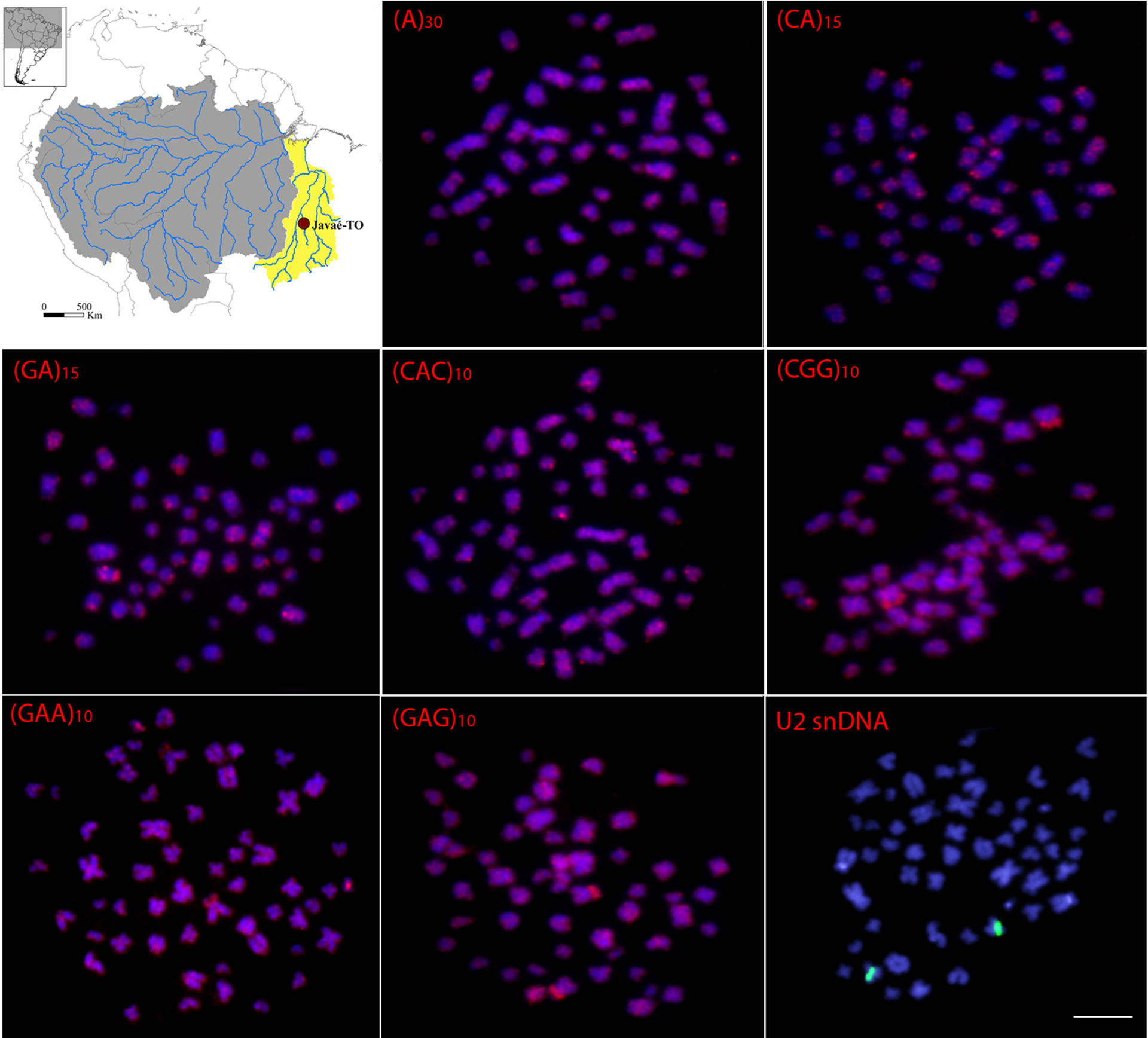
Microsatellites and U2 snDNA distribution. The (GC)15, (CAA)10, (CAC)10, (CAG)10, (CAT)10, (CGG)10, (GAA)10 and (GAG)10 repeats displayed scattered hybridization signals throughout the genome of all individuals from all populations (Figs. 3-4). Specifically, (CGG)10 repeats displayed conspicuous signals corresponding to the NORs (Figs. 3-4). No significant inter/intrapopulation variation regarding the microsatellite distribution was observed. The U2 snDNA was mapped onto the interstitial position of one small acrocentric pair in individuals from all populations. To demonstrate these patterns, an individual from a representative population from each basin, i.e., Javaé (To-Ar) and Mamirauá (MAM), were selected and here presented. The other results can be found in the supplementary figures S1, S2, S3 and S4.
Metaphase plates of Arapaima gigasfrom Javaé (JAV) population (Araguaia-Tocantins River basin) hybridized with repetitive DNA sequences, including mono-, di- and trinucleotide microsatellites and the multigene families U2 snDNA. Bar=5 µm.
Metaphase plates of Arapaima gigasfrom Mamirauá (MAM) population (Amazon River basin) hybridized with repetitive DNA sequences, including mono-, di- and trinucleotide microsatellites and the multigene families U2 snDNA. Bar=5 µm.
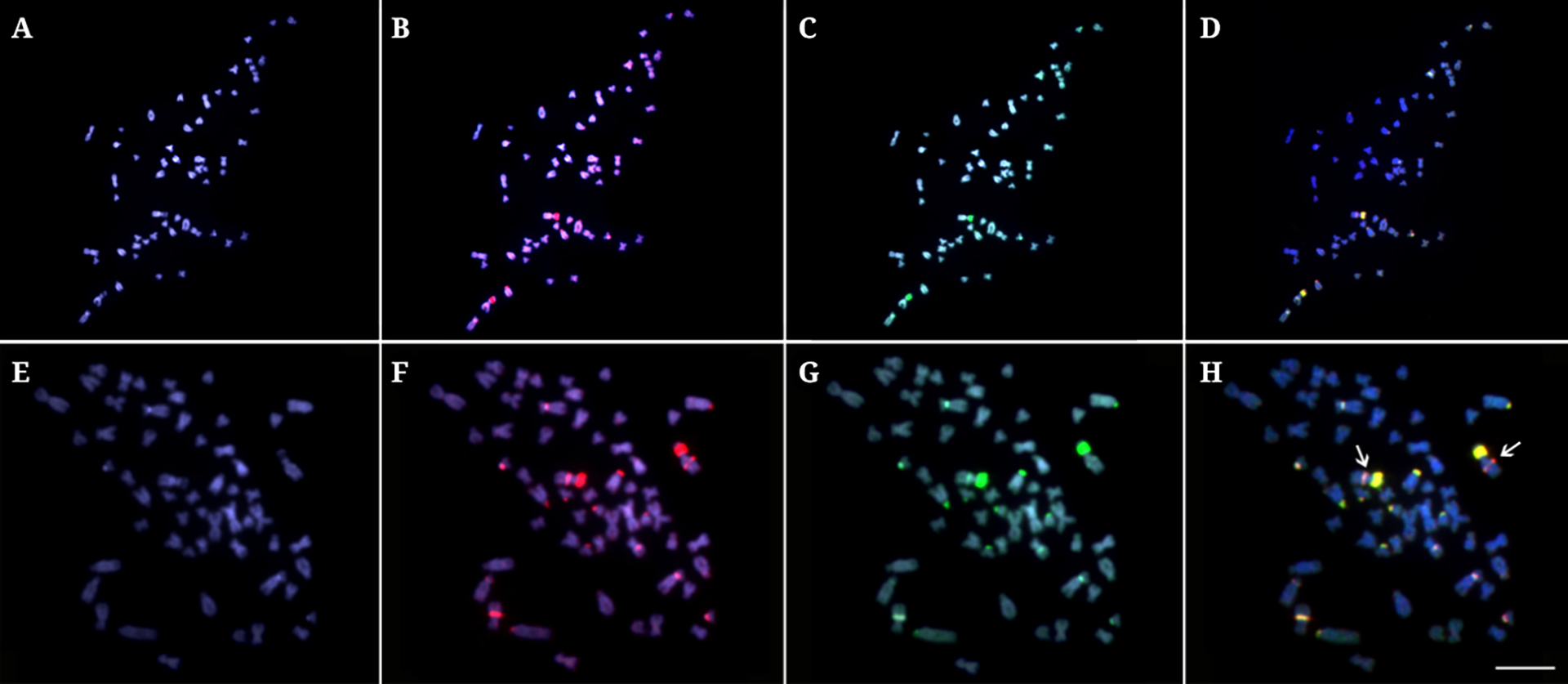
Comparative Genomic Hybridization. The comparative hybridization of gDNAs probes of individuals from JAV (To-Ar) and MAM (Am) populations produced essentially a large number of overlapping signals, highlighting the heterochromatic blocks that occurred in the centromeric and terminal regions of some chromosomes. Some of these signals were considerably stronger and linked to the major rDNA sites. However, the hybridization against metaphase chromosomes of individuals from the MAM population particularly highlighted some over-represented signals, probably linked to a higher copy number of repetitive sequences in some chromosomal regions (Fig. 5).
Comparative genomic hybridization (CGH) experiments on metaphase chromosomes of Arapaima individuals from the Javaé (JAV) (A-D) and Mamirauá (MAM) populations (E-H). First column (A and E): DAPI images of chromosomes; Second column (B and F): Hybridization pattern using gDNA of A. gigasfrom MAM population (red): Third column (C and G): Hybridization pattern using the gDNA of A. gigasfrom JAV population (green); Fourth column (D and H) overlap of the images. The shared regions are highlighted in yellow. The arrows indicate the higher abundance of some repetitions in individuals from MAM population. Bar=5 µm.
DISCUSSION
Cytogenetic and genomic characteristics among basins. The same cytogenetic characteristics, i.e., the same karyotype structures and the same patterns of chromosomal mapping of repetitive DNAs, were found for both sexes from all the populations in both basins. Therefore, our results agree with the previous reports of Marques et al. (2006)Marques DK, Venere PC, Galetti PM. Chromosomal characterization of the bonytongueArapaima gigas (Osteoglossiformes: Arapaimidae). Neotrop Ichthyol. 2006; 4(2):215-18. https://doi.org/10.1590/S1679-62252006000200007
https://doi.org/10.1590/S1679-6225200600...
, Rosa et al. (2009)Rosa R, Rubert M, Caetano-Filho M, Giuliano-Caetano L. Conserved cytogenetic features in the Amazonian Arapaima, Arapaima gigas(Schinz 1822) from Jamari River, Rondônia-Brazil. Open Biol. 2009; 2:91-94. http://doi.org/10.2174/1874196700902010091
http://doi.org/10.2174/18741967009020100...
and Oliveira et al. (2019)Oliveira EA, Bertollo LAC, Ráb P, Ezaz T, Yano CF, Hatanaka T, Jegede OI, Tanomtong A, Liehr T, Sember A, Maruyama SR, Feldberg E, Viana PF, Cioffi MB. Cytogenetics, genomics and biodiversity of the South American and African Arapaimidae fish family (Teleostei, Osteoglossiformes). PLoS ONE. 2019; 14(3):e0214225. https://doi.org/10.1371/journal.pone.0214225
https://doi.org/10.1371/journal.pone.021...
. The CGH comparison, an appropriate approach for comparing the genome divergence between closely related species and/or populations, as well as hybrids (Symonová et al., 2015Symonová R, Sember A, Majtánová Z, Ráb P. Characterization of fish genomes by GISH and CGH. In: Ozouf-Costaz C, Pisano E, Foresti F, Toledo LFA, editors. Fish cytogenetic techniques: ray-fin fishes and chondrichthyans. Boca Raton: CRC Press; 2015. p.118-31.; Moraes et al., 2017Moraes RLR, Bertollo LAC, Marinho MMF, Yano CF, Hatanaka T, Barby FF, Troy WP, Cioffi MB. Evolutionary relationships and cytotaxonomy considerations in the genus Pyrrhulina (Characiformes, Lebiasinidae). Zebrafish. 2017; 14(6):536-46. https://doi.org/10.1089/zeb.2017.1465
https://doi.org/10.1089/zeb.2017.1465...
; Sember et al., 2018Sember A, Bertollo LAC, Ráb P, Yano CF, Hatanaka T, Oliveira EA, Cioffi MB. Sex chromosome evolution and genomic divergence in the fish Hoplias malabaricus (Characiformes, Erythrinidae). Front Genet. 2018; 9(71):1-12. https://doi.org/10.3389/fgene.2018.00071
https://doi.org/10.3389/fgene.2018.00071...
; Sassi et al., 2019Sassi FMC, Oliveira EA, Bertollo LAC, Nirchio M, Hatanaka T, Marinho MMF, Moreira-Filho O, Aroutiounian R, Liehr T, Al-Rikabi ABH, Cioffi MB. Chromosomal evolution and evolutionary relationships of Lebiasina species (Characiformes, Lebiasinidae). Int J Mol Sci. 2019; 20(2944):1-17. https://doi.org/10.3390/ijms20122944
https://doi.org/10.3390/ijms20122944...
), showed a low genomic divergence between the populations from the To-Ar and Am basins.
Karyotypic and morphological patterns are often indicators of the lifestyle of a species (Pellestor et al., 2011Pellestor F, Anahory T, Lefort G, Puechberty J, Liehr T, Hédon B, Sarda P. Complex chromosomal rearrangements: origin and meiotic behavior. Hum Reprod Update. 2011; 17(4):476-94. https://doi.org/10.1093/humupd/dmr010
https://doi.org/10.1093/humupd/dmr010...
; Rabosky et al., 2013Rabosky DL, Santini F, Eastman J, Smith SA, Sidlauskas B, Chang J, Alfaro ME. Rates of speciation and morphological evolution are correlated across the largest vertebrate radiation. Nat Commun. 2013; 4(1958):1-8. https://doi.org/10.1038/ncomms2958
https://doi.org/10.1038/ncomms2958...
). Arapaima fishes are considered sedentary animals having low migratory activities, with a preference for low-oxygenated lentic environments, specialized parental care and a high degree of endogamy (Hrbek et al., 2005Hrbek T, Farias IP, Crossa M, Sampaio I, Porto JIR, Meyer A. Population genetic analysis of Arapaima gigas, one of the largest freshwater fishes of the Amazon basin: implications for its conservation. Anim Conserv. 2005; 8(3):297-308. https://doi.org/10.1017/S1367943005002210
https://doi.org/10.1017/S136794300500221...
).Fish species with such behavioral characteristics usually display a high level of karyotype diversity among populations, as observed in the genus ChannaScopoli, 1777 (Channidae) (Cioffi et al., 2015Cioffi MB, Bertollo LAC, Villa MA, Oliveira EA, Tanomtong A, Yano CF, Supiwong W, Chaveerach A. Genomic organization of repetitive DNA elements and its implications for the chromosomal evolution of channid fishes (Actinopterygii, Perciformes). PLoS ONE. 2015; 10(6):e0130199. https://doi.org/10.1371/journal.pone.0130199
https://doi.org/10.1371/journal.pone.013...
), in the Erythrinidae species Hoplias malabaricus(Bloch, 1794) and Erythrinus erythrinus(Bloch & Schneider, 1801) (reviewed in Bertollo, 2007Bertollo LAC. Chromosome evolution in the Neotropical Erythrinidae fish family: an overview. In: Pisano E, Ozouf-Costaz C, Foresti F, Kapoor BG, editors. Fish cytogenetics. Boca Raton: CRC Press; 2007. p.195-211. and Cioffi et al., 2012Cioffi MB, Moreira-Filho O, Almeida-Toledo LF, Bertollo LAC. The contrasting role of heterochromatin in the differentiation of sex chromosomes: an overview from Neotropical fishes. J Fish Biol. 2012; 80(6):2125-39. https://doi.org/10.1111/j.1095-8649.2012.03272.x
https://doi.org/10.1111/j.1095-8649.2012...
), and in the Synbranchidae species Synbranchus marmoratus Bloch, 1795 and Monopterus albus (Zuiew, 1793) (reviewed in Supiwong et al., 2019Supiwong W, Pinthong K, Seetapan K, Saenjundaeng P, Bertollo LAC, Oliveira EA, Yano CF, Liehr T, Phimphan S, Tanomtong A, Cioffi MB. Karyotype diversity and evolutionary trends in the Asian swamp eel Monopterus albus (Synbranchiformes, Synbranchidae): a case of chromosomal speciation?. BMC Genet. 2019; 19(73):1-9. https://doi.org/10.1186/s12862-019-1393-4
https://doi.org/10.1186/s12862-019-1393-...
), among others. However, due their complicated and not fully solved taxonomy, Arapaima seems to represent another case of higher taxonomic diversity, which is not associated with differentiated cytogenetic parameters. Examples of these processes can be observed in the genus Esox Linnaeus, 1758 (Symonová et al., 2017Symonová R, Ocalewicz K, Kirtiklis L, Delmastro GB, Pelikánová Š, Garcia S, Kovařík A. Higher-order organisation of extremely amplified, potentially functional and massively methylated 5S rDNA in European pikes (Esox sp.). BMC Genomics. 2017; 18(391):1-16. https://doi.org/10.1186/s12864-017-3774-7
https://doi.org/10.1186/s12864-017-3774-...
), in Leuciscidae family (Ayata et al., 2019Ayata MK, Yüksel E, Gaffaroğlu M. Ag-NOR karyotypes of five endemic Pseudophoxinus Bleeker, 1860 (Teleostei: Leuciscidae) species from Anatolia. Genet Aquat Org. 2019; 3(1):27-30. http://doi.org/10.4194/2459-1831-v3_1_04
http://doi.org/10.4194/2459-1831-v3_1_04...
) and in many marine fish groups (reviewed in Molina, 2007Molina WF. Chromosomal changes and stasis in marine fish groups. In: Pisano E, Ozouf-Costaz C, Foresti F, Kapoor BG, editors. Fish cytogenetics. Boca Raton: CRC Press; 2007. p.69-110.).
However, it is noteworthy that this low karyotype chromosomal differentiation in both karyotype structure and repetitive DNA levels is not substantiated by single nucleotide polymorphism (SNPs) dataset analyses. Indeed, our previous studies demonstrated a low genetic diversity for A. gigas populations from the To-Ar basin, but not for those from the Am basin, where a higher diversity level comparable to the SNP diversity of other taxa from the same region was detected (Oliveira et al., 2020Oliveira EA, Perez MF, Bertollo LAC, Gestich CC, Ráb P, Ezaz T, Souza FHS, Viana P, Feldberg E, Oliveira EHC, Cioffi MB. Historical demography and climate driven distributional changes in a widespread Neotropical freshwater species within high economic importance. Ecography. 2020; 43(9):1291-304. https://doi.org/10.1111/ecog.04874
https://doi.org/10.1111/ecog.04874...
). This lower genetic diversity for the To-Ar populations, especially from those of the upper section of the basin, was also found by additional studies (Alencar-Leão, 2009Alencar-Leão AS. Análise da variabilidade genética das populações de pirarucu (Arapaima gigas, Schinz1822) dos principais tributários do rio Amazonas através do uso de marcadores microssatélites. [Master Dissertation]. Manaus: Instituto Nacional de Pesquisas da Amazônia; 2009. Available from: https://bdtd.inpa.gov.br/handle/tede/2108
https://bdtd.inpa.gov.br/handle/tede/210...
; Vitorino et al., 2017Vitorino CA, Nogueira F, Souza IL, Araripe J, Venere PC. Low genetic diversity and structuring of the Arapaima (Osteoglossiformes, Arapaimidae) population of the Araguaia-Tocantins basin. Front Genet. 2017; 8(159):1-10. https://doi.org/10.3389/fgene.2017.00159
https://doi.org/10.3389/fgene.2017.00159...
; Torati et al., 2019Torati LS, Taggart JB, Varela ES, Araripe J, Wehner S, Migaud H. Genetic diversity and structure in Arapaima gigaspopulations from Amazon and Araguaia-Tocantins river basins. BMC Genet. 2019; 20(13):1-13. https://doi.org/10.1186/s12863-018-0711-y
https://doi.org/10.1186/s12863-018-0711-...
).
These contrasting cytogenetic and sequence patterns presented by pirarucu populations may be associated with multiple factors. Among them, a population homogenizing effect over time can be attributed to the hydrological dynamics of the region where these fishes are currently found. Indeed, the Brazilian Amazon region shows long flooding duration and flow cycles allowing the fish dispersion for long periods, a process known as lateral migration (Castello, 2008Castello L. Lateral migration of Arapaima gigas in floodplains of the Amazon. Ecol Freshw Fish. 2008; 17(1):38-46. https://doi.org/10.1111/j.1600-0633.2007.00255.x
https://doi.org/10.1111/j.1600-0633.2007...
). Besides, rivers from the To-Ar basin are situated in an active tectonic depression, which resulted in the development of extensive alluvial plains. In this scenario, there is evidence for ichthyofaunal exchanges among the headwaters of the Tocantins River and all other major drainages on its boundaries, including several ones from the Am basin (e.g., Xingu River) (Rossetti, Valeriano, 2007Rossetti DF, Valeriano MM. Evolution of the lowest Amazon basin modeled from the integration of geological and SRTM topographic data. Catena. 2007; 70(2):253-65. https://doi.org/10.1016/j.catena.2006.08.009
https://doi.org/10.1016/j.catena.2006.08...
; Lima, Ribeiro, 2011Lima FC, Ribeiro AC. Continental-scale tectonic controls of biogeography and ecology. In: Albert JS, Reis R, editors. Historical Biogeography of Neotropical Freshwater Fishes. Oakland: University of California Press; 2011. p.145-64.). Besides that, along with several other fishes, Arapaima has shown a population decline due to the loss of natural habitats and commercial over-exploitation (Allan et al., 2005Allan JD, Abell R, Hogan Z, Revenga C, Taylor BW, Welcomme RL, Winemiller K. Overfishing of inland waters. BioScience. 2005; 55(12):1041-51. https://doi.org/10.1641/0006-3568(2005)055[1041:OOIW]2.0.CO;2
https://doi.org/10.1641/0006-3568(2005)0...
; Castello et al., 201Castello L, McGrath DG, Beck PSA. Resource sustainability in small-scale fisheries in the Lower Amazon floodplains. Fish Res. 2011; 110(2):356-64. https://doi.org/10.1016/j.fishres.2011.05.002
https://doi.org/10.1016/j.fishres.2011.0...
1). In addition to this recent decline due to anthropogenic activities, paleogeographic reconstructions (Oliveira et al., 2019Oliveira EA, Bertollo LAC, Ráb P, Ezaz T, Yano CF, Hatanaka T, Jegede OI, Tanomtong A, Liehr T, Sember A, Maruyama SR, Feldberg E, Viana PF, Cioffi MB. Cytogenetics, genomics and biodiversity of the South American and African Arapaimidae fish family (Teleostei, Osteoglossiformes). PLoS ONE. 2019; 14(3):e0214225. https://doi.org/10.1371/journal.pone.0214225
https://doi.org/10.1371/journal.pone.021...
) indicated that during the last glacial period the pirarucu fishes distribution was constrained under severe climatic conditions, with scattered suitable habitats and restricted areas of refuge. Additionally, a deep leaning model analysis indicates that the Tocantins-Araguaia river basin was colonized by the population from the Amazon river basin (Oliveira et al., 2020Oliveira EA, Perez MF, Bertollo LAC, Gestich CC, Ráb P, Ezaz T, Souza FHS, Viana P, Feldberg E, Oliveira EHC, Cioffi MB. Historical demography and climate driven distributional changes in a widespread Neotropical freshwater species within high economic importance. Ecography. 2020; 43(9):1291-304. https://doi.org/10.1111/ecog.04874
https://doi.org/10.1111/ecog.04874...
).
Altogether, these features possibly played a major role in shaping modern genetic diversity and chromosomal conservatism among populations. In this sense, the pattern of genetic differentiation among distinct populations most likely arose due to natural and human actions reducing their size and distribution area providing differentiation over time. Under these size fluctuations, an accentuated genetic drift and bottleneck effects are not also ruled out (Wright, 1931Wright S. Evolution in Mendelian populations. Genetics. 1931; 16:97-159. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1201091/
https://www.ncbi.nlm.nih.gov/pmc/article...
; Lande, 1988Lande R. Genetics and demography in biological conservation. Science. 1988; 241(4872):1455-60. https://doi.org/10.1126/science.3420403
https://doi.org/10.1126/science.3420403...
). On the other hand, chromosomal differentiation usually requires more time and more specific conditions to be fixed, such as complete geographic isolation of small populations without gene flow among them. In this respect, the regional hydrological dynamism may act in the opposite direction preventing major changes in the karyotypes.
Despite the high economic importance of pirarucu fishes, research on their evolutionary, genetic and chromosomal characteristics deserves more in-depth studies. This study focused, for the first time, on a comparative cytogenetic survey of pirarucu populations from the Amazon and Tocantins-Araguaia rivers basins, in order to characterize possible karyotype differentiation among them. It was shown that those populations retain a remarkable karyotype and/or chromosomal stability, as revealed both by conventional and molecular cytogenetic markers.
ACKNOWLEDGEMENTS
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (401962/2016-4 and 302449/2018-3 to MBC); Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (2018/22033-1 to MBC; and 2017/10240-0 to MFP); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)/Alexander von Humboldt Foundation (88881.136128/2017-01 to MBC). Petr Ráb was supported by the project EXCELLENCE (CZ.02.1.01/0.0/0.0/15_003/0000460 OP). This study was financed in part by the CAPES (Finance Code 001).
REFERENCES
- Alencar-Leão AS. Análise da variabilidade genética das populações de pirarucu (Arapaima gigas, Schinz1822) dos principais tributários do rio Amazonas através do uso de marcadores microssatélites. [Master Dissertation]. Manaus: Instituto Nacional de Pesquisas da Amazônia; 2009. Available from: https://bdtd.inpa.gov.br/handle/tede/2108
» https://bdtd.inpa.gov.br/handle/tede/2108 - Allan JD, Abell R, Hogan Z, Revenga C, Taylor BW, Welcomme RL, Winemiller K. Overfishing of inland waters. BioScience. 2005; 55(12):1041-51. https://doi.org/10.1641/0006-3568(2005)055[1041:OOIW]2.0.CO;2
» https://doi.org/10.1641/0006-3568(2005)055[1041:OOIW]2.0.CO;2 - Artoni RF, Shibatta OA, Gross MC, Schneider CH, Almeida MC, Vicari MR, Bertollo LAC. Astyanax aff. fasciatus Cuvier, 1819 (Teleostei; Characidae): evidences of a species complex in the upper rioTibagi basin (Paraná, Brazil). Neotrop Ichthyol. 2006; 4(2):197-202. https://doi.org/10.1590/S1679-62252006000200005
» https://doi.org/10.1590/S1679-62252006000200005 - Ayata MK, Yüksel E, Gaffaroğlu M. Ag-NOR karyotypes of five endemic Pseudophoxinus Bleeker, 1860 (Teleostei: Leuciscidae) species from Anatolia. Genet Aquat Org. 2019; 3(1):27-30. http://doi.org/10.4194/2459-1831-v3_1_04
» http://doi.org/10.4194/2459-1831-v3_1_04 - Barby FF, Bertollo LAC, Oliveira EA, Yano CF, Hatanaka T, Ráb P, Sember A, Ezaz T, Artoni RF, Liehr T, Al-Rikabi ABH, Trifonov V, Oliveira EHC, Molina WF, Jegede OI, Tanomtong A, Cioffi MB. Emerging patterns of genome organization in Notopteridae species (Teleostei, Osteoglossiformes) as revealed by Zoo-FISH and Comparative Genomic Hybridization (CGH). Sci Rep. 2019; 9(1112):1-21. https://doi.org/10.1038/s41598-019-38617-4
» https://doi.org/10.1038/s41598-019-38617-4 - Bertollo LAC. Chromosome evolution in the Neotropical Erythrinidae fish family: an overview. In: Pisano E, Ozouf-Costaz C, Foresti F, Kapoor BG, editors. Fish cytogenetics. Boca Raton: CRC Press; 2007. p.195-211.
- Bertollo LAC, Cioffi MB, Moreira-Filho O. Direct chromosome preparation from freshwater teleost fishes. In: Ozouf-Costaz C, Pisano E, Foresti F, Toledo LFA, editors. Fish cytogenetic techniques: Ray-Fin Fishes and Chondrichthyans. Boca Raton: CRC Press; 2015. p.21-26.
- Castello L. Lateral migration of Arapaima gigas in floodplains of the Amazon. Ecol Freshw Fish. 2008; 17(1):38-46. https://doi.org/10.1111/j.1600-0633.2007.00255.x
» https://doi.org/10.1111/j.1600-0633.2007.00255.x - Castello L, Viana JP, Watkins G, Pinedo-Vasquez M, Luzadis VA. Lessons from integrating fishers of Arapaima in small-scale fisheries management at the Mamirauá Reserve, Amazon. Environ Manage. 2009; 43:197-209. https://doi.org/10.1007/s00267-008-9220-5
» https://doi.org/10.1007/s00267-008-9220-5 - Castello L, McGrath DG, Beck PSA. Resource sustainability in small-scale fisheries in the Lower Amazon floodplains. Fish Res. 2011; 110(2):356-64. https://doi.org/10.1016/j.fishres.2011.05.002
» https://doi.org/10.1016/j.fishres.2011.05.002 - Cioffi MB, Martins C, Bertollo LAC. Comparative chromosome mapping of repetitive sequences. Implications for genomic evolution in the fish, Hoplias malabaricus BMC Genet. 2009; 10(34):1-11. https://doi.org/10.1186/1471-2156-10-34
» https://doi.org/10.1186/1471-2156-10-34 - Cioffi MB, Moreira-Filho O, Almeida-Toledo LF, Bertollo LAC. The contrasting role of heterochromatin in the differentiation of sex chromosomes: an overview from Neotropical fishes. J Fish Biol. 2012; 80(6):2125-39. https://doi.org/10.1111/j.1095-8649.2012.03272.x
» https://doi.org/10.1111/j.1095-8649.2012.03272.x - Cioffi MB, Bertollo LAC, Villa MA, Oliveira EA, Tanomtong A, Yano CF, Supiwong W, Chaveerach A. Genomic organization of repetitive DNA elements and its implications for the chromosomal evolution of channid fishes (Actinopterygii, Perciformes). PLoS ONE. 2015; 10(6):e0130199. https://doi.org/10.1371/journal.pone.0130199
» https://doi.org/10.1371/journal.pone.0130199 - Cioffi MB, Moreira-Filho O, Ráb P, Sember A, Molina WF, Bertollo LAC. Conventional cytogenetic approaches - useful and indispensable tools in discovering fish biodiversity. Curr Genet Med Rep. 2018; 6:176-86. https://doi.org/10.1007/s40142-018-0148-7
» https://doi.org/10.1007/s40142-018-0148-7 - Cioffi MB, Ráb P, Ezaz T, Bertollo LAC, Lavoué S, Oliveira EA, Sember A, Molina WF, Souza FHS, Majtánová Z, Liehr T, Al-Rikabi ABH, Yano CF, Viana P, Feldberg E, Unmack P, Hatanaka T, Tanomtong A, Perez MF. Deciphering the evolutionary history of arowana fishes (Teleostei, Osteoglossiformes, Osteoglossidae): insight from comparative cytogenomics. Int J Mol Sci. 2019; 20(4296):1-19. https://doi.org/10.3390/ijms20174296
» https://doi.org/10.3390/ijms20174296 - Farias IP, Willis S, Leão A, Verba JT, Crossa M, Foresti F, Porto-Foresti F, Sampaio I, Hrbek T. The largest fish in the world’s biggest river: Genetic connectivity and conservation of Arapaima gigasin the Amazon and Araguaia-Tocantins drainages. PLoS ONE. 2019; 14(8):e0220882. https://doi.org/10.1371/journal.pone.0220882
» https://doi.org/10.1371/journal.pone.0220882 - Günther A. Contribution to the anatomy of Hatteria (Rhynchocephalus, Owen). Philos Trans R Soc Lond B Biol Sci. 1867; 157:595-629. https://doi.org/10.1098/rstl.1867.0019
» https://doi.org/10.1098/rstl.1867.0019 - Hrbek T, Farias IP, Crossa M, Sampaio I, Porto JIR, Meyer A. Population genetic analysis of Arapaima gigas, one of the largest freshwater fishes of the Amazon basin: implications for its conservation. Anim Conserv. 2005; 8(3):297-308. https://doi.org/10.1017/S1367943005002210
» https://doi.org/10.1017/S1367943005002210 - Kubat Z, Hobza R, Vyskot B, Kejnovsky E. Microsatellite accumulation on the Y chromosome in Silene latifolia Genome. 2008; 51(5):350-56. https://doi.org/10.1139/G08-024
» https://doi.org/10.1139/G08-024 - Lande R. Genetics and demography in biological conservation. Science. 1988; 241(4872):1455-60. https://doi.org/10.1126/science.3420403
» https://doi.org/10.1126/science.3420403 - Levan A, Fredga K, Sandberg AA. Nomenclature for centromeric position on chromosomes. Hereditas. 1964; 52(2):201-20. https://doi.org/10.1111/j.1601-5223.1964.tb01953.x
» https://doi.org/10.1111/j.1601-5223.1964.tb01953.x - Lima FC, Ribeiro AC. Continental-scale tectonic controls of biogeography and ecology. In: Albert JS, Reis R, editors. Historical Biogeography of Neotropical Freshwater Fishes. Oakland: University of California Press; 2011. p.145-64.
- Lowe-McConnell RH. Ecological studies in Tropical Fish communities. Cambridge: Cambridge University Press; 1987.
- Maldonado AG, Lopes PFM, Fernández CAR, Lasso-Alcala CA, Summalia UR. Transboundary fisheries management in the Amazon: Assessing current policies for the management of the ornamental silver arawana (Osteoglossum bicirrhosum). Mar Policy. 2017; 76:192-99. https://doi.org/10.1016/j.marpol.2016.11.021
» https://doi.org/10.1016/j.marpol.2016.11.021 - Marques DK, Venere PC, Galetti PM. Chromosomal characterization of the bonytongueArapaima gigas (Osteoglossiformes: Arapaimidae). Neotrop Ichthyol. 2006; 4(2):215-18. https://doi.org/10.1590/S1679-62252006000200007
» https://doi.org/10.1590/S1679-62252006000200007 - Medipally SR, Yusoff FM, Sharifhuddin N, Shariff M. Sustainable aquaculture of Asian arowana - a review. J Environ Biol. 2016; 37:829-38.
- Molina WF. Chromosomal changes and stasis in marine fish groups. In: Pisano E, Ozouf-Costaz C, Foresti F, Kapoor BG, editors. Fish cytogenetics. Boca Raton: CRC Press; 2007. p.69-110.
- Moraes RLR, Bertollo LAC, Marinho MMF, Yano CF, Hatanaka T, Barby FF, Troy WP, Cioffi MB. Evolutionary relationships and cytotaxonomy considerations in the genus Pyrrhulina (Characiformes, Lebiasinidae). Zebrafish. 2017; 14(6):536-46. https://doi.org/10.1089/zeb.2017.1465
» https://doi.org/10.1089/zeb.2017.1465 - Nelson JS, Grande TC, Wilson MVH. Fishes of the World. Hoboken: John Wiley & Sons; 2016.
- Oliveira EA, Bertollo LAC, Ráb P, Ezaz T, Yano CF, Hatanaka T, Jegede OI, Tanomtong A, Liehr T, Sember A, Maruyama SR, Feldberg E, Viana PF, Cioffi MB. Cytogenetics, genomics and biodiversity of the South American and African Arapaimidae fish family (Teleostei, Osteoglossiformes). PLoS ONE. 2019; 14(3):e0214225. https://doi.org/10.1371/journal.pone.0214225
» https://doi.org/10.1371/journal.pone.0214225 - Oliveira EA, Perez MF, Bertollo LAC, Gestich CC, Ráb P, Ezaz T, Souza FHS, Viana P, Feldberg E, Oliveira EHC, Cioffi MB. Historical demography and climate driven distributional changes in a widespread Neotropical freshwater species within high economic importance. Ecography. 2020; 43(9):1291-304. https://doi.org/10.1111/ecog.04874
» https://doi.org/10.1111/ecog.04874 - Pansonato-Alves JC, Serrano EA, Utsunomia R, Scacchetti PC, Oliveira C, Foresti F. Mapping five repetitive DNA classes in sympatric species of Hypostomus (Teleostei: Siluriformes: Loricariidae): analysis of chromosomal variability. Rev Fish Biol Fish. 2013; 23:477-89. https://doi.org/10.1007/s11160-013-9303-0
» https://doi.org/10.1007/s11160-013-9303-0 - Pazza R, Dergam JA, Kavalco KF. Trends in karyotype evolution in Astyanax (Teleostei, Characiformes, Characidae): insights from molecular data. Front Genet. 2018; 9(131):1-11. https://doi.org/10.3389/fgene.2018.00131
» https://doi.org/10.3389/fgene.2018.00131 - Pellestor F, Anahory T, Lefort G, Puechberty J, Liehr T, Hédon B, Sarda P. Complex chromosomal rearrangements: origin and meiotic behavior. Hum Reprod Update. 2011; 17(4):476-94. https://doi.org/10.1093/humupd/dmr010
» https://doi.org/10.1093/humupd/dmr010 - Pendás AM, Moran P, Freije JP, Garcia-Vázquez E. Chromosomal mapping and nucleotide sequence of two tandem repeats of Atlantic salmon 5S rDNA. Cytogenet Genome Res. 1994; 67(1):31-36. https://doi.org/10.1159/000133792
» https://doi.org/10.1159/000133792 - Queiroz HL. Natural history and conservation of Pirarucu, Arapaima gigas, at the Amazonian Várzea: Red giants in muddy waters. [PhD Thesis]. St Andrews: University of St. Andrews; 2000. Available from: http://hdl.handle.net/10023/2818
» http://hdl.handle.net/10023/2818 - Rabosky DL, Santini F, Eastman J, Smith SA, Sidlauskas B, Chang J, Alfaro ME. Rates of speciation and morphological evolution are correlated across the largest vertebrate radiation. Nat Commun. 2013; 4(1958):1-8. https://doi.org/10.1038/ncomms2958
» https://doi.org/10.1038/ncomms2958 - Reis RE, Albert JS, Di Dario F, Mincarone MM, Petry P, Rocha LA. Fish biodiversity and conservation in South America. J Fish Biol. 2016; 89(1):12-47. https://doi.org/10.1111/jfb.13016
» https://doi.org/10.1111/jfb.13016 - Rosa R, Rubert M, Caetano-Filho M, Giuliano-Caetano L. Conserved cytogenetic features in the Amazonian Arapaima, Arapaima gigas(Schinz 1822) from Jamari River, Rondônia-Brazil. Open Biol. 2009; 2:91-94. http://doi.org/10.2174/1874196700902010091
» http://doi.org/10.2174/1874196700902010091 - Rossetti DF, Valeriano MM. Evolution of the lowest Amazon basin modeled from the integration of geological and SRTM topographic data. Catena. 2007; 70(2):253-65. https://doi.org/10.1016/j.catena.2006.08.009
» https://doi.org/10.1016/j.catena.2006.08.009 - Sassi FMC, Oliveira EA, Bertollo LAC, Nirchio M, Hatanaka T, Marinho MMF, Moreira-Filho O, Aroutiounian R, Liehr T, Al-Rikabi ABH, Cioffi MB. Chromosomal evolution and evolutionary relationships of Lebiasina species (Characiformes, Lebiasinidae). Int J Mol Sci. 2019; 20(2944):1-17. https://doi.org/10.3390/ijms20122944
» https://doi.org/10.3390/ijms20122944 - Sassi FMC, Hatanaka T, Moraes RLR, Toma GA, Oliveira EA, Liehr T, Ráb P, Bertollo LAC, Viana PF, Feldberg E, Nirchio M, Marinho MMF, Souza JFS, Cioffi MB. An insight into the chromosomal evolution of Lebiasinidae (Teleostei, Characiformes). Genes. 2020; 11(365):1-14. https://doi.org/10.3390/genes11040365
» https://doi.org/10.3390/genes11040365 - Sember A, Bertollo LAC, Ráb P, Yano CF, Hatanaka T, Oliveira EA, Cioffi MB. Sex chromosome evolution and genomic divergence in the fish Hoplias malabaricus (Characiformes, Erythrinidae). Front Genet. 2018; 9(71):1-12. https://doi.org/10.3389/fgene.2018.00071
» https://doi.org/10.3389/fgene.2018.00071 - Silva DMZA, Utsunomia R, Pansonato-Alves JC, Oliveira C, Foresti F. Chromosomal mapping of repetitive DNA sequences in five species of Astyanax (Characiformes, Characidae) reveals independent location of U1 and U2 snRNA sites and association of U1 snRNA and 5S rDNA. Cytogenet Genome Res. 2015; 146:144-52. https://doi.org/10.1159/000438813
» https://doi.org/10.1159/000438813 - Stewart DJ. A New Species of Arapaima (Osteoglossomorpha: Osteoglossidae) from the Solimões River, Amazonas State, Brazil. Copeia. 2013a; 2013(3):470-76. https://doi.org/10.1643/CI-12-017
» https://doi.org/10.1643/CI-12-017 - Stewart DJ. Re-description of Arapaima agassizii (Valenciennes), a rare fish from Brazil (Osteoglossomorpha: Osteoglossidae). Copeia. 2013b; 2013(1):38-51. https://doi.org/10.1643/CI-12-013
» https://doi.org/10.1643/CI-12-013 - Sumner AT. A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res. 1972; 75(1):304-06. https://doi.org/10.1016/0014-4827(72)90558-7
» https://doi.org/10.1016/0014-4827(72)90558-7 - Supiwong W, Pinthong K, Seetapan K, Saenjundaeng P, Bertollo LAC, Oliveira EA, Yano CF, Liehr T, Phimphan S, Tanomtong A, Cioffi MB. Karyotype diversity and evolutionary trends in the Asian swamp eel Monopterus albus (Synbranchiformes, Synbranchidae): a case of chromosomal speciation?. BMC Genet. 2019; 19(73):1-9. https://doi.org/10.1186/s12862-019-1393-4
» https://doi.org/10.1186/s12862-019-1393-4 - Symonová R, Sember A, Majtánová Z, Ráb P. Characterization of fish genomes by GISH and CGH. In: Ozouf-Costaz C, Pisano E, Foresti F, Toledo LFA, editors. Fish cytogenetic techniques: ray-fin fishes and chondrichthyans. Boca Raton: CRC Press; 2015. p.118-31.
- Symonová R, Ocalewicz K, Kirtiklis L, Delmastro GB, Pelikánová Š, Garcia S, Kovařík A. Higher-order organisation of extremely amplified, potentially functional and massively methylated 5S rDNA in European pikes (Esox sp.). BMC Genomics. 2017; 18(391):1-16. https://doi.org/10.1186/s12864-017-3774-7
» https://doi.org/10.1186/s12864-017-3774-7 - Torati LS, Taggart JB, Varela ES, Araripe J, Wehner S, Migaud H. Genetic diversity and structure in Arapaima gigaspopulations from Amazon and Araguaia-Tocantins river basins. BMC Genet. 2019; 20(13):1-13. https://doi.org/10.1186/s12863-018-0711-y
» https://doi.org/10.1186/s12863-018-0711-y - Vitorino CA, Nogueira F, Souza IL, Araripe J, Venere PC. Low genetic diversity and structuring of the Arapaima (Osteoglossiformes, Arapaimidae) population of the Araguaia-Tocantins basin. Front Genet. 2017; 8(159):1-10. https://doi.org/10.3389/fgene.2017.00159
» https://doi.org/10.3389/fgene.2017.00159 - Wright S. Evolution in Mendelian populations. Genetics. 1931; 16:97-159. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1201091/
» https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1201091/ - Yano CF, Bertollo LAC, Rebordinos L, Merlo MA, Liehr T, Portela-Bens S, Cioffi MB. Evolutionary dynamics of rDNAs and U2 small nuclear DNAs in Triportheus(Characiformes, Triportheidae): high variability and particular syntenic organization. Zebrafish. 2017; 14(2):146-54. https://doi.org/10.1089/zeb.2016.1351
» https://doi.org/10.1089/zeb.2016.1351 - Zwick MS, Hanson RE, Islam-Faridi MN, Stelly DM, Wing RA, Price HJ, McKnight TD. A rapid procedure for the isolation of C0t-1 DNA from plants. Genome. 1997; 40(1):138-42. https://doi.org/10.1139/g97-020
» https://doi.org/10.1139/g97-020
ADDITIONAL NOTES
-
HOW TO CITE THIS ARTICLE
Oliveira EA, Sassi FMC, Perez MF, Bertollo LAC, Ráb P, Ezaz T, Hatanaka T, Viana PF, Feldberg E, de Oliveira EHC, Cioffi MB. Comparative cytogenetic survey of the giant bonytongue Arapaima fish (Osteoglossiformes: Arapaimidae), across different Amazonian and Tocantins/Araguaia River basins. Neotrop Ichthyol. 2020; 18(4):e200055. https://doi.org/10.1590/1982-0224-2020-0055
Edited by
Publication Dates
-
Publication in this collection
16 Nov 2020 -
Date of issue
2020
History
-
Received
29 June 2020 -
Accepted
18 Sept 2020